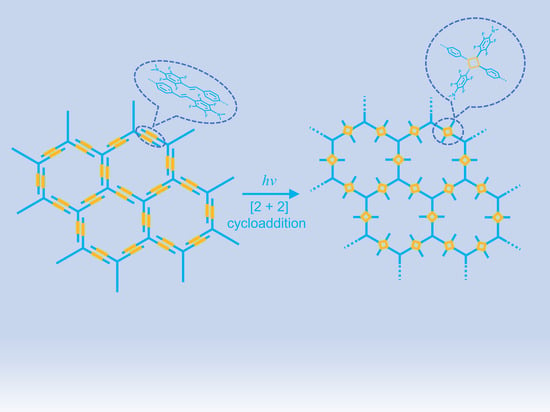
Interfacial Synthesis of an Ultrathin Two-Dimensional Polymer Film via [2 + 2] Photocycloaddition
Abstract
1. Introduction
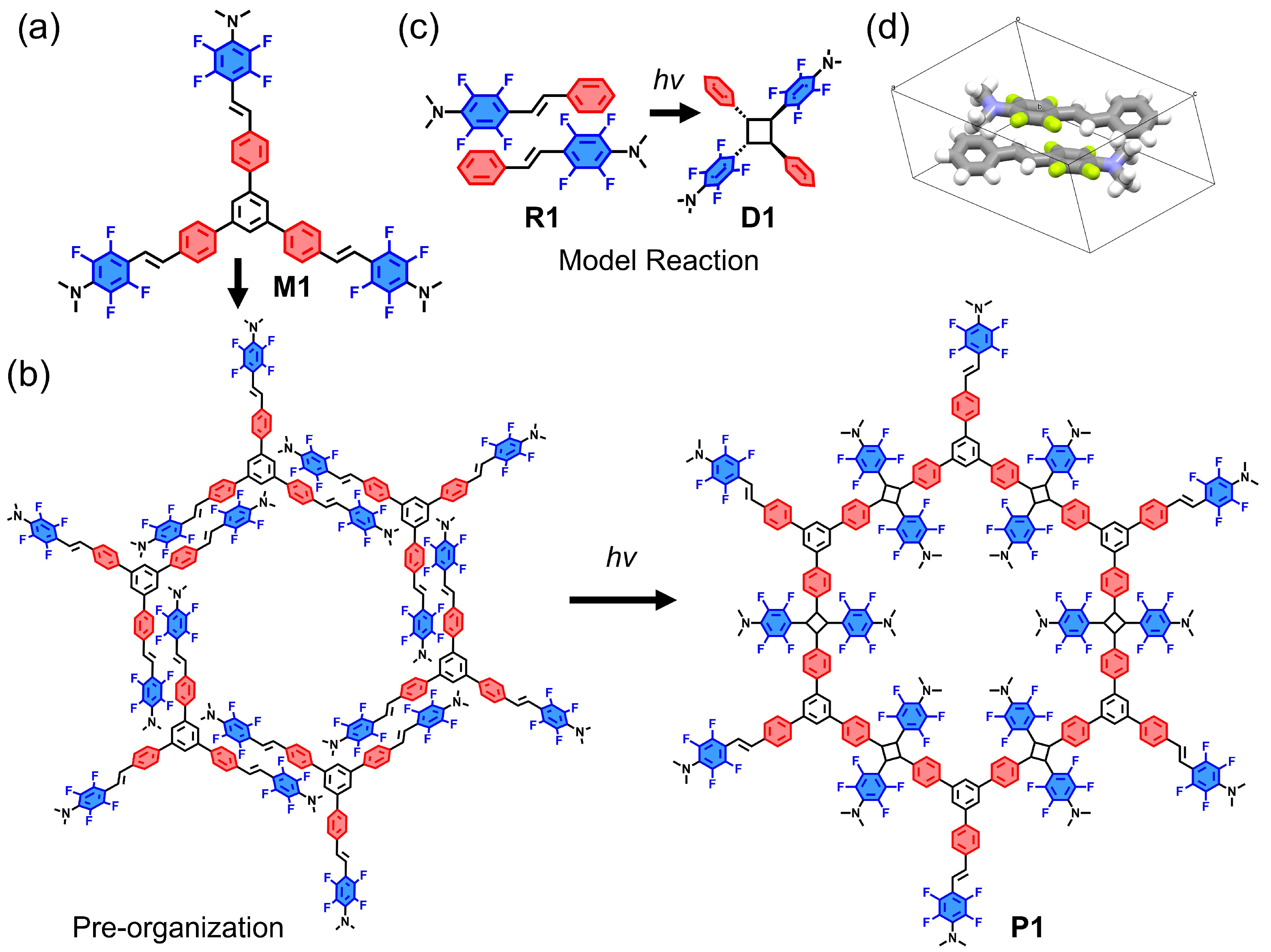
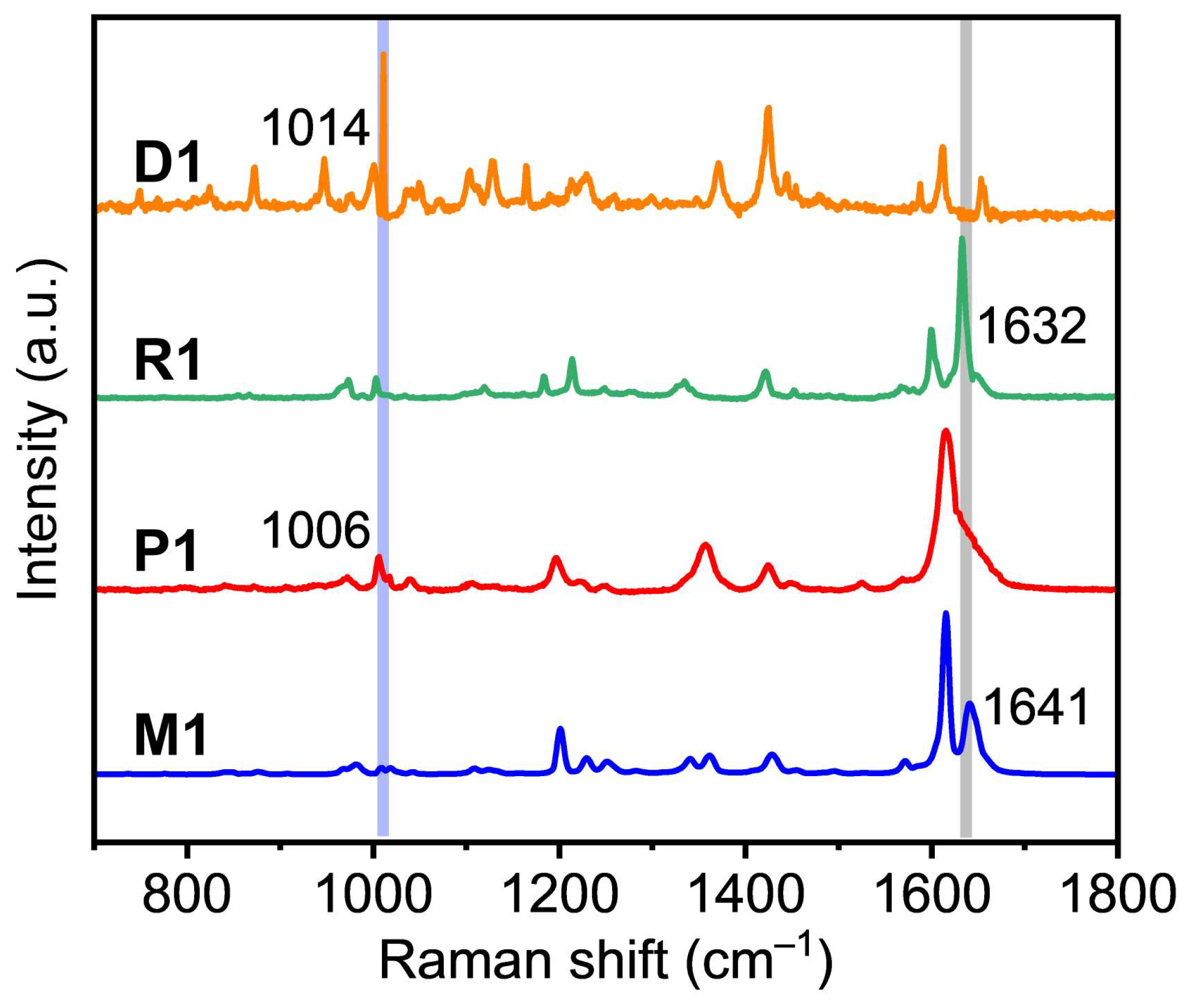
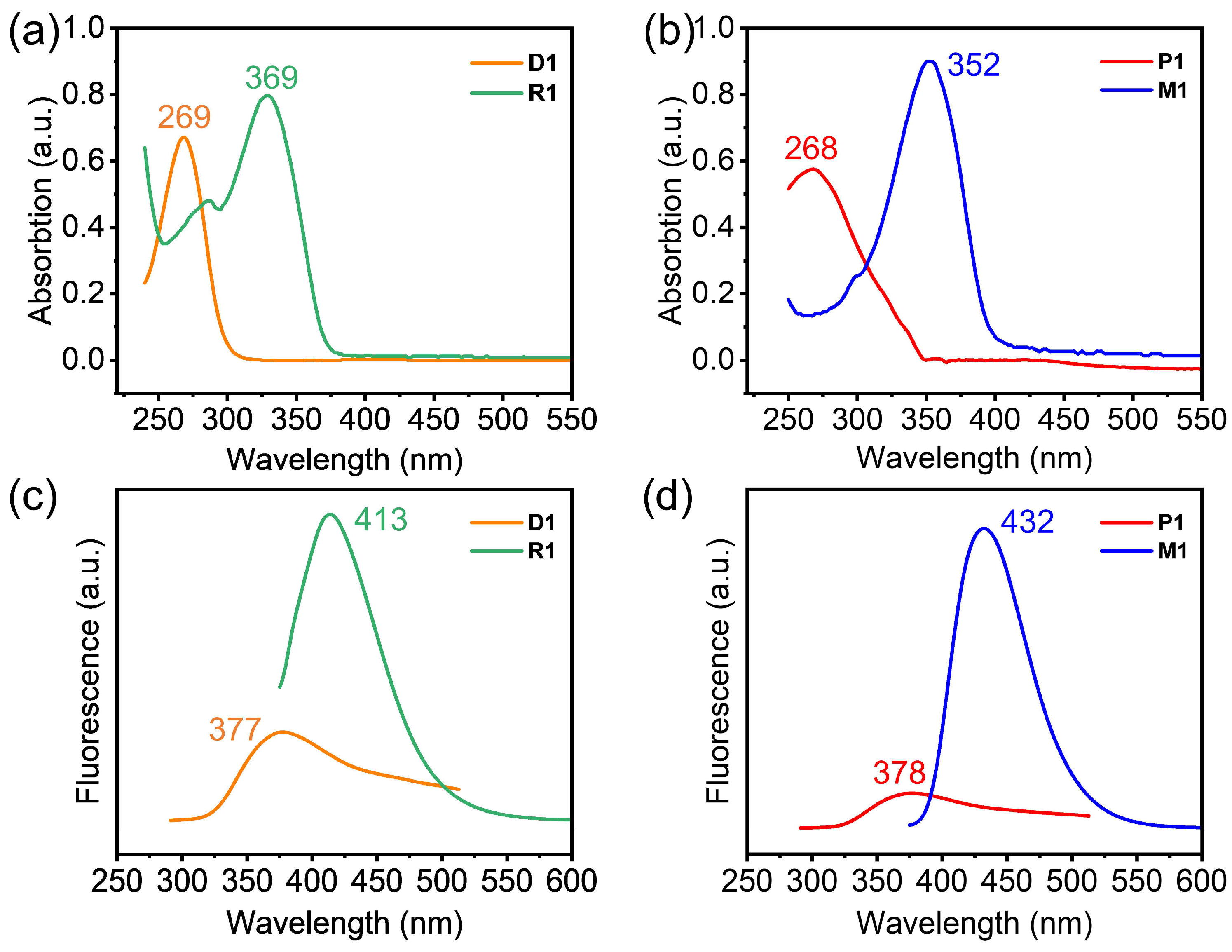
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterizations
3.3. Synthesis Details
3.3.1. Compound (1)
3.3.2. Compound R1
3.3.3. Compound D1
3.3.4. Compound R2
3.3.5. Compound M1
3.3.6. The Preparation of the 2D Polymer Film P1
3.3.7. Film P1 Transfer and Spanning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sakamoto, J.; van Heijst, J.; Lukin, O.; Schluter, A.D. Two-dimensional polymers: Just a dream of synthetic chemists? Angew. Chem. Int. Ed. Engl. 2009, 48, 1030–1069. [Google Scholar] [CrossRef] [PubMed]
- Colson, J.W.; Dichtel, W.R. Rationally synthesized two-dimensional polymers. Nat. Chem. 2013, 5, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Cao, D.; Dai, L. Well-defined two dimensional covalent organic polymers: Rational design, controlled syntheses, and potential applications. Polym. Chem. 2015, 6, 1896–1911. [Google Scholar] [CrossRef]
- Payamyar, P.; King, B.T.; Ottinger, H.C.; Schluter, A.D. Two-dimensional polymers: Concepts and perspectives. Chem. Commun. 2016, 52, 18–34. [Google Scholar] [CrossRef]
- Rodriguez-San-Miguel, D.; Amo-Ochoa, P.; Zamora, F. MasterChem: Cooking 2D-polymers. Chem. Commun. 2016, 52, 4113–4127. [Google Scholar] [CrossRef]
- Liu, H.; Kan, X.-N.; Wu, C.-Y.; Pan, Q.-Y.; Li, Z.-B.; Zhao, Y.-J. Synthetic Two-dimensional Organic Structures. Chin. J. Polym. Sci. 2017, 36, 425–444. [Google Scholar] [CrossRef]
- Wang, W.; Schluter, A.D. Synthetic 2D Polymers: A Critical Perspective and a Look into the Future. Macromol. Rapid Commun. 2019, 40, e1800719. [Google Scholar] [CrossRef]
- Evans, A.M.; Strauss, M.J.; Corcos, A.R.; Hirani, Z.; Ji, W.; Hamachi, L.S.; Aguilar-Enriquez, X.; Chavez, A.D.; Smith, B.J.; Dichtel, W.R. Two-Dimensional Polymers and Polymerizations. Chem. Rev. 2022, 122, 442–564. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, T.; Feng, X. Interface-Assisted Synthesis of 2D Materials: Trend and Challenges. Chem. Rev. 2018, 118, 6189–6235. [Google Scholar] [CrossRef]
- Liang, R.R.; Jiang, S.Y.; Ru-Han, A.; Zhao, X. Two-dimensional covalent organic frameworks with hierarchical porosity. Chem. Soc. Rev. 2020, 49, 3920–3951. [Google Scholar] [CrossRef]
- Niu, T.; Hua, C.; Zhou, M. On-Surface Synthesis toward Two-Dimensional Polymers. J. Phys. Chem. Lett. 2022, 13, 8062–8077. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, T.; Xue, Q. Recent advances in single-crystalline two-dimensional polymers: Synthesis, characterization and challenges. Chin. Chem. Lett. 2022, 33, 4989–5000. [Google Scholar] [CrossRef]
- Cai, S.L.; Zhang, W.G.; Zuckermann, R.N.; Li, Z.T.; Zhao, X.; Liu, Y. The Organic Flatland-Recent Advances in Synthetic 2D Organic Layers. Adv. Mater. 2015, 27, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tsujimoto, M.; Packwood, D.; Duong, N.T.; Nishiyama, Y.; Kadota, K.; Kitagawa, S.; Horike, S. Construction of a Hierarchical Architecture of Covalent Organic Frameworks via a Postsynthetic Approach. J. Am. Chem. Soc. 2018, 140, 2602–2609. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruna, H.D.; Dichtel, W.R. Beta-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef]
- Shinde, D.B.; Sheng, G.; Li, X.; Ostwal, M.; Emwas, A.H.; Huang, K.W.; Lai, Z. Crystalline 2D Covalent Organic Framework Membranes for High-Flux Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2018, 140, 14342–14349. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wang, J.; Li, X. Two-Dimensional Covalent Organic Frameworks (COFs) for Membrane Separation: A Mini Review. Ind. Eng. Chem. Res. 2019, 58, 15394–15406. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, H.; Zhao, Y.; Chen, S.; Xue, B.; Kan, X.; Huang, X.; Liu, J.; Li, Z. Preparation of N-Graphdiyne Nanosheets at Liquid/Liquid Interface for Photocatalytic NADH Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 2740–2744. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Z.; Xu, P.; Li, L.; Zeng, G.; Xiao, R.; Tang, Z.; Huang, D.; Tang, L.; Lai, C.; et al. Recent progress in covalent organic framework thin films: Fabrications, applications and perspectives. Chem. Soc. Rev. 2019, 48, 488–516. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Cheng, Y.; Pu, Y.; Zhao, D. Two-Dimensional Membranes: New Paradigms for High-Performance Separation Membranes. Chem. Asian J. 2020, 15, 2241–2270. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Bradshaw, N.P.; Litchfield, B.; Strauss, M.J.; Seckman, B.; Ryder, M.R.; Castano, I.; Gilmore, C.; Gianneschi, N.C.; Mulzer, C.R.; et al. High-Sensitivity Acoustic Molecular Sensors Based on Large-Area, Spray-Coated 2D Covalent Organic Frameworks. Adv. Mater. 2020, 32, e2004205. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cui, L.; Zhao, H.; Zhang, X. In Situ Generation of Regularly Ordered 2D Ultrathin Covalent Organic Framework Films for Highly Sensitive Photoelectrochemical Bioanalysis. ACS Appl. Mater. Interfaces 2020, 12, 47090–47098. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ye, J.; Shen, Y.; Fang, Q.; Liu, F. Covalent triazine frameworks composite membrane (CdS/CTF-1) with enhanced photocatalytic in-situ cleaning and disinfection properties for sustainable separation. Chem. Eng. J. 2021, 421, 127784. [Google Scholar] [CrossRef]
- Matsumoto, M.; Valentino, L.; Stiehl, G.M.; Balch, H.B.; Corcos, A.R.; Wang, F.; Ralph, D.C.; Mariñas, B.J.; Dichtel, W.R. Lewis-Acid-Catalyzed Interfacial Polymerization of Covalent Organic Framework Films. Chemistry 2018, 4, 308–317. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Sun, Q.; Shao, F.; Pan, Q.; Zhuang, T.; Zhao, Y. Interfacial Synthesis of a Monolayered Fluorescent Two-Dimensional Polymer through Dynamic Imine Chemistry. ChemistryOpen 2020, 9, 381–385. [Google Scholar] [CrossRef]
- Chen, R.; Wang, D.; Hao, W.; Shao, F.; Zhao, Y. Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition. Chem. Commun. 2021, 57, 5794–5797. [Google Scholar] [CrossRef]
- Ou, Z.; Liang, Z.; Dong, X.; Tan, F.; Gong, L.; Zhao, P.; Wang, H.; Liu, W.; Zheng, Z. Surfactants Mediated Synthesis of Highly Crystalline Thin Films of Imine-Linked Covalent Organic Frameworks on Water Surface. Chin. J. Chem. 2021, 39, 3322–3328. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, Y.; Leist, C.; Ou, Z.; Polozij, M.; Wang, Z.; Mucke, D.; Dong, R.; Zheng, Z.; Heine, T.; et al. Optimal acceleration voltage for near-atomic resolution imaging of layer-stacked 2D polymer thin films. Nat. Commun. 2022, 13, 3948. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Sun, B.; Cai, S.; Chen, Z.; Lv, Y.; Zhang, J.; Liu, Y. Expeditious synthesis of covalent organic frameworks: A review. J. Mater. Chem. A 2020, 8, 16045–16060. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Lyle, S.J.; Waller, P.J.; Yaghi, O.M. Covalent Organic Frameworks: Organic Chemistry Extended into Two and Three Dimensions. Trends Chem. 2019, 1, 172–184. [Google Scholar] [CrossRef]
- Smith, B.J.; Parent, L.R.; Overholts, A.C.; Beaucage, P.A.; Bisbey, R.P.; Chavez, A.D.; Hwang, N.; Park, C.; Evans, A.M.; Gianneschi, N.C.; et al. Colloidal Covalent Organic Frameworks. ACS Cent. Sci. 2017, 3, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hu, Y.; Ortiz, M.; Huang, S.; Ge, Y.; Zhang, W. Confined growth of ordered organic frameworks at an interface. Chem. Soc. Rev. 2020, 49, 4637–4666. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, R.; Su, Y.; Shi, B.; You, X.; Guo, W.; Ma, Y.; Yuan, J.; Wang, F.; Jiang, Z. Polydopamine-modulated covalent organic framework membranes for molecular separation. J. Mater. Chem. A 2019, 7, 18063–18071. [Google Scholar] [CrossRef]
- Tao, Y.; Ji, W.; Ding, X.; Han, B.-H. Exfoliated covalent organic framework nanosheets. J. Mater. Chem. A 2021, 9, 7336–7365. [Google Scholar] [CrossRef]
- Berlanga, I.; Ruiz-Gonzalez, M.L.; Gonzalez-Calbet, J.M.; Fierro, J.L.; Mas-Balleste, R.; Zamora, F. Delamination of layered covalent organic frameworks. Small 2011, 7, 1207–1211. [Google Scholar] [CrossRef]
- Chandra, S.; Kandambeth, S.; Biswal, B.P.; Lukose, B.; Kunjir, S.M.; Chaudhary, M.; Babarao, R.; Heine, T.; Banerjee, R. Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination. J. Am. Chem. Soc. 2013, 135, 17853–17861. [Google Scholar] [CrossRef]
- Khayum, M.A.; Kandambeth, S.; Mitra, S.; Nair, S.B.; Das, A.; Nagane, S.S.; Mukherjee, R.; Banerjee, R. Chemically Delaminated Free-Standing Ultrathin Covalent Organic Nanosheets. Angew. Chem. Int. Ed. Engl. 2016, 55, 15604–15608. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Yin, S.; Sun, J.; Wang, C. Twist Building Blocks from Planar to Tetrahedral for the Synthesis of Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 3718–3723. [Google Scholar] [CrossRef]
- Xu, S.; Li, Y.; Biswal, B.P.; Addicoat, M.A.; Paasch, S.; Imbrasas, P.; Park, S.; Shi, H.; Brunner, E.; Richter, M.; et al. Luminescent sp2-Carbon-Linked 2D Conjugated Polymers with High Photostability. Chem. Mater. 2020, 32, 7985–7991. [Google Scholar]
- Liu, K.; Wang, L.; Dong, R. Two-dimensional conjugated polymer films via liquid-interface-assisted synthesis toward organic electronic devices. J. Mater. Chem. C 2020, 8, 10696–10718. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline Graphdiyne Nanosheets Produced at a Gas/Liquid or Liquid/Liquid Interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef]
- Kan, X.; Ban, Y.; Wu, C.; Pan, Q.; Liu, H.; Song, J.; Zuo, Z.; Li, Z.; Zhao, Y. Interfacial Synthesis of Conjugated Two-Dimensional N-Graphdiyne. ACS Appl. Mater. Interfaces 2018, 10, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Han, S.; Peng, D.; Wang, H.; Yang, J.; Zhao, P.; Ye, X.; Dong, X.; Zheng, Y.; Zheng, N.; et al. Nanoporous and Highly Thermal Conductive Thin Film of Single-Crystal Covalent Organic Frameworks Ribbons. J. Am. Chem. Soc. 2021, 143, 3927–3933. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Q.; Fu, H.; Wu, L.Z.; Feng, X. Controlled growth of organic 2D layered material thin films via interfacial methods. Chem. Commun. 2022, 58, 12384–12398. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Z.B.; Qi, Q.Y.; Yao, J.; Zhao, X. Polyamide Covalent Organic Framework Membranes for Molecular Sieving. ACS Appl. Mater. Interfaces 2022, 14, 37019–37027. [Google Scholar] [CrossRef] [PubMed]
- Rapakousiou, A.; Sakamoto, R.; Shiotsuki, R.; Matsuoka, R.; Nakajima, U.; Pal, T.; Shimada, R.; Hossain, A.; Masunaga, H.; Horike, S.; et al. Liquid/Liquid Interfacial Synthesis of a Click Nanosheet. Chemistry 2017, 23, 8443–8449. [Google Scholar] [PubMed]
- Sun, Q.; Pan, Q.; Ban, Y.; Liu, H.; Fan, C.; Sun, L.; Zhao, Y. Donor-Acceptor Interactions Induced Interfacial Synthesis of an Ultrathin Fluoric 2D Polymer by Photochemical [2 + 2] Cycloaddition. Chemistry 2021, 27, 3661–3664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, C.; Pan, Q.; Shao, F.; Sun, Q.; Chen, S.; Li, Z.; Zhao, Y. Interfacial synthesis of crystalline two-dimensional cyano-graphdiyne. Chem. Commun. 2020, 56, 3210–3213. [Google Scholar] [CrossRef] [PubMed]
- Valentino, L.; Matsumoto, M.; Dichtel, W.R.; Marinas, B.J. Development and Performance Characterization of a Polyimine Covalent Organic Framework Thin-Film Composite Nanofiltration Membrane. Environ. Sci. Technol. 2017, 51, 14352–14359. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 6, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Yan, Y.; Xia, F.; Huang, A.; Xian, Y. Highly Fluorescent Polyimide Covalent Organic Nanosheets as Sensing Probes for the Detection of 2,4,6-Trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9, 13415–13421. [Google Scholar] [CrossRef]
- Tao, P.; Yao, S.; Liu, F.; Wang, B.; Huang, F.; Wang, M. Recent advances in exfoliation techniques of layered and non-layered materials for energy conversion and storage. J. Mater. Chem. A 2019, 7, 23512–23536. [Google Scholar] [CrossRef]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef]
- Payamyar, P.; Kaja, K.; Ruiz-Vargas, C.; Stemmer, A.; Murray, D.J.; Johnson, C.J.; King, B.T.; Schiffmann, F.; Vandevondele, J.; Renn, A.; et al. Synthesis of a covalent monolayer sheet by photochemical anthracene dimerization at the air/water interface and its mechanical characterization by AFM indentation. Adv. Mater. 2014, 26, 2052–2058. [Google Scholar] [CrossRef]
- Murray, D.J.; Patterson, D.D.; Payamyar, P.; Bhola, R.; Song, W.; Lackinger, M.; Schluter, A.D.; King, B.T. Large area synthesis of a nanoporous two-dimensional polymer at the air/water interface. J. Am. Chem. Soc. 2015, 137, 3450–3453. [Google Scholar] [CrossRef]
- Khan, S.; Dutta, B.; Mir, M.H. Impact of solid-state photochemical [2 + 2] cycloaddition on coordination polymers for diverse applications. Dalton Trans. 2020, 49, 9556–9563. [Google Scholar]
- Rath, B.B.; Vittal, J.J. Photoreactive Crystals Exhibiting [2 + 2] Photocycloaddition Reaction and Dynamic Effects. Acc. Chem. Res. 2022, 55, 1445–1455. [Google Scholar] [CrossRef]
- Sarkar, D.; Bera, N.; Ghosh, S. [2 + 2] Photochemical Cycloaddition in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 1310–1326. [Google Scholar] [CrossRef]
- Sicignano, M.; Rodriguez, R.I.; Aleman, J. Recent Visible Light and Metal Free Strategies in [2 + 2] and [4 + 2] Photocycloadditions. Eur. J. Org. Chem. 2021, 2021, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- Kole, G.K.; Mir, M.H. Isolation of elusive cyclobutane ligands via a template-assisted photochemical [2 + 2] cycloaddition reaction and their utility in engineering crystalline solids. CrystEngComm 2022, 24, 3993–4007. [Google Scholar] [CrossRef]
- Braslau, A.; Deutsch, M.; Pershan, P.S.; Weiss, A.H.; Als-Nielsen, J.; Bohr, J. Surface roughness of water measured by X-ray refelctivity. Phys. Rev. Lett. 1985, 54, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Cantin, S.; Fichet, O.; Perrot, F.; Teyssié, D. Spontaneous Polymerization at the Air−Water Interface: A Brewster Angle Microscopy Study. Langmuir 2007, 23, 12243–12248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, H.; Ban, X.; Yuan, G.; Sun, Y.; Huang, B.; Duan, L.; Qiu, Y. Alcohol-soluble electron-transport small molecule for fully solution-processed multilayer white electrophosphorescent devices. Org. Lett. 2014, 16, 1140–1143. [Google Scholar] [CrossRef]
- Li, Z.; Twieg, R.J. Photocyclodehydrofluorination. Chemistry 2015, 21, 15534–15539. [Google Scholar] [CrossRef]
- Dai, W.; Shao, F.; Szczerbinski, J.; McCaffrey, R.; Zenobi, R.; Jin, Y.; Schluter, A.D.; Zhang, W. Synthesis of a Two-Dimensional Covalent Organic Monolayer through Dynamic Imine Chemistry at the Air/Water Interface. Angew. Chem. Int. Ed. Engl. 2016, 55, 213–217. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, Y.; Wang, H.; Xiao, Z.; Sun, L.; Pan, Q.; Zhao, Y. Interfacial Synthesis of an Ultrathin Two-Dimensional Polymer Film via [2 + 2] Photocycloaddition. Molecules 2023, 28, 1930. https://doi.org/10.3390/molecules28041930
Ban Y, Wang H, Xiao Z, Sun L, Pan Q, Zhao Y. Interfacial Synthesis of an Ultrathin Two-Dimensional Polymer Film via [2 + 2] Photocycloaddition. Molecules. 2023; 28(4):1930. https://doi.org/10.3390/molecules28041930
Chicago/Turabian StyleBan, Yanqi, Hui Wang, Zixuan Xiao, Lishui Sun, Qingyan Pan, and Yingjie Zhao. 2023. "Interfacial Synthesis of an Ultrathin Two-Dimensional Polymer Film via [2 + 2] Photocycloaddition" Molecules 28, no. 4: 1930. https://doi.org/10.3390/molecules28041930
APA StyleBan, Y., Wang, H., Xiao, Z., Sun, L., Pan, Q., & Zhao, Y. (2023). Interfacial Synthesis of an Ultrathin Two-Dimensional Polymer Film via [2 + 2] Photocycloaddition. Molecules, 28(4), 1930. https://doi.org/10.3390/molecules28041930