CVD Growth of Hematite Thin Films for Photoelectrochemical Water Splitting: Effect of Precursor-Substrate Distance on Their Final Properties
Abstract
:1. Introduction
2. Results and Discussion
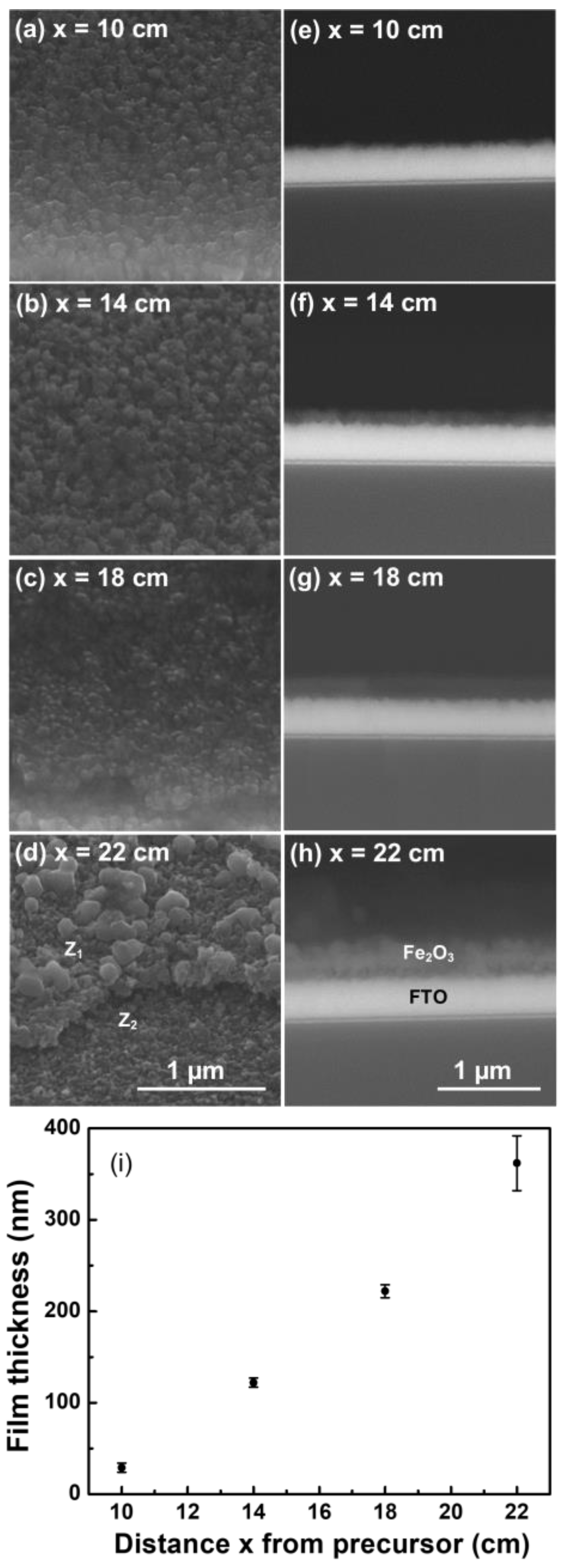
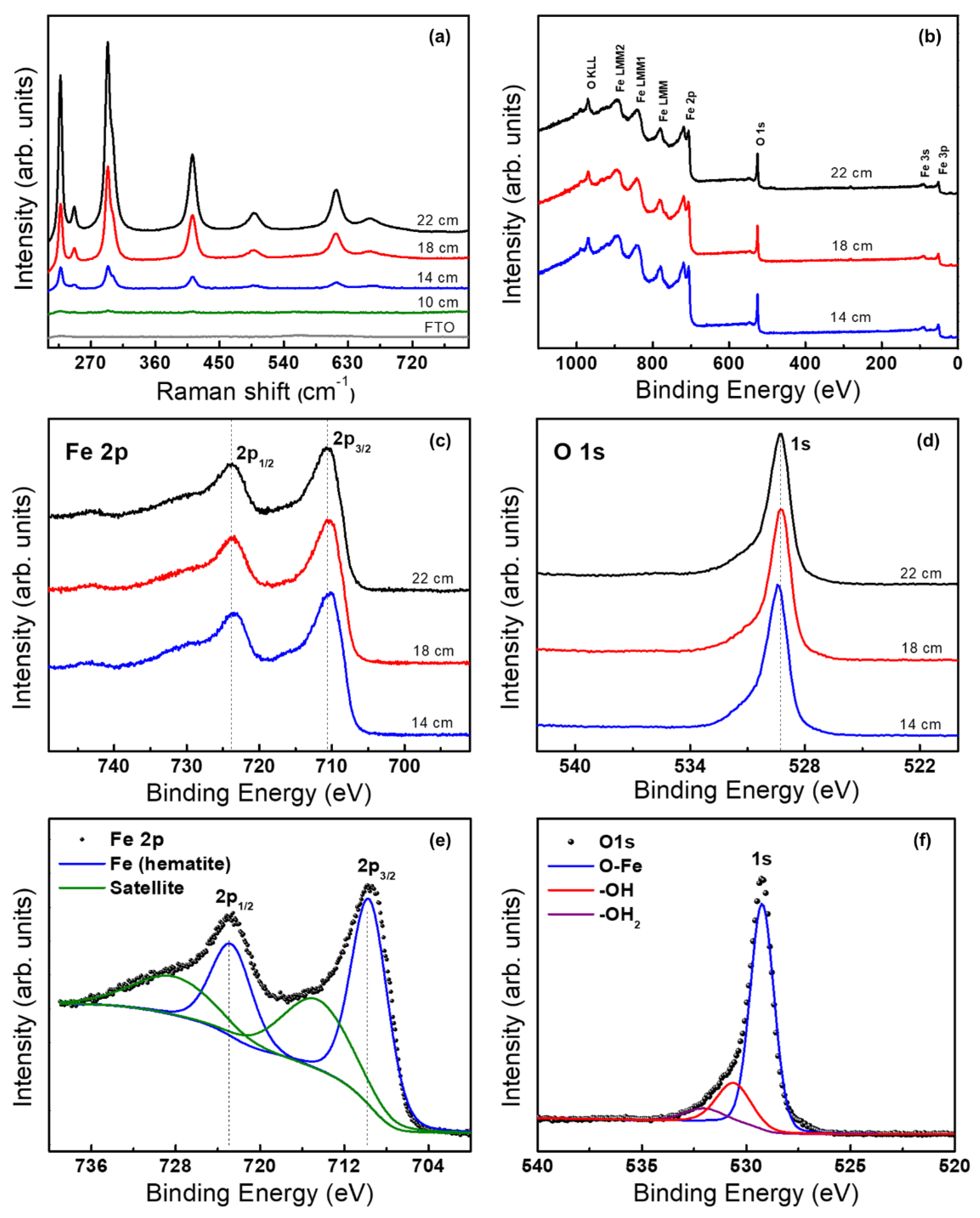
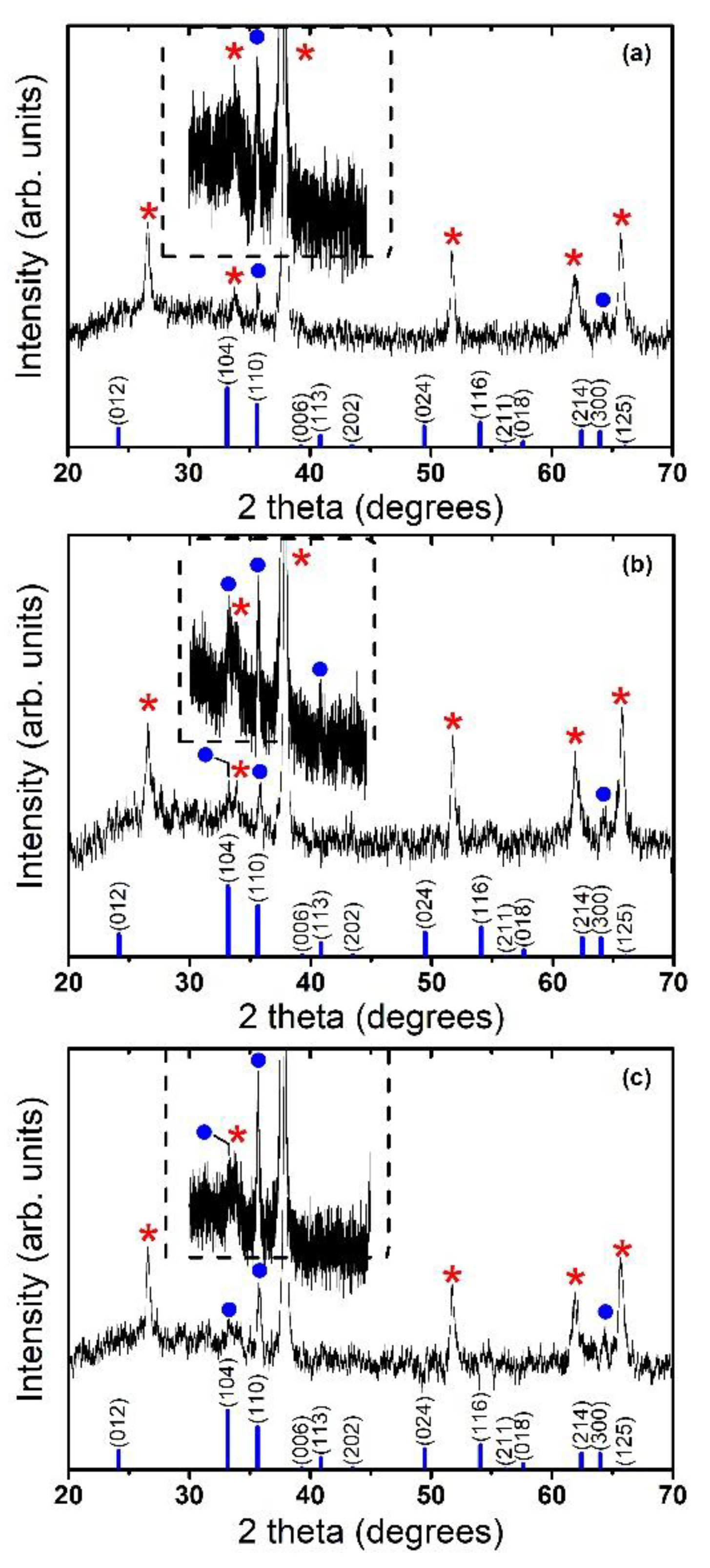
2.1. Morphological and Structural Characterization of the Hematite Thin Films
2.2. Optical Characterization of the Hematite Thin Films
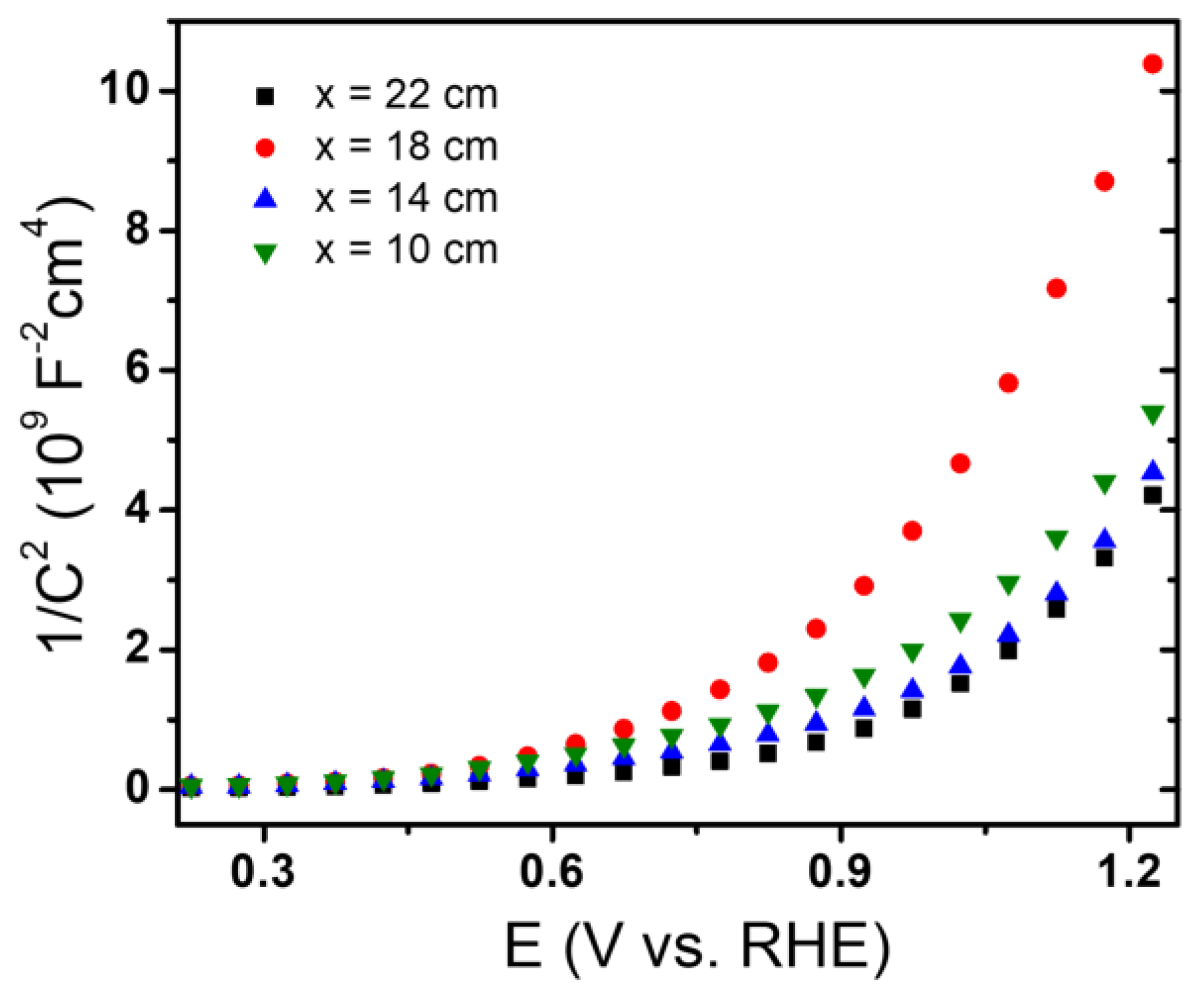
2.3. Photoelectrochemical Properties of the Hematite Thin Films
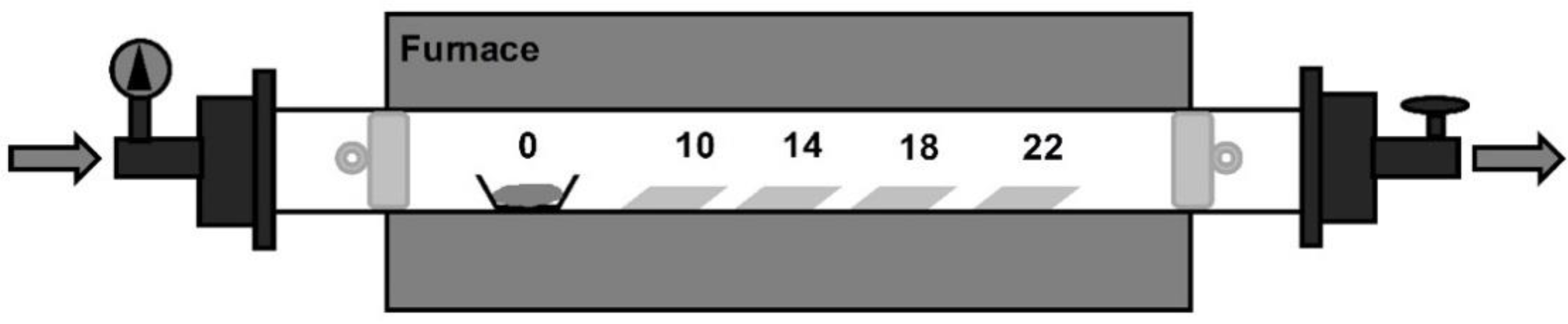
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schrebler, R.; Llewelyn, C.; Vera, F.; Cury, P.; Muñoz, E.; Del Rio, R.; Meier, H.G.; Córdova, R.; Dalchiele, E.A. An Electrochemical Deposition Route for Obtaining α-Fe2O3 Thin Films. Electrochem. Solid State Lett. 2007, 10, D95–D99. [Google Scholar] [CrossRef]
- Cesar, I.; Kay, A.; Martinez, J.A.G.; Grätzel, M. Translucent Thin Film Fe2O3 Photoanodes for Efficient Water Splitting by Sunlight: Nanostructure-Directing Effect of Si-Doping. J. Am. Chem. Soc. 2006, 128, 4582–4583. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, H.; Ding, S.; Xie, Q. Promoting photocarrier separation for photoelectrochemical water splitting in α-Fe2O3@C. J. Nanoparticle Res. 2019, 21, 153. [Google Scholar] [CrossRef]
- Le Formal, F.; Grätzel, M.; Sivula, K. Controlling Photoactivity in Ultrathin Hematite Films for Solar Water-Splitting. Adv. Funct. Mater. 2010, 20, 1099–1107. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; John, S.E.; Banerjee, S.; Misra, M. Water Photooxidation by Smooth and Ultrathin α-Fe2O3 Nanotube Arrays. Chem. Mater. 2009, 21, 3048–3055. [Google Scholar] [CrossRef]
- Kleiman-Shwarsctein, A.; Hu, Y.-S.; Forman, A.J.; Stucky, G.D.; McFarland, E.W. Electrodeposition of α-Fe2O3 Doped with Mo or Cr as Photoanodes for Photocatalytic Water Splitting. J. Phys. Chem. C 2008, 112, 15900–15907. [Google Scholar] [CrossRef]
- Choi, H.; Hong, Y.; Ryu, H.; Lee, W.-J. Photoelectrochemical properties of hematite thin films grown by MW-CBD. Surf. Coat. Technol. 2018, 333, 259–266. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.-X.; Zhang, W.-D. Photoelectrochemical properties of Ni-doped Fe2O3 thin films prepared by electrodeposition. Electrochim. Acta 2012, 59, 121–127. [Google Scholar] [CrossRef]
- Ruan, G.; Wu, S.; Wang, P.; Liu, J.; Cai, Y.; Tian, Z.; Ye, Y.; Liang, C.; Shao, G. Simultaneous doping and growth of Sn-doped hematite nanocrystalline films with improved photoelectrochemical performance. RSC Adv. 2014, 4, 63408–63413. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bansode, R.A.; Bhosale, C.H.; Rajpure, K.Y. Physical properties of hematite α-Fe2O3thin films: Application to photoelectrochemical solar cells. J. Semicond. 2011, 32, 013001. [Google Scholar] [CrossRef]
- Campo, L.; Pereyra, C.J.; Amy, L.; Elhordoy, F.; Marotti, R.E.; Martín, F.; Ramos-Barrado, J.R.; Dalchiele, E.A. Electrochemically Grown ZnO Nanorod Arrays Decorated with CdS Quantum Dots by Using a Spin-Coating Assisted Successive-Ionic-Layer-Adsorption and Reaction Method for Solar Cell Applications. ECS J. Solid State Sci. Technol. 2013, 2, 26–28. [Google Scholar] [CrossRef]
- Guerguerian, G.; Elhordoy, F.; Pereyra, C.J.; Marotti, R.E.; Martin, F.; Leinen, D.; Ramos-Barrado, J.R.; Dalchiele, E.A. ZnO nanorod/CdS nanocrystal core/shell-type heterostructures for solar cell applications. Nanotechnology 2011, 22, 505401. [Google Scholar] [CrossRef]
- Enache, C.; Liang, Y.; van de Krol, R. Characterization of structured α-Fe2O3 photoanodes prepared via electrodeposition and thermal oxidation of iron. Thin Solid Films 2011, 520, 1034–1040. [Google Scholar] [CrossRef]
- Pina, J.; Dias, P.; Serpa, C.; Azevedo, J.; Mendes, A.; de Melo, J.S.S. Phenomenological Understanding of Hematite Photoanode Performance. J. Phys. Chem. C 2021, 125, 8274–8284. [Google Scholar] [CrossRef]
- Berardi, S.; Cristino, V.; Bignozzi, C.A.; Grandi, S.; Caramori, S. Hematite-based photoelectrochemical interfaces for solar fuel production. Inorganica Chim. Acta 2022, 535, 120862. [Google Scholar] [CrossRef]
- Baldovi, H. Optimization of α-Fe2O3 Nanopillars Diameters for Photoelectrochemical Enhancement of α-Fe2O3-TiO2 Heterojunction. Nanomaterials 2021, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, H.; Liu, C.; Xie, Q.; Xu, L.; Li, H.; Wang, J.; Li, S. Enhanced photoelectrochemical water oxidation in Hematite: Accelerated charge separation with Co doping. Appl. Surf. Sci. 2021, 568, 150606. [Google Scholar] [CrossRef]
- Talibawo, J.; Kyesmen, P.I.; Cyulinyana, M.C.; Diale, M. Facile Zn and Ni Co-doped hematite nanorods for efficient photocatalytic water oxidation. Nanomaterials 2022, 12, 2961. [Google Scholar] [CrossRef]
- Jubb, A.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Y.; Kong, T.; Shang, Y.; Niu, F.; Diao, Z.; Shen, S. Protected Hematite Nanorod Arrays with Molecular Complex Co-Catalyst for Efficient and Stable Photoelectrochemical Water Oxidation. Eur. J. Inorg. Chem. 2018, 2019, 2078–2085. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, K. Optical properties of nanocrystallite films of α-Fe2O3 and α-Fe2-xCrxO3 (0.0 ≤ x ≤ 0.9) deposited on glass substrates. Mater. Res. Express 2017, 4, 075003. [Google Scholar] [CrossRef]
- Akl, A.A. Optical properties of crystalline and non-crystalline iron oxide thin films deposited by spray pyrolysis. Appl. Surf. Sci. 2004, 233, 307–319. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol–gel method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Leduc, J.; Goenuellue, Y.; Ghamgosar, P.; You, S.; Mouzon, J.; Choi, H.; Vomiero, A.; Grosch, M.; Mathur, S. Electronically-Coupled Phase Boundaries in α-Fe2O3/Fe3O4 Nanocomposite Photoanodes for Enhanced Water Oxidation. ACS Appl. Nano Mater. 2019, 2, 334–342. [Google Scholar] [CrossRef]
- Stadler, D.; Brede, T.; Schwarzbach, D.; Maccari, F.; Fischer, T.; Gutfleisch, O.; Volkert, C.A.; Mathur, S. Anisotropy control in magnetic nanostructures through field-assisted chemical vapor deposition. Nanoscale Adv. 2019, 1, 4290–4295. [Google Scholar] [CrossRef] [Green Version]
- Liardet, L.; Katz, J.E.; Luo, J.; Grätzel, M.; Hu, X. An ultrathin cobalt–iron oxide catalyst for water oxidation on nanostructured hematite photoanodes. J. Mater. Chem. A 2019, 7, 6012–6020. [Google Scholar] [CrossRef]
- Lévy-Clément, C.; Elias, J. Optimization of the Design of Extremely Thin Absorber Solar Cells Based on Electrodeposited ZnO Nanowires. ChemPhysChem 2013, 14, 2321–2330. [Google Scholar] [CrossRef]
- Li, S.; Zhang, P.; Song, X.; Gao, L. Ultrathin Ti-doped hematite photoanode by pyrolysis of ferrocene. Int. J. Hydrogen Energy 2014, 39, 14596–14603. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.-N.; Hwang, B.-J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Li, W.; Chen, H.; Fang, W.; Tian, P.; Xiao, F.; Zhao, L. Hematite nanorod arrays top-decorated with an MIL-101 layer for photoelectrochemical water oxidation. Chem. Commun. 2019, 55, 11382–11385. [Google Scholar] [CrossRef]
- Gasparotto, A.; Carraro, G.; Maccato, C.; Barreca, D. On the use of Fe(dpm)3 as precursor for the thermal-CVD growth of hematite nanostructures. Phys. Status Solidi (A) 2017, 214, 1600779. [Google Scholar] [CrossRef]
- Fornari, C.I.; Fornari, G.; Rappl, P.H.D.O.; Abramof, E.; Travelho, J.D.S. Monte Carlo Simulation of Epitaxial Growth. In Epitaxy; IntechOpen: Rijeka, Croatia, 2018; pp. 113–129. [Google Scholar] [CrossRef] [Green Version]
- Ohring, M. The Materials Science of thin Films; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Mansour, H.; Letifi, H.; Bargougui, R.; De Almeida-Didry, S.; Negulescu, B.; Autret-Lambert, C.; Gadri, A.; Ammar, S. Structural, optical, magnetic and electrical properties of hematite (α-Fe2O3) nanoparticles synthesized by two methods: Polyol and precipitation. Appl. Phys. A 2017, 123, 787. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D.; Zhang, X.; Jin, W.; Kashkarov, P.; Zhang, H. Synthesis and characterization of single-crystalline α-Fe2O3 nanoleaves. Phys. E Low Dimens. Syst. Nanostructures 2009, 41, 806–811. [Google Scholar] [CrossRef]
- Ingler, W.B.; Khan, S.U. Photoresponse of spray pyrolytically synthesized copper-doped p-Fe2O3 thin film electrodes in water splitting. Int. J. Hydrogen Energy 2005, 30, 821–827. [Google Scholar] [CrossRef]
- Powder Diffraction File, Joint Committee for Powder Diffraction Studies (JCPDS) File No. JCPDS 86-0550 (Rhombohedrally Centered Hexagonal Structure of Fe2O3, Hematite), n.d.
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: Reading, MA, USA, 1956. [Google Scholar]
- Nasibulin, A.G.; Rackauskas, S.; Jiang, H.; Tian, Y.; Mudimela, P.R.; Shandakov, S.D.; Nasibulina, L.I.; Jani, S.; Kauppinen, E.I. Simple and rapid synthesis of α-Fe2O3 nanowires under ambient conditions. Nano Res. 2009, 2, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Liao, L.; Zheng, Z.; Yan, B.; Zhang, J.X.; Gong, H.; Li, J.C.; Liu, C.; Shen, Z.X.; Yu, T. Morphology Controllable Synthesis of α-Fe2O3 1D Nanostructures: Growth Mechanism and Nanodevice Based on Single Nanowire. J. Phys. Chem. C 2008, 112, 10784–10788. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors, 2nd ed.; Dover Publications: New York, NY, USA, 1976. [Google Scholar]
- Sapoval, B.; Hermann, C. Physics of Semiconductors; Springer: New York, NY, USA, 1995. [Google Scholar]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Mohanty, S.; Ghose, J. Studies on some α-Fe2O3 photoelectrodes. J. Phys. Chem. Solids 1992, 53, 81–91. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Liston, D.B.; Lovejoy, J.A.; Deng, H.; Zhang, J.Z. Ultrafast Studies of Photoexcited Electron Dynamics in γ- and α-Fe2O3 Semiconductor Nanoparticles. J. Phys. Chem. B 1998, 102, 770–776. [Google Scholar] [CrossRef]
- Duret, A.; Grätzel, M. Visible Light-Induced Water Oxidation on Mesoscopic α-Fe2O3 Films Made by Ultrasonic Spray Pyrolysis. J. Phys. Chem. B 2005, 109, 17184–17191. [Google Scholar] [CrossRef]
- Glasscock, J.; Barnes, P.; Plumb, I.; Bendavid, A.; Martin, P. Structural, optical and electrical properties of undoped polycrystalline hematite thin films produced using filtered arc deposition. Thin Solid Films 2008, 516, 1716–1724. [Google Scholar] [CrossRef]
- Gilbert, B.; Frandsen, C.; Maxey, E.R.; Sherman, D.M. Band-gap measurements of bulk and nanoscale hematite by soft X-ray spectroscopy. Phys. Rev. B 2009, 79, 035108. [Google Scholar] [CrossRef] [Green Version]
- Rühle, S.; Anderson, A.Y.; Barad, H.-N.; Kupfer, B.; Bouhadana, Y.; Rosh-Hodesh, E.; Zaban, A. All-Oxide Photovoltaics. J. Phys. Chem. Lett. 2012, 3, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuhaili, M.; Saleem, M.; Durrani, S. Optical properties of iron oxide (α-Fe2O3) thin films deposited by the reactive evaporation of iron. J. Alloy. Compd. 2012, 521, 178–182. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis, structural, morphological, optical and magnetic characterization of iron oxide (α-Fe2O3) nanoparticles by precipitation method: Effect of varying the nature of precursor. Phys. E Low Dimens. Syst. Nanostructures 2018, 97, 328–334. [Google Scholar] [CrossRef]
- Kung, H.H.; Jarrett, H.S.; Sleight, A.W.; Ferretti, A. Semiconducting oxide anodes in photoassisted electrolysis of water. J. Appl. Phys. 1977, 48, 2463–2469. [Google Scholar] [CrossRef]
- Merchant, P.; Collins, R.; Kershaw, R.; Dwight, K.; Wold, A. The electrical, optical and photoconducting properties of Fe2−xCrxO3 (0 ≤ x ≤ 0.47). J. Solid State Chem. 1979, 27, 307–315. [Google Scholar] [CrossRef]
- Shinar, R.; Kennedy, J.H. Photoactivity of doped αFe2O3 electrodes. Sol. Energy Mater. 1982, 6, 323–335. [Google Scholar] [CrossRef]
- Bojorge, C.D.; Kent, V.R.; Teliz, E.; Cánepa, H.R.; Henríquez, R.; Gómez, H.; Marotti, R.E.; Dalchiele, E.A. Zinc-oxide nanowires electrochemically grown onto sol-gel spin-coated seed layers. Phys. Status Solidi (A) 2011, 208, 1662–1669. [Google Scholar] [CrossRef]
- Guerguerian, G.; Elhordoy, F.; Pereyra, C.J.; Marotti, R.E.; Martín, F.; Leinen, D.; Ramos-Barrado, J.R.; Dalchiele, E.A. ZnO/Cu2O heterostructure nanopillar arrays: Synthesis, structural and optical properties. J. Phys. D Appl. Phys. 2012, 45, 245301. [Google Scholar] [CrossRef]
- Piccinin, S. The band structure and optical absorption of hematite (α-Fe2O3): A first-principles GW-BSE study. Phys. Chem. Chem. Phys. 2019, 21, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rawat, N.; Hang, D.-R.; Lee, H.-N.; Kumar, R. Controlling band gap and refractive index in dopant-free α-Fe2O3 films. Electron. Mater. Lett. 2015, 11, 13–23. [Google Scholar] [CrossRef]
- Marotti, R.; Guerra, D.; Bello, C.; Machado, G.; Dalchiele, E. Bandgap energy tuning of electrochemically grown ZnO thin films by thickness and electrodeposition potential. Sol. Energy Mater. Sol. Cells 2004, 82, 85–103. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, C.; Zhang, X. Ti, Zn co-doped hematite photoanode for solar driven photoelectrochemical water oxidation. J. Energy Chem. 2019, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]

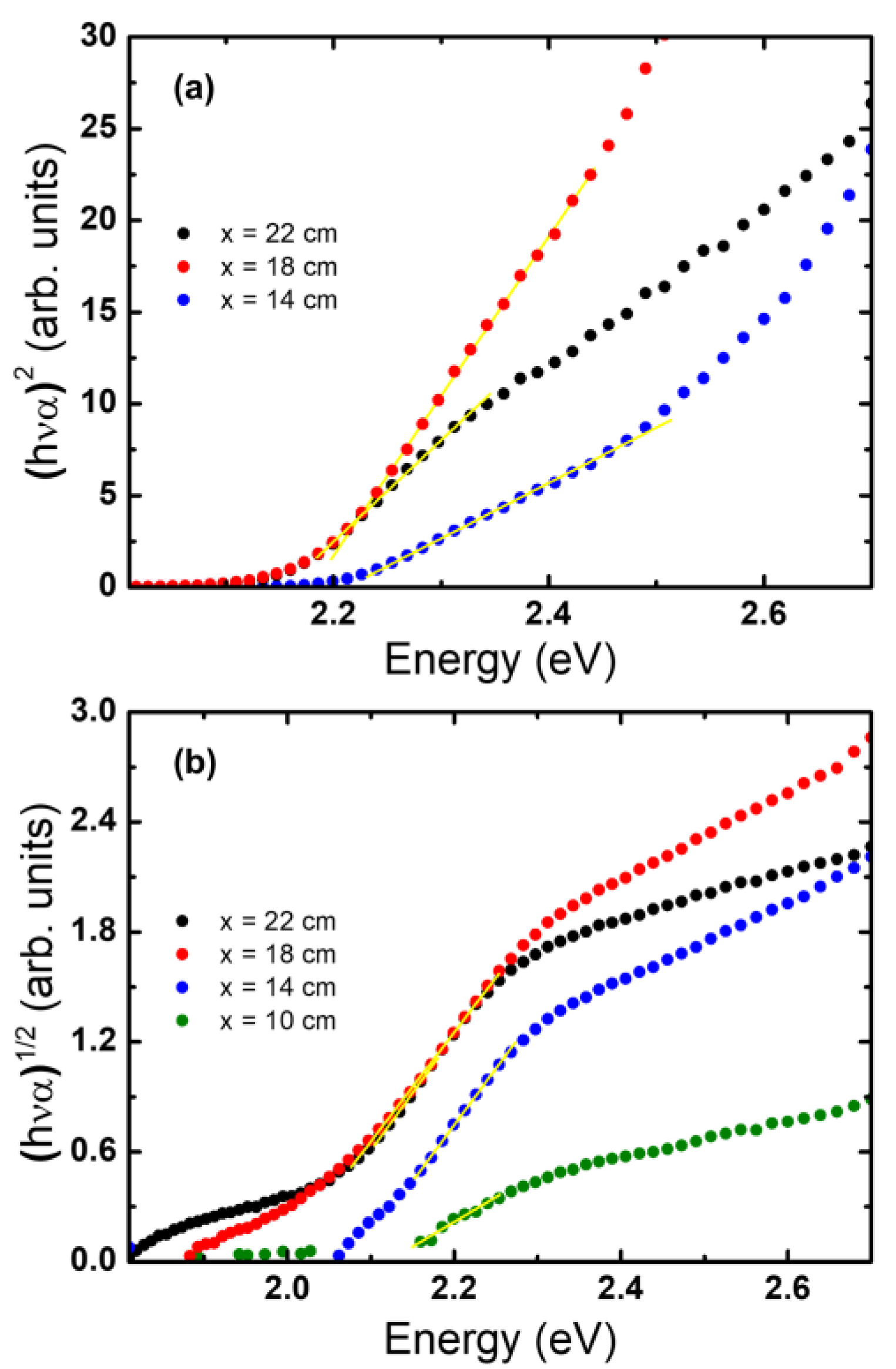

| Precursor-Substrate Distance Xsp (cm) | Direct Optical Bandgap (eV) | Indirect Optical Bandgap (eV) |
|---|---|---|
| 22 | 2.16 | 2.00 |
| 18 | 2.18 | 1.99 |
| 14 | 2.21 | 2.08 |
| 10 | 2.31 | 2.12 |
| Precursor-Substrate Distance xsp (cm) | Thin-Film Thickness (nm) | AE (%) | Eff. (%) |
|---|---|---|---|
| 22 | 362 | 65 | 0.46 |
| 18 | 222 | 75 | 1.35 |
| 14 | 122 | 56 | 0.50 |
| 10 | 29 | 13 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Izquierdo, L.; Spera, E.L.; Durán, B.; Marotti, R.E.; Dalchiele, E.A.; del Rio, R.; Hevia, S.A. CVD Growth of Hematite Thin Films for Photoelectrochemical Water Splitting: Effect of Precursor-Substrate Distance on Their Final Properties. Molecules 2023, 28, 1954. https://doi.org/10.3390/molecules28041954
Fernandez-Izquierdo L, Spera EL, Durán B, Marotti RE, Dalchiele EA, del Rio R, Hevia SA. CVD Growth of Hematite Thin Films for Photoelectrochemical Water Splitting: Effect of Precursor-Substrate Distance on Their Final Properties. Molecules. 2023; 28(4):1954. https://doi.org/10.3390/molecules28041954
Chicago/Turabian StyleFernandez-Izquierdo, Leunam, Enzo Luigi Spera, Boris Durán, Ricardo Enrique Marotti, Enrique Ariel Dalchiele, Rodrigo del Rio, and Samuel A. Hevia. 2023. "CVD Growth of Hematite Thin Films for Photoelectrochemical Water Splitting: Effect of Precursor-Substrate Distance on Their Final Properties" Molecules 28, no. 4: 1954. https://doi.org/10.3390/molecules28041954
APA StyleFernandez-Izquierdo, L., Spera, E. L., Durán, B., Marotti, R. E., Dalchiele, E. A., del Rio, R., & Hevia, S. A. (2023). CVD Growth of Hematite Thin Films for Photoelectrochemical Water Splitting: Effect of Precursor-Substrate Distance on Their Final Properties. Molecules, 28(4), 1954. https://doi.org/10.3390/molecules28041954







