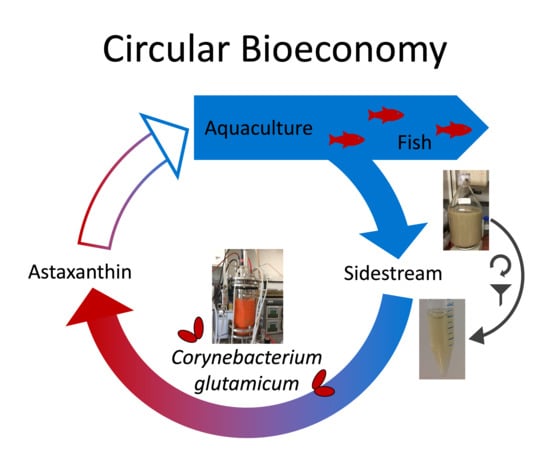
From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium glutamicum Using Aquaculture Sidestream
Abstract
1. Introduction
2. Results
2.1. Analysis of the Untreated Aquaculture Sidestream
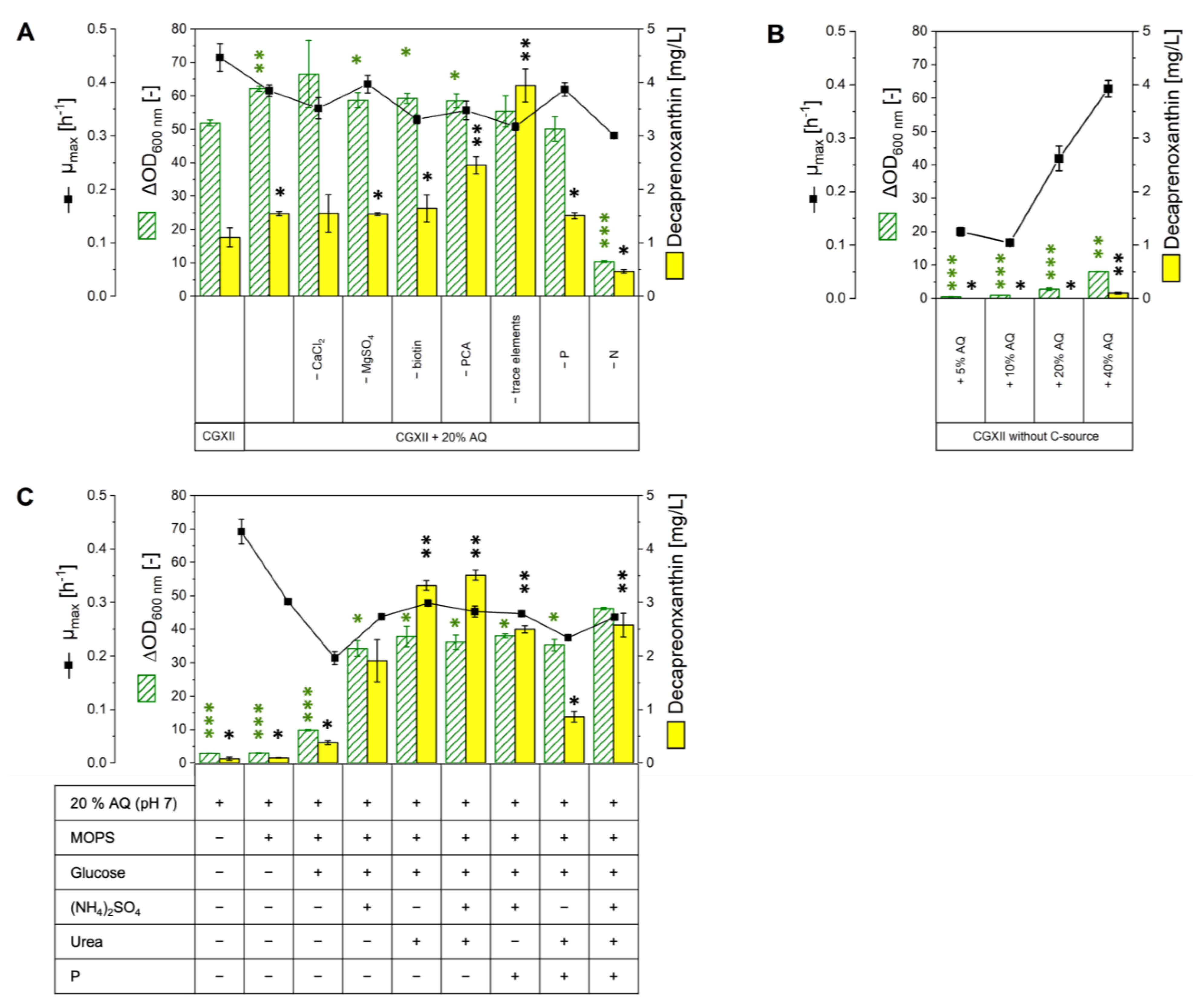
2.2. Growth in Various Media Based on or Supplemented with AQ
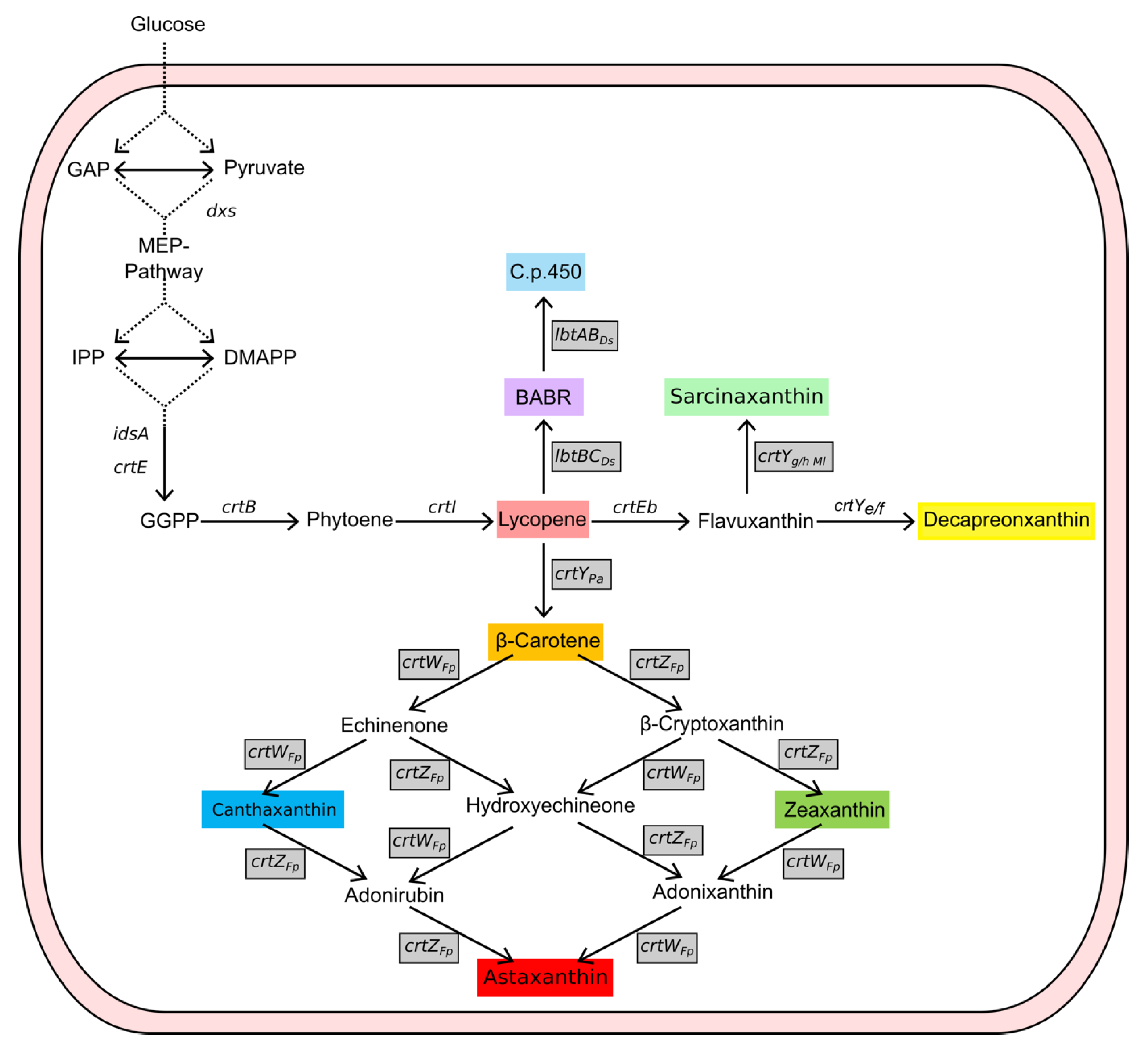
2.3. Carotenoid Production in AQ Supplemented Media
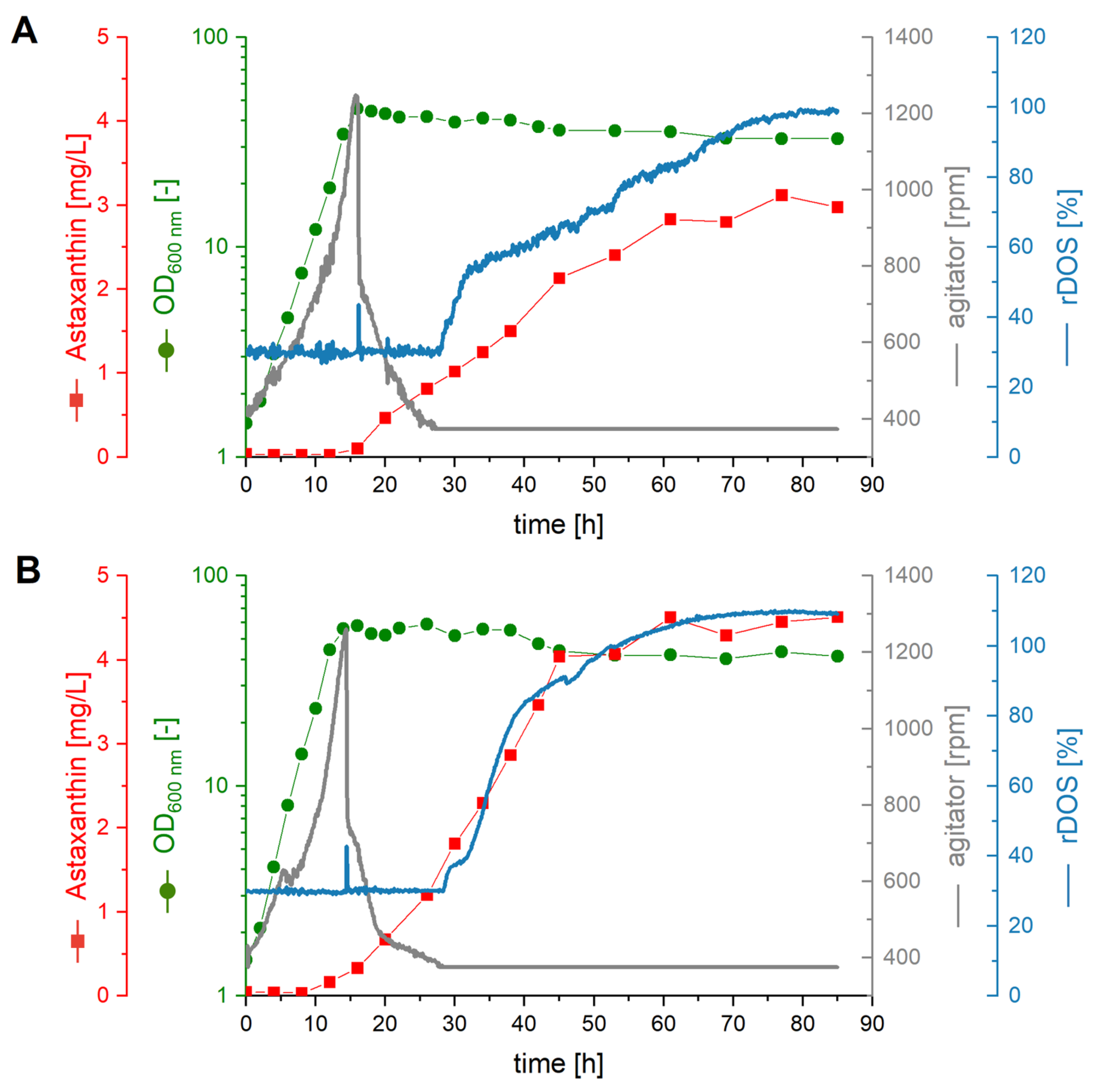
2.4. Fermentative Production of Astaxanthin in AQ Supplemented Media
3. Discussion
4. Materials and Methods
4.1. Preprocessing of the Aquaculture Sidestream
4.2. Microorangisms and Cultivation Conditions
4.3. Fermentative Production
4.4. Recombinant DNA Work
4.5. Quantification of Macro- and Micronutrients
4.6. High-Performance Liquid Chromatography (HPLC) Analysis
4.6.1. Quantification of Amino Acids and Amines
4.6.2. Quantification of Carbohydrates and Organic Acids
4.6.3. Quantification of Carotenoids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. A review on recirculating aquaculture system: Influence of stocking density on fish and crustacean behavior, growth performance, and immunity. Ann. Anim. Sci. 2022, 22, 873–884. [Google Scholar] [CrossRef]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.Á.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The Future of Food from the Sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Bostock, J.; Fletcher, D. Review of Recirculation Aquaculture System Technologies and Their Commercial Application; Stirling Aquaculture, Institute of Aquaculture, University of Stirling: Stirling, UK, 2014. [Google Scholar]
- Verdegem, M.C.J.; Bosma, R.H.; Verreth, J.A.J. Reducing Water Use for Animal Production through Aquaculture. Int. J. Water Resour. Dev. 2006, 22, 101–113. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New Developments in Recirculating Aquaculture Systems in Europe: A Perspective on Environmental Sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Masser, M.P.; Rakocy, J.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems. South. Reg. Aquac. Cent. Publ. 1992, 13. [Google Scholar]
- Campanati, C.; Willer, D.; Schubert, J.; Aldridge, D.C. Sustainable Intensification of Aquaculture through Nutrient Recycling and Circular Economies: More Fish, Less Waste, Blue Growth. Rev. Fish. Sci. Aquac. 2022, 30, 143–169. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso Jr, J.R.; et al. Achieving Sustainable Aquaculture: Historical and Current Perspectives and Future Needs and Challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- van Rijn, J. Waste Treatment in Recirculating Aquaculture Systems. Aquac. Eng. 2013, 53, 49–56. [Google Scholar] [CrossRef]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste Production in Aquaculture: Sources, Components and Managements in Different Culture Systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal Cultivation Using Aquaculture Wastewater: Integrated Biomass Generation and Nutrient Remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Milhazes-Cunha, H.; Otero, A. Valorisation of Aquaculture Effluents with Microalgae: The Integrated Multi-Trophic Aquaculture Concept. Algal Res. 2017, 24, 416–424. [Google Scholar] [CrossRef]
- Roy, E.D. Phosphorus Recovery and Recycling with Ecological Engineering: A Review. Ecol. Eng. 2017, 98, 213–227. [Google Scholar] [CrossRef]
- Khatoon, H.; Banerjee, S.; Syakir Syahiran, M.; Mat Noordin, N.Bt.; Munafi Ambok Bolong, A.; Endut, A. Re-Use of Aquaculture Wastewater in Cultivating Microalgae as Live Feed for Aquaculture Organisms. Desalination Water Treat. 2016, 57, 29295–29302. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Queiroz, M.I.; Hornes, M.O.; Gonçalves da Silva Manetti, A.; Zepka, L.Q.; Jacob-Lopes, E. Fish Processing Wastewater as a Platform of the Microalgal Biorefineries. Biosyst. Eng. 2013, 115, 195–202. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.-M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated Utilization of Microalgae Cultured in Aquaculture Wastewater: Wastewater Treatment and Production of Valuable Fatty Acids and Tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef]
- Grassi, T.L.M.; do Espírito Santo, E.F.; de Siqueira Marcos, M.T.; Cavazzana, J.F.; Oliveira, D.L.; Bossolani, I.L.C.; Ponsano, E.H.G. Bacterial Pigment for Nile Tilapia Feeding. Aquacult. Int. 2016, 24, 647–660. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khosravi, S.; Chang, K.H.; Lee, S.-M. Effects of Dietary Inclusion of Astaxanthin on Growth, Muscle Pigmentation and Antioxidant Capacity of Juvenile Rainbow Trout (Oncorhynchus mykiss). Prev. Nutr. Food Sci. 2016, 21, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.Md.; Shariff, M.; Kamarudin, M.S. Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Pereira da Costa, D.; Campos Miranda-Filho, K. The Use of Carotenoid Pigments as Food Additives for Aquatic Organisms and Their Functional Roles. Rev. Aquac. 2020, 12, 1567–1578. [Google Scholar] [CrossRef]
- Maoka, T.; Tanimoto, F.; Sano, M.; Tsurukawa, K.; Tsuno, T.; Tsujiwaki, S.; Ishimaru, K.; Takii, K. Effects of Dietary Supplementation of Ferulic Acid and γ-Oryzanol on Integument Color and Suppression of Oxidative Stress in Cultured Red Sea Bream, Pagrus major. J. Oleo Sci. 2008, 57, 133–137. [Google Scholar] [CrossRef]
- Paibulkichakul, C.; Piyatiratitivorakul, S.; Sorgeloos, P.; Menasveta, P. Improved Maturation of Pond-Reared, Black Tiger Shrimp (Penaeus monodon) Using Fish Oil and Astaxanthin Feed Supplements. Aquaculture 2008, 282, 83–89. [Google Scholar] [CrossRef]
- Johnson, E.A.; Villa, T.G.; Lewis, M.J. Phaffia rhodozyma as an Astaxanthin Source in Salmonid Diets. Aquaculture 1980, 20, 123–134. [Google Scholar] [CrossRef]
- Kheirabadi, E.P.; Shekarabi, P.H.; Yadollahi, F.; Soltani, M.; Najafi, E.; von Hellens, J.; Flores, C.L.; Salehi, K.; Faggio, C. Red Yeast (Phaffia rhodozyma) and Its Effect on Growth, Antioxidant Activity and Color Pigmentation of Rainbow Trout (Oncorhynchus mykiss). Aquac. Rep. 2022, 23, 101082. [Google Scholar] [CrossRef]
- Choubert, G.; Mendes-Pinto, M.M.; Morais, R. Pigmenting Efficacy of Astaxanthin Fed to Rainbow Trout Oncorhynchus mykiss: Effect of Dietary Astaxanthin and Lipid Sources. Aquaculture 2006, 257, 429–436. [Google Scholar] [CrossRef]
- Li, M.; Wu, W.; Zhou, P.; Xie, F.; Zhou, Q.; Mai, K. Comparison Effect of Dietary Astaxanthin and Haematococcus pluvialis on Growth Performance, Antioxidant Status and Immune Response of Large Yellow Croaker Pseudosciaena crocea. Aquaculture 2014, 434, 227–232. [Google Scholar] [CrossRef]
- Xie, J.; Fang, H.; He, X.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Study on Mechanism of Synthetic Astaxanthin and Haematococcus pluvialis Improving the Growth Performance and Antioxidant Capacity under Acute Hypoxia Stress of Golden Pompano (Trachinotus ovatus) and Enhancing Anti-Inflammatory by Activating Nrf2-ARE Pathway to Antagonize the NF-ΚB Pathway. Aquaculture 2020, 518, 734657. [Google Scholar] [CrossRef]
- Breitenbach, J.; Nogueira, M.; Farré, G.; Zhu, C.; Capell, T.; Christou, P.; Fleck, G.; Focken, U.; Fraser, P.D.; Sandmann, G. Engineered Maize as a Source of Astaxanthin: Processing and Application as Fish Feed. Transgenic Res. 2016, 25, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Authority (EFSA), E.F.S. Opinion of the Scientific Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP) on Safety and Efficacy of Panaferd-AX (Red Carotenoid-Rich Bacterium Paracoccus carotinifaciens) as Feed Additive for Salmon and Trout. EFSA J. 2007, 5, 546. [Google Scholar] [CrossRef]
- Grand-View-Research. Astaxanthin Market Size, Share & Trends Analysis Report By Product (Oil, Softgel, Liquid), By Source (Natural, Synthetic), By Application (Aquaculture & Animal Feed, Nutraceuticals), By Region, And Segment Forecasts, 2021–2028; Grand View Research Inc.: San Francisco, MA, USA, 2021. [Google Scholar]
- Butler, T.; Golan, Y. Astaxanthin Production from Microalgae. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 175–242. ISBN 9789811501692. [Google Scholar]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Moretti, V.M.; Mentasti, T.; Bellagamba, F.; Luzzana, U.; Caprino, F.; Turchini, G.M.; Giani, I.; Valfrè, F. Determination of Astaxanthin Stereoisomers and Colour Attributes in Flesh of Rainbow Trout (Oncorhynchus mykiss) as a Tool to Distinguish the Dietary Pigmentation Source. Food Addit. Contam. 2006, 23, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Moody, A.J.; Serwata, R.D.; Bowen, J.; Soutar, C.; Young, A.J.; Davies, S.J. The Degree of Carotenoid Esterification Influences the Absorption of Astaxanthin in Rainbow Trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutr. 2003, 9, 247–251. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Huang, S.; Stephanopoulos, G. Targeting Pathway Expression to Subcellular Organelles Improves Astaxanthin Synthesis in Yarrowia lipolytica. Metab. Eng. 2021, 68, 152–161. [Google Scholar] [CrossRef]
- Tramontin, L.R.R.; Kildegaard, K.R.; Sudarsan, S.; Borodina, I. Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway. Microorganisms 2019, 7, 472. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishibashi, T.; Kuwahara, D.; Hirasawa, K. Commercial Production of Astaxanthin with Paracoccus carotinifaciens. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; pp. 11–20. ISBN 9789811573606. [Google Scholar]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic Engineering of Escherichia coli for High-Level Astaxanthin Production with High Productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.-P. Multidimensional Heuristic Process for High-Yield Production of Astaxanthin and Fragrance Molecules in Escherichia coli. Nat Commun 2018, 9, 1858. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yang, Z.; Wang, Y.; Yao, M.; Chen, Y.; Xiao, W.; Yuan, Y. Enhanced Astaxanthin Production in Yeast via Combined Mutagenesis and Evolution. Biochem. Eng. J. 2020, 156, 107519. [Google Scholar] [CrossRef]
- Henke, N.A.; Wendisch, V.F. Improved Astaxanthin Production with Corynebacterium glutamicum by Application of a Membrane Fusion Protein. Mar. Drugs 2019, 17, 621. [Google Scholar] [CrossRef]
- Krubasik, P.; Takaichi, S.; Maoka, T.; Kobayashi, M.; Masamoto, K.; Sandmann, G. Detailed Biosynthetic Pathway to Decaprenoxanthin Diglucoside in Corynebacterium glutamicum and Identification of Novel Intermediates. Arch. Microbiol. 2001, 176, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.E.; Peters-Wendisch, P.; Netzer, R.; Stafnes, M.; Brautaset, T.; Wendisch, V.F. Production and Glucosylation of C50 and C40 Carotenoids by Metabolically Engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2014, 98, 1223–1235. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Peters-Wendisch, P.; Wendisch, V.F. Carotenoid Biosynthesis and Overproduction in Corynebacterium glutamicum. BMC Microbiol. 2012, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-K.; Eom, J.-H.; Kim, Y.; Um, Y.; Woo, H.M. Biosynthesis of Pinene from Glucose Using Metabolically-Engineered Corynebacterium glutamicum. Biotechnol. Lett. 2014, 36, 2069–2077. [Google Scholar] [CrossRef]
- Frohwitter, J.; Heider, S.A.E.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. Production of the Sesquiterpene (+)-Valencene by Metabolically Engineered Corynebacterium glutamicum. J. Biotechnol. 2014, 191, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Woo, H.M.; Choi, J.-I. Analysis of Novel Antioxidant Sesquarterpenes (C35 Terpenes) Produced in Recombinant Corynebacterium glutamicum. Appl. Biochem. Biotechnol. 2018, 186, 525–534. [Google Scholar] [CrossRef]
- Henke, N.A.; Wichmann, J.; Baier, T.; Frohwitter, J.; Lauersen, K.J.; Risse, J.M.; Peters-Wendisch, P.; Kruse, O.; Wendisch, V.F. Patchoulol Production with Metabolically Engineered Corynebacterium glutamicum. Genes 2018, 9, 219. [Google Scholar] [CrossRef]
- Lim, H.; Park, J.; Woo, H.M. Overexpression of the Key Enzymes in the Methylerythritol 4-Phosphate Pathway in Corynebacterium glutamicum for Improving Farnesyl Diphosphate-Derived Terpene Production. J. Agric. Food Chem. 2020, 68, 10780–10786. [Google Scholar] [CrossRef]
- Li, C.; Swofford, C.A.; Rückert, C.; Chatzivasileiou, A.O.; Ou, R.W.; Opdensteinen, P.; Luttermann, T.; Zhou, K.; Stephanopoulos, G.; Jones Prather, K.L.; et al. Heterologous Production of α-Carotene in Corynebacterium glutamicum Using a Multi-Copy Chromosomal Integration Method. Bioresour. Technol. 2021, 341, 125782. [Google Scholar] [CrossRef]
- Taniguchi, H.; Henke, N.A.; Heider, S.A.E.; Wendisch, V.F. Overexpression of the Primary Sigma Factor Gene SigA Improved Carotenoid Production by Corynebacterium glutamicum: Application to Production of β-Carotene and the Non-Native Linear C50 Carotenoid Bisanhydrobacterioruberin. Metab. Eng. Commun. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Henke, N.A.; Heider, S.A.E.; Hannibal, S.; Wendisch, V.F.; Peters-Wendisch, P. Isoprenoid Pyrophosphate-Dependent Transcriptional Regulation of Carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 2017, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Henke, N.A.; Heider, S.; Peters-Wendisch, P.; Wendisch, V. Production of the Marine Carotenoid Astaxanthin by Metabolically Engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Wolf, N.; Hofemeier, A.; Peters-Wendisch, P.; Wendisch, V.F. Optimization of the IPP Precursor Supply for the Production of Lycopene, Decaprenoxanthin and Astaxanthin by Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2014, 2, 28. [Google Scholar] [CrossRef]
- Henke, N.A.; Austermeier, S.; Grothaus, I.L.; Götker, S.; Persicke, M.; Peters-Wendisch, P.; Wendisch, V.F. Corynebacterium glutamicum CrtR and Its Orthologs in Actinobacteria: Conserved Function and Application as Genetically Encoded Biosensor for Detection of Geranylgeranyl Pyrophosphate. Int. J. Mol. Sci. 2020, 21, 5482. [Google Scholar] [CrossRef]
- Göttl, V.L.; Schmitt, I.; Braun, K.; Peters-Wendisch, P.; Wendisch, V.F.; Henke, N.A. CRISPRi-Library-Guided Target Identification for Engineering Carotenoid Production by Corynebacterium glutamicum. Microorganisms 2021, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Kato, Y.; Matsuda, M.; Chen, C.-Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Lutein Production with Chlorella sorokiniana MB-1-M12 Using Novel Two-Stage Cultivation Strategies–Metabolic Analysis and Process Improvement. Bioresour. Technol. 2021, 334, 125200. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; You, J.; Qiao, T.; Zhong, D.; Yu, X. Sodium Chloride Stimulates the Biomass and Astaxanthin Production by Haematococcus pluvialis via a Two-Stage Cultivation Strategy. Bioresour. Technol. 2022, 344, 126214. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of Carotenoids Production by Rhodotorula mucilaginosa (MTCC-1403) Using Agro-Industrial Waste in Bioreactor: A Statistical Approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing Biomass and Lutein Production From Scenedesmus almeriensis: Effect of Carbon Dioxide Concentration and Culture Medium Reuse. Front. Plant Sci. 2020, 11, 415. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Tan, T. Lipid and Carotenoid Production by Rhodotorula glutinis under Irradiation/High-Temperature and Dark/Low-Temperature Cultivation. Bioresour. Technol. 2014, 157, 149–153. [Google Scholar] [CrossRef]
- Ahirwar, A.; Meignen, G.; Khan, M.J.; Sirotiya, V.; Harish; Scarsini, M.; Roux, S.; Marchand, J.; Schoefs, B.; Vinayak, V. Light Modulates Transcriptomic Dynamics Upregulating Astaxanthin Accumulation in Haematococcus: A Review. Bioresour. Technol. 2021, 340, 125707. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of Temperature on the Astaxanthin Productivity and Light Harvesting Characteristics of the Green Alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, A.A.; Abd El-Razek, A.M.; El-Mahdy, A.R. Some Factors Affecting the Production of Carotenoids by Rhodotorula Glutinis Var. Glutinis. Food Nutr. Sci. 2012, 3, 64–71. [Google Scholar] [CrossRef]
- Han, S.-I.; Chang, S.H.; Lee, C.; Jeon, M.S.; Heo, Y.M.; Kim, S.; Choi, Y.-E. Astaxanthin Biosynthesis Promotion with PH Shock in the Green Microalga, Haematococcus Lacustris. Bioresour. Technol. 2020, 314, 123725. [Google Scholar] [CrossRef]
- Harith, Z.T.; de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimised Production and Extraction of Astaxanthin from the Yeast Xanthophyllomyces dendrorhous. Microorganisms 2020, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Nakano, S. New Insights into the Stoichiometric Regulation of Carotenoid Production in Chlorella vulgaris. Bioresour. Technol. Rep. 2022, 20, 101227. [Google Scholar] [CrossRef]
- Marinho, Y.F.; Malafaia, C.B.; de Araújo, K.S.; da Silva, T.D.; dos Santos, A.P.F.; de Moraes, L.B.; Gálvez, A.O. Evaluation of the Influence of Different Culture Media on Growth, Life Cycle, Biochemical Composition, and Astaxanthin Production in Haematococcus pluvialis. Aquacult. Int. 2021, 29, 757–778. [Google Scholar] [CrossRef]
- Martins, V.; Dias, C.; Caldeira, J.; Duarte, L.C.; Reis, A.; Lopes da Silva, T. Carob Pulp Syrup: A Potential Mediterranean Carbon Source for Carotenoids Production by Rhodosporidium toruloides NCYC 921. Bioresour. Technol. Rep. 2018, 3, 177–184. [Google Scholar] [CrossRef]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and Carotenoid Production from Wheat Straw Hydrolysates by Different Oleaginous Yeasts. J. Clean. Prod. 2020, 249, 119308. [Google Scholar] [CrossRef]
- Pedras, B.M.; Gonçalves, C.; Figueira, D.R.; Simões, P.; Gonçalves, P.; Paiva, A.; Barreiros, S.; Salema-Oom, M. White Wine Grape Pomace as a Suitable Carbon Source for Lipid and Carotenoid Production by Fructophilic Rhodorotula Babjevae. J. Appl. Microbiol. 2022, 133, 656–664. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Brito, L.F.; Gil Lopez, M.; Hennig, G.; Pfeifenschneider, J.; Sgobba, E.; Veldmann, K.H. The Flexible Feedstock Concept in Industrial Biotechnology: Metabolic Engineering of Escherichia coli, Corynebacterium glutamicum, Pseudomonas, Bacillus and Yeast Strains for Access to Alternative Carbon Sources. J. Biotechnol. 2016, 234, 139–157. [Google Scholar] [CrossRef]
- Wendisch, V.F. Metabolic Engineering Advances and Prospects for Amino Acid Production. Metab. Eng. 2020, 58, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Sasaki, M.; Vertès, A.A.; Inui, M.; Yukawa, H. Engineering of an L-Arabinose Metabolic Pathway in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2008, 77, 1053–1062. [Google Scholar] [CrossRef]
- Schneider, J.; Niermann, K.; Wendisch, V.F. Production of the Amino Acids L-Glutamate, l-Lysine, l-Ornithine and l-Arginine from Arabinose by Recombinant Corynebacterium glutamicum. J. Biotechnol. 2011, 154, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Huang, Z.; Bao, J. High-Titer Glutamic Acid Production from Lignocellulose Using an Engineered Corynebacterium glutamicum with Simultaneous Co-Utilization of Xylose and Glucose. ACS Sustain. Chem. Eng. 2020, 8, 6315–6322. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Vertès, A.A.; Okino, S.; Inui, M.; Yukawa, H. Engineering of a Xylose Metabolic Pathway in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2006, 72, 3418–3428. [Google Scholar] [CrossRef]
- Meiswinkel, T.M.; Gopinath, V.; Lindner, S.N.; Nampoothiri, K.M.; Wendisch, V.F. Accelerated Pentose Utilization by Corynebacterium glutamicum for Accelerated Production of Lysine, Glutamate, Ornithine and Putrescine. Microb. Biotechnol. 2013, 6, 131–140. [Google Scholar] [CrossRef]
- Mindt, M.; Heuser, M.; Wendisch, V.F. Xylose as Preferred Substrate for Sarcosine Production by Recombinant Corynebacterium glutamicum. Bioresour. Technol. 2019, 281, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Uhde, A.; Youn, J.-W.; Maeda, T.; Clermont, L.; Matano, C.; Krämer, R.; Wendisch, V.F.; Seibold, G.M.; Marin, K. Glucosamine as Carbon Source for Amino Acid-Producing Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2013, 97, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Matano, C.; Uhde, A.; Youn, J.-W.; Maeda, T.; Clermont, L.; Marin, K.; Krämer, R.; Wendisch, V.F.; Seibold, G.M. Engineering of Corynebacterium glutamicum for Growth and l-Lysine and Lycopene Production from N-Acetyl-Glucosamine. Appl. Microbiol. Biotechnol. 2014, 98, 5633–5643. [Google Scholar] [CrossRef] [PubMed]
- Vortmann, M.; Stumpf, A.K.; Sgobba, E.; Dirks-Hofmeister, M.E.; Krehenbrink, M.; Wendisch, V.F.; Philipp, B.; Moerschbacher, B.M. A Bottom-up Approach towards a Bacterial Consortium for the Biotechnological Conversion of Chitin to l-Lysine. Appl. Microbiol. Biotechnol. 2021, 105, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Meiswinkel, T.M.; Wendisch, V.F.; Nampoothiri, K.M. Amino Acid Production from Rice Straw and Wheat Bran Hydrolysates by Recombinant Pentose-Utilizing Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2011, 92, 985–996. [Google Scholar] [CrossRef]
- Mindt, M.; Hannibal, S.; Heuser, M.; Risse, J.M.; Sasikumar, K.; Nampoothiri, K.M.; Wendisch, V.F. Fermentative Production of N-Alkylated Glycine Derivatives by Recombinant Corynebacterium glutamicum Using a Mutant of Imine Reductase DpkA From Pseudomonas putida. Front. Bioeng. Biotechnol. 2019, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Eng, T.; Herbert, R.A.; Trinh, J.; Chen, Y.; Rodriguez, A.; Gladden, J.; Simmons, B.A.; Petzold, C.J.; Mukhopadhyay, A. Engineering Corynebacterium glutamicum to Produce the Biogasoline Isopentenol from Plant Biomass Hydrolysates. Biotechnol. Biofuels 2019, 12, 41. [Google Scholar] [CrossRef]
- Meiswinkel, T.M.; Rittmann, D.; Lindner, S.N.; Wendisch, V.F. Crude Glycerol-Based Production of Amino Acids and Putrescine by Corynebacterium glutamicum. Bioresour. Technol. 2013, 145, 254–258. [Google Scholar] [CrossRef]
- Lange, J.; Müller, F.; Bernecker, K.; Dahmen, N.; Takors, R.; Blombach, B. Valorization of Pyrolysis Water: A Biorefinery Sidestream, for 1,2-Propanediol Production with Engineered Corynebacterium glutamicum. Biotechnol. Biofuels 2017, 10, 277. [Google Scholar] [CrossRef]
- Burgardt, A.; Prell, C.; Wendisch, V.F. Utilization of a Wheat Sidestream for 5-Aminovalerate Production in Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2021, 9, 732271. [Google Scholar] [CrossRef]
- Prell, C.; Burgardt, A.; Meyer, F.; Wendisch, V.F. Fermentative Production of L-2-Hydroxyglutarate by Engineered Corynebacterium glutamicum via Pathway Extension of l-Lysine Biosynthesis. Front. Bioeng. Biotechnol. 2021, 8, 630476. [Google Scholar] [CrossRef]
- Eggeling, L.; Bott, M. Handbook of Corynebacterium glutamicum, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kind, S.; Jeong, W.K.; Schröder, H.; Wittmann, C. Systems-Wide Metabolic Pathway Engineering in Corynebacterium glutamicum for Bio-Based Production of Diaminopentane. Metab. Eng. 2010, 12, 341–351. [Google Scholar] [CrossRef]
- Schneider, J.; Wendisch, V.F. Putrescine Production by Engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2010, 88, 859–868. [Google Scholar] [CrossRef]
- Haupka, C.; Delépine, B.; Irla, M.; Heux, S.; Wendisch, V.F. Flux Enforcement for Fermentative Production of 5-Aminovalerate and Glutarate by Corynebacterium glutamicum. Catalysts 2020, 10, 1065. [Google Scholar] [CrossRef]
- Platzen, L.; Koch-Koerfges, A.; Weil, B.; Brocker, M.; Bott, M. Role of Flavohaemoprotein Hmp and Nitrate Reductase NarGHJI of Corynebacterium glutamicum for Coping with Nitrite and Nitrosative Stress. FEMS Microbiol. Lett. 2014, 350, 239–248. [Google Scholar] [CrossRef]
- Takeno, S.; Ohnishi, J.; Komatsu, T.; Masaki, T.; Sen, K.; Ikeda, M. Anaerobic Growth and Potential for Amino Acid Production by Nitrate Respiration in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2007, 75, 1173–1182. [Google Scholar] [CrossRef]
- Brailo, M.; Schreier, H.J.; McDonald, R.; Maršić-Lučić, J.; Gavrilović, A.; Pećarević, M.; Jug-Dujaković, J. Bacterial Community Analysis of Marine Recirculating Aquaculture System Bioreactors for Complete Nitrogen Removal Established from a Commercial Inoculum. Aquaculture 2019, 503, 198–206. [Google Scholar] [CrossRef]
- Ruiz, P.; Vidal, J.M.; Sepúlveda, D.; Torres, C.; Villouta, G.; Carrasco, C.; Aguilera, F.; Ruiz-Tagle, N.; Urrutia, H. Overview and Future Perspectives of Nitrifying Bacteria on Biofilters for Recirculating Aquaculture Systems. Rev. Aquac. 2020, 12, 1478–1494. [Google Scholar] [CrossRef]
- Koyama, T. Molecular Analysis of Prenyl Chain Elongating Enzymes. Biosci. Biotechnol. Biochem. 1999, 63, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.-H.; Ko, T.-P.; Wang, A.H.-J. Structure, Mechanism and Function of Prenyltransferases: Structure, Mechanism and Function of Prenyltransferases. Eur. J. Biochem. 2002, 269, 3339–3354. [Google Scholar] [CrossRef]
- Liang, P.-H. Reaction Kinetics, Catalytic Mechanisms, Conformational Changes, and Inhibitor Design for Prenyltransferases. Biochemistry 2009, 48, 6562–6570. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.E.; Peters-Wendisch, P.; Beekwilder, J.; Wendisch, V.F. IdsA Is the Major Geranylgeranyl Pyrophosphate Synthase Involved in Carotenogenesis in Corynebacterium glutamicum. FEBS J. 2014, 281, 4906–4920. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, T.; Jiang, Y.; Yang, B. Substrate Specificity Change of a Flavonoid Prenyltransferase AhPT1 Induced by Metal Ion. Int. J. Biol. Macromol. 2020, 153, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, F.; Keller, Y.; d’Harlingue, A.; Camara, B. Xanthophyll Biosynthesis: Molecular and Functional Characterization of Carotenoid Hydroxylases from Pepper Fruits (Capsicum annuum L.). Biochim. Biophys. Acta 1998, 1391, 320–328. [Google Scholar] [CrossRef]
- Buzzini, P.; Martini, A.; Gaetani, M.; Turchetti, B.; Pagnoni, U.M.; Davoli, P. Optimization of Carotenoid Production by Rhodotorula graminis DBVPG 7021 as a Function of Trace Element Concentration by Means of Response Surface Analysis. Enzym. Microb. Technol. 2005, 36, 687–692. [Google Scholar] [CrossRef]
- Rusinova-Videva, S.; Dimitrova, S.; Georgieva, K.; Katsarova, M.; Pavlova, K. Effect of Zn2+, Cu2+ and Fe2+ Ions for Accumulation of Ergosterol, β–Carotene and Coenzyme Q10 by Antarctic Yeast Strain Sporobolomyces salmonicolor AL1. Compt. Rend. Acad. Bulg. Sci. 2016, 69, 1005–1012. [Google Scholar]
- Chen, D.; Han, Y.; Gu, Z. Application of Statistical Methodology to the Optimization of Fermentative Medium for Carotenoids Production by Rhodobacter sphaeroides. Process Biochem. 2006, 41, 1773–1778. [Google Scholar] [CrossRef]
- Nasri Nasrabadi, M.R.; Razavi, S.H. Enhancement of Canthaxanthin Production from Dietzia natronolimnaea HS-1 in a Fed-Batch Process Using Trace Elements and Statistical Methods. Braz. J. Chem. Eng. 2010, 27, 517–529. [Google Scholar] [CrossRef]
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.M.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; De Pasquale, P.; Dugo, G.; Cicero, N. Astaxanthin Production by Xanthophyllomyces dendrorhous Growing on a Low Cost Substrate. Agroforest. Syst. 2020, 94, 1229–1234. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A. Mussel Processing Wastewater: A Low-Cost Substrate for the Production of Astaxanthin by Xanthophyllomyces dendrorhous. Microb. Cell Factories 2015, 14, 177. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Kucek, L.A.; Hill, E.; Pinchuk, G.E.; Mundree, S.G.; Beliaev, A.S. Conversion of Stranded Waste-Stream Carbon and Nutrients into Value-Added Products via Metabolically Coupled Binary Heterotroph-Photoautotroph System. Bioresour. Technol. 2018, 260, 68–75. [Google Scholar] [CrossRef]
- Lai, J.-X.; Chen, X.; Bu, J.; Hu, B.-B.; Zhu, M.-J. Direct Production of Astaxanthin from Food Waste by Phaffia rhodozyma. Process Biochem. 2022, 113, 224–233. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Nghiem, N.P.; Latona, R.J. Xylose-Enriched Ethanol Fermentation Stillage from Sweet Sorghum for Xylitol and Astaxanthin Production. Fermentation 2019, 5, 84. [Google Scholar] [CrossRef]
- Qi, D.-D.; Jin, J.; Liu, D.; Jia, B.; Yuan, Y.-J. In Vitro and in Vivo Recombination of Heterologous Modules for Improving Biosynthesis of Astaxanthin in Yeast. Microb. Cell Factories 2020, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gong, Z.; Tang, J.; Lu, F.; Li, Q.; Zhang, X. Improving Astaxanthin Production in Escherichia coli by Co-Utilizing CrtZ Enzymes with Different Substrate Preference. Microb. Cell Factories 2022, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Wan-Mohtar, W.A.A.Q.I.; Ibrahim, M.F.; Rasdi, N.W.; Zainorahim, N.; Taufek, N.M. Microorganisms as a Sustainable Aquafeed Ingredient: A Review. Aquac. Res. 2022, 53, 746–766. [Google Scholar] [CrossRef]
- Alloul, A.; Wille, M.; Lucenti, P.; Bossier, P.; Van Stappen, G.; Vlaeminck, S.E. Purple Bacteria as Added-Value Protein Ingredient in Shrimp Feed: Penaeus vannamei Growth Performance, and Tolerance against Vibrio and Ammonia Stress. Aquaculture 2021, 530, 735788. [Google Scholar] [CrossRef]
- Delamare-Deboutteville, J.; Batstone, D.J.; Kawasaki, M.; Stegman, S.; Salini, M.; Tabrett, S.; Smullen, R.; Barnes, A.C.; Hülsen, T. Mixed Culture Purple Phototrophic Bacteria Is an Effective Fishmeal Replacement in Aquaculture. Water Res. X 2019, 4, 100031. [Google Scholar] [CrossRef] [PubMed]
- Mora-Sánchez, B.; Balcázar, J.L.; Pérez-Sánchez, T. Effect of a Novel Postbiotic Containing Lactic Acid Bacteria on the Intestinal Microbiota and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss). Biotechnol. Lett. 2020, 42, 1957–1962. [Google Scholar] [CrossRef]
- Biswas, A.; Takakuwa, F.; Yamada, S.; Matsuda, A.; Saville, R.M.; LeBlanc, A.; Silverman, J.A.; Sato, N.; Tanaka, H. Methanotroph (Methylococcus capsulatus, Bath) Bacteria Meal as an Alternative Protein Source for Japanese Yellowtail, Seriola quinqueradiata. Aquaculture 2020, 529, 735700. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, S.; Zhang, S.; Dong, X.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Evaluation of Methanotroph (Methylococcus capsulatus, Bath) Bacteria Meal on Body Composition, Lipid Metabolism, Protein Synthesis and Muscle Metabolites of Pacific White Shrimp (Litopenaeus vannamei). Aquaculture 2022, 547, 737517. [Google Scholar] [CrossRef]
- Henke, N.A.; Wiebe, D.; Pérez-García, F.; Peters-Wendisch, P.; Wendisch, V.F. Coproduction of Cell-Bound and Secreted Value-Added Compounds: Simultaneous Production of Carotenoids and Amino Acids by Corynebacterium glutamicum. Bioresour. Technol. 2018, 247, 744–752. [Google Scholar] [CrossRef]
- Craig, S.; Helfrich, L.; Kuhn, D.D.; Schwarz, M.H. Understanding Fish Nutrition, Feeds, and Feeding. VCE Publ. 2017, 269, 6. [Google Scholar]
- Li, X.; Zheng, S.; Wu, G. Nutrition and Functions of Amino Acids in Fish. In Amino Acids in Nutrition and Health: Amino Acids in the Nutrition of Companion, Zoo and Farm Animals; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 133–168. ISBN 978-3-030-54462-1. [Google Scholar]
- Feeda, P.; Bampidis, V.; Azimonti, G.; de Bastos, M.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Safety and Efficacy of a Feed Additive Consisting of L-Lysine Sulfate Produced by Corynebacterium glutamicum KCCM 80227 for All Animal Species (Daesang Europe BV). EFSA J. 2021, 19, e06706. [Google Scholar] [CrossRef]
- Bertani, G. STUDIES ON LYSOGENESIS I: The Mode of Phage Liberation by Lysogenic Escherichia Coli. J Bacteriol 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Studies on Transformation of Escherichia coli with Plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Cho, J.-C.; Giovannoni, S.J. Fulvimarina pelagi Gen. Nov., Sp. Nov., a Marine Bacterium That Forms a Deep Evolutionary Lineage of Descent in the Order “Rhizobiales.”. Int. J. Syst. Evol. Microbiol. 2003, 53, 1853–1859. [Google Scholar] [CrossRef]
- Abe, S.; Takayama, K.-I.; Kinoshita, S. Taxonomical Studies on Glutamic Acid-Producing Bacteria. J. Gen. Appl. Microbiol. 1967, 13, 279–301. [Google Scholar] [CrossRef]
- Baumgart, M.; Unthan, S.; Rückert, C.; Sivalingam, J.; Grünberger, A.; Kalinowski, J.; Bott, M.; Noack, S.; Frunzke, J. Construction of a Prophage-Free Variant of Corynebacterium glutamicum ATCC 13032 for Use as a Platform Strain for Basic Research and Industrial Biotechnology. Appl. Environ. Microbiol. 2013, 79, 6006–6015. [Google Scholar] [CrossRef]
- Taniguchi, H.; Wendisch, V.F. Exploring the Role of Sigma Factor Gene Expression on Production by Corynebacterium glutamicum: Sigma Factor H and FMN as Example. Front. Microbiol. 2015, 6, 740. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic Assembly of DNA Molecules up to Several Hundred Kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Hanahan, D.; Jessee, J.; Bloom, F.R. Plasmid Transformation of Escherichia coli and Other Bacteria. In Methods in Enzymology; Bacterial Genetic Systems; Academic Press: Cambridge, MA, USA, 1991; Volume 204, pp. 63–113. [Google Scholar]
- van der Rest, M.E.; Lange, C.; Molenaar, D. A Heat Shock Following Electroporation Induces Highly Efficient Transformation of Corynebacterium glutamicum with Xenogeneic Plasmid DNA. Appl. Microbiol. Biotechnol. 1999, 52, 541–545. [Google Scholar] [CrossRef]
- Prell, C.; Vonderbank, S.-A.; Meyer, F.; Pérez-García, F.; Wendisch, V.F. Metabolic Engineering of Corynebacterium glutamicum for de Novo Production of 3-Hydroxycadaverine. Curr. Res. Biotechnol. 2022, 4, 32–46. [Google Scholar] [CrossRef]


| Strain/Plasmid | Characteristics | Reference |
|---|---|---|
| Escherichia coli DH5α | F- thi−1 endA1 hsdR17(r-, m-) supE44 ∆lacU169 (Φ80lacZ∆M15) recA1 gyrA96 | [131] |
| Fulvimarina pelagi | Type strain, HTCC 2506; DSM No. 15513 | [132] |
| Corynebacterium glutamicum strains | ||
| WT | wild type, ATCC 13032 | [133] |
| MB001 | ATCC 13032 with in-frame deletion of prophages cgp1 (cg1507-cg1524), cgp2 (cg1746-cg1752), cgp3 (cg1890-cg2071) | [134] |
| MB001∆crtR | MB001 derivative with deletion of crtR (cg0725) | [57] |
| LYC5 | MB001 derivative with deletion of crtYefEb (cg0717-cg0719), and chromosomal integration of Ptuf-dxs and Ptuf-crtEBI | [58] |
| LYC6 | LYC5 derivative with deletion of crtR (cg0725) | [57] |
| BABR1 | LYC5 carrying pVWEx1_lbtBC and pEKEx3_sigA | [56] |
| CP1 | LYC6 carrying pEKEx3_lbtABC | [57] |
| SAX1 | LYC6 carrying pEKEx3_crtE2Y | [57] |
| BETA4 | LYC6 with chromosomal integration of crtY from P. ananatis under the control of tuf promotor | [57] |
| ZEA5 | BETA4 carrying pSH2_crtZFp | this work |
| CAN5 | BETA4 carrying pSH2_crtWFp | this work |
| ASTA* | BETA4 carrying pSH1_crtZ~WFp | [46] |
| BETALYS | GRLys1 with the following modifications: ∆ldhA (cg3219), ∆sugR (cg2115), ∆crtR (cg0725), ∆crtYefEb (cg0717-cg0719), chromosomal integration of Ptuf-crtEBI and Ptuf-crtYPa | [126] |
| ASTALYS* | BETALYS carrying pSH1_crtZ~WFp | this work |
| Plasmids | ||
| pEKEx3_sigA | SpecR; pBL1 oriVCg, E. coli/C. glutamicum shuttle vector; for IPTG-inducible expression of sigA from C. glutamicum | [135] |
| pEKEx3_crtE2Y | SpecR; pBL1 oriVCg, E. coli/C. glutamicum shuttle vector; for IPTG-inducible expression of crtE2 and crtYg/h from M. luteus containing an artificial ribosome binding site in front of crtE2 | [48] |
| pEKEx3_lbtABC | SpecR; pBL1 oriVCg, E. coli/C. glutamicum shuttle vector for IPTG-inducible expression of codon optimized lbtABC from Dietzia sp. CQ4 containing artificial ribosome binding sites in front of each gene | [48] |
| pVWEx1_lbtBC | KmR; pCG1 oriVCg, E. coli/C. glutamicum shuttle vector for IPTG-inducible expression of lbtBC from Dietzia sp. CQ4 | [56] |
| pSH1_crtZ~WFp | KmR; pHM519 oriVCg; E. coli/C. glutamicum shuttle vector, Ptuf, encoding a fusion protein comprising CrtZ and CrtW from F. pelagi | [46] |
| pSH2 | pSH1 derivative with mutation in repA | this work |
| pSH2_crtZFp | pSH2 derivative for constitutive expression of crtZ from F. pelagi | this work |
| pSH2_crtWFp | pSH2 derivative for constitutive expression of crtW from F. pelagi | this work |
| Oligonucleotide | Sequence (5′→3′) |
|---|---|
| HA36 | AAAATCGCTTGACCATTGCAGGTTG |
| HA37 | CTTTAGCTTTCCTAGCTTGTCGTTGAC |
| HA34 | CATGCCTGCAGGTCGACTCTAGAGGAAAGGAGGCCCTTCAGATGACGATCTGGACTCTCTACTAC |
| HA35 | ATTCGAGCTCGGTACCCGGGGATCTTACCGAACCGGCGCGT |
| FpW1 | CATGCCTGCAGGTCGACTCTAGAGGAAAGGAGGCCCTTCAGATGACCCTCAGCCCAACCTC |
| FpW4 | ATTCGAGCTCGGTACCCGGGGATCTTAGGACTGGCGAGTATGCG |
| PD5 | CGCTCACCGGCTCCAGATTTATCAG |
| 582 | ATCTTCTCTCATCCGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, I.; Meyer, F.; Krahn, I.; Henke, N.A.; Peters-Wendisch, P.; Wendisch, V.F. From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium glutamicum Using Aquaculture Sidestream. Molecules 2023, 28, 1996. https://doi.org/10.3390/molecules28041996
Schmitt I, Meyer F, Krahn I, Henke NA, Peters-Wendisch P, Wendisch VF. From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium glutamicum Using Aquaculture Sidestream. Molecules. 2023; 28(4):1996. https://doi.org/10.3390/molecules28041996
Chicago/Turabian StyleSchmitt, Ina, Florian Meyer, Irene Krahn, Nadja A. Henke, Petra Peters-Wendisch, and Volker F. Wendisch. 2023. "From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium glutamicum Using Aquaculture Sidestream" Molecules 28, no. 4: 1996. https://doi.org/10.3390/molecules28041996
APA StyleSchmitt, I., Meyer, F., Krahn, I., Henke, N. A., Peters-Wendisch, P., & Wendisch, V. F. (2023). From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium glutamicum Using Aquaculture Sidestream. Molecules, 28(4), 1996. https://doi.org/10.3390/molecules28041996