Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components
Abstract
:1. Introduction
2. Biological Activity of Green Tea Catechins
3. Anticancer Activity of Green Tea Catechins
4. Anticancer Activity of Catechins Combined with Natural Compounds
5. Anticarcinogenic Antioxidant/Prooxidant Effects of Catechins
6. Challenges, Future Recommendations, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating Intrinsic and Non-Intrinsic Cancer Risk Factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Cancer Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 January 2023).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praetorius, N.P.; Mandal, T.K. Engineered Nanoparticles in Cancer Therapy. Recent Pat. Drug Deliv. Formul. 2007, 1, 37–51. [Google Scholar] [CrossRef]
- Donnenberg, V.S.; Donnenberg, A.D. Multiple Drug Resistance in Cancer Revisited: The Cancer Stem Cell Hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877. [Google Scholar] [CrossRef]
- Velasco-Velázquez, M.A.; Homsi, N.; De La Fuente, M.; Pestell, R.G. Breast Cancer Stem Cells. Int. J. Biochem. Cell Biol. 2012, 44, 573–577. [Google Scholar] [CrossRef] [Green Version]
- LaVan, D.A.; McGuire, T.; Langer, R. Small-Scale Systems for in Vivo Drug Delivery. Nat. Biotechnol. 2003, 21, 1184–1191. [Google Scholar] [CrossRef]
- Illouz, F.; Braun, D.; Briet, C.; Schweizer, U.; Rodien, P. Endocrine Side-Effects of Anti-Cancer Drugs: Thyroid Effects of Tyrosine Kinase Inhibitors. Eur. J. Endocrinol. 2014, 171, R91–R99. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Yang, T.; Chen, Y.; Miao, Y.; Xu, Y.; Jiang, H.; Yang, M.; Mao, C. Emulating Interactions between Microorganisms and Tumor Microenvironment to Develop Cancer Theranostics. Theranostics 2022, 12, 2833–2859. [Google Scholar] [CrossRef]
- Stork, G.; Schultz, A.G. The Total Synthesis of Dl-Camptothecin. J. Am. Chem. Soc. 1971, 93, 4074–4075. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, R.; Danishefsky, S.; Eggler, J.; Solomon, D.M. Total Synthesis of (+-)-Camptothecine. J. Am. Chem. Soc. 1971, 93, 5576–5577. [Google Scholar] [CrossRef]
- Ejima, A.; Terasawa, H.; Sugimori, M.; Tagawa, H. Asymmetric Synthesis of (S)-Camptothecin. Tetrahedron Lett. 1989, 30, 2639–2640. [Google Scholar] [CrossRef]
- Bennasar, M.-L.; Juan, C.; Bosch, J. A Short Synthesis of Camptothecin via A2-Fluoro-1,4-Dihydropyridine. Chem. Commun. 2000, 2459–2460. [Google Scholar] [CrossRef]
- Bennasar, M.-L.; Zulaica, E.; Juan, C.; Alonso, Y.; Bosch, J. Addition of Ester Enolates to N-Alkyl-2-Fluoropyridinium Salts: Total Synthesis of (±)-20-Deoxycamptothecin and (+)-Camptothecin. J. Org. Chem. 2002, 67, 7465–7474. [Google Scholar] [CrossRef]
- Lin, L.-G.; Xie, H.; Li, H.-L.; Tong, L.-J.; Tang, C.-P.; Ke, C.-Q.; Liu, Q.-F.; Lin, L.-P.; Geng, M.-Y.; Jiang, H.; et al. Naturally Occurring Homoisoflavonoids Function as Potent Protein Tyrosine Kinase Inhibitors by C-Src-Based High-Throughput Screening. J. Med. Chem. 2008, 51, 4419–4429. [Google Scholar] [CrossRef]
- Yang, S.-P.; Zhang, X.-W.; Ai, J.; Gan, L.-S.; Xu, J.-B.; Wang, Y.; Su, Z.-S.; Wang, L.; Ding, J.; Geng, M.-Y.; et al. Potent HGF/c-Met Axis Inhibitors from Eucalyptus Globulus: The Coupling of Phloroglucinol and Sesquiterpenoid Is Essential for the Activity. J. Med. Chem. 2012, 55, 8183–8187. [Google Scholar] [CrossRef]
- Li, M.-H.; Miao, Z.-H.; Tan, W.-F.; Yue, J.-M.; Zhang, C.; Lin, L.-P.; Zhang, X.-W.; Ding, J. Pseudolaric Acid B Inhibits Angiogenesis and Reduces Hypoxia-Inducible Factor 1α by Promoting Proteasome-Mediated Degradation. Clin. Cancer Res. 2004, 10, 8266–8274. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Li, M.-H.; Wang, W.; Wang, Y.-Q.; Jiang, Y.; Yang, S.-P.; Yue, J.-M.; Ding, J.; Miao, Z.-H. Pseudolaric Acid B-Driven Phosphorylation of c-Jun Impairs Its Role in Stabilizing HIF-1alpha: A Novel Function-Converter Model. J. Mol. Med. 2012, 90, 971–981. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, C.; Tian, C.; Li, C.; Nie, F.; Song, X.; Zeng, R.; Wu, D.; Hao, X.; Li, L. The Plant Sesquiterpene Lactone Parthenolide Inhibits Wnt/β-Catenin Signaling by Blocking Synthesis of the Transcriptional Regulators TCF4/LEF1. J. Biol. Chem. 2018, 293, 5335–5344. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Gu, K.J.; Li, G. An Overview of Cancer Prevention: Chemoprevention and Immunoprevention. J. Cancer Prev. 2020, 25, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.R.; Berlinck, R.G.S.; Nascimento, G.G.F.; Fortier, S.C.; Pessoa, C.; de Moraes, M.O. Antibacterial Activity against Resistant Bacteria and Cytotoxicity of Four Alkaloid Toxins Isolated from the Marine Sponge Arenosclera Brasiliensis. Toxicon 2002, 40, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Dobiáš, L.; Černá, M.; Rössner, P.; Šrám, R. Genotoxicity and Carcinogenicity of Metronidazole. Mutat. Res./Rev. Genet. Toxicol. 1994, 317, 177–194. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef] [Green Version]
- Shanmugham, V.; Subban, R. Capsanthin-Loaded Micelles: Preparation, Characterization and in Vitro Evaluation of Cytotoxicity Using MDA-MB-231 Breast Cancer Cell Line. Food Technol. Biotechnol. 2022, 60, 350–360. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.; Meng, H.; Li, W.; Li, Q.; Luo, Y.; Wang, C.; Xie, J.; Wu, L.; Zhang, X.; et al. Characteristics of the Emulsion Stabilized by Polysaccharide Conjugates Alkali-Extracted from Green Tea Residue and Its Protective Effect on Catechins. Ind. Crops Prod. 2019, 140, 111611. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, Z.; Zhang, Y.; Zhang, N.; Zhu, X.; Chen, X.; Shao, Y.; Cheng, Y.; Wang, C.; Luo, Y.; et al. Effect of Metal Ions and PH on the Emulsifying Properties of Polysaccharide Conjugates Prepared from Low-Grade Green Tea. Food Hydrocoll. 2020, 102, 105624. [Google Scholar] [CrossRef]
- Guo, Y.; Zhi, F.; Chen, P.; Zhao, K.; Xiang, H.; Mao, Q.; Wang, X.; Zhang, X. Green Tea and the Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine 2017, 96, e6426. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Bai, J.; Wang, X.; Li, C. Association of Tea Consumption and the Risk of Thyroid Cancer: A Meta-Analysis. Int. J. Clin. Exp. Med. 2015, 8, 14345–14351. [Google Scholar]
- Najaf Najafi, M.; Salehi, M.; Ghazanfarpour, M.; Hoseini, Z.S.; Khadem-Rezaiyan, M. The Association between Green Tea Consumption and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Phytother. Res. 2018, 32, 1855–1864. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Modulation of Signaling Pathways in Prostate Cancer by Green Tea Polyphenols. Biochem. Pharmacol. 2013, 85, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Sawada, N. Risk and Preventive Factors for Prostate Cancer in Japan: The Japan Public Health Center-Based Prospective (JPHC) Study. J. Epidemiol. 2017, 27, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ho, C.-T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Du, Y.; Wu, L.; Xie, J.; Chen, X.; Hu, B.; Wu, Z.; Yao, Q.; Li, Q. Effects of Tea-Polysaccharide Conjugates and Metal Ions on Precipitate Formation by Epigallocatechin Gallate and Caffeine, the Key Components of Green Tea Infusion. J. Agric. Food Chem. 2019, 67, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M. Possible Mechanisms of Green Tea and Its Constituents against Cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, H.S.; Chen, T.; de Villiers, W.J.S. Green Tea Polyphenols and Sulfasalazine Have Parallel Anti-Inflammatory Properties in Colitis Models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A Prodrug of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (Pro-EGCG) Serves as a Novel Angiogenesis Inhibitor in Endometrial Cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Kale, A.; Gawande, S.; Kotwal, S.; Netke, S.; Roomi, W.; Ivanov, V.; Niedzwiecki, A.; Rath, M. Studies on the Effects of Oral Administration of Nutrient Mixture, Quercetin and Red Onions on the Bioavailability of Epigallocatechin Gallate from Green Tea Extract. Phytother. Res. 2010, 24 (Suppl. S1), S48–S55. [Google Scholar] [CrossRef]
- Bimonte, S.; Albino, V.; Piccirillo, M.; Nasto, A.; Molino, C.; Palaia, R.; Cascella, M. Epigallocatechin-3-Gallate in the Prevention and Treatment of Hepatocellular Carcinoma: Experimental Findings and Translational Perspectives. Drug Des. Dev. Ther. 2019, 13, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.F.; Maier, C.S. The Chemistry of Gut Microbial Metabolism of Polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A Review on Anti-Cancer Effect of Green Tea Catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, H. Green Tea Polyphenols and Cancer: Biologic Mechanisms and Practical Implications. Nutr. Rev. 1999, 57, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Tadano, N.; Du, C.-K.; Yumoto, F.; Morimoto, S.; Ohta, M.; Xie, M.-F.; Nagata, K.; Zhan, D.-Y.; Lu, Q.-W.; Miwa, Y.; et al. Biological Actions of Green Tea Catechins on Cardiac Troponin C. Br. J. Pharmacol. 2010, 161, 1034–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Jin, C.H.; Row, K.H. Separation of Catechin Compounds from Different Teas. Biotechnol. J. 2006, 1, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.-T.; Ho, C.K.; Choi, S.-W.; Chung, W.-Y.; Benzie, I.F.F. Comparison of Catechin Profiles in Human Plasma and Urine after Single Dosing and Regular Intake of Green Tea (Camellia sinensis). Br. J. Nutr. 2013, 109, 2199–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Hamilton-Miller, J.M.T. Anti-Cariogenic Properties of Tea (Camellia sinensis). J. Med. Microbiol. 2001, 50, 299–302. [Google Scholar] [CrossRef]
- Maity, R.; Chatterjee, M.; Banerjee, A.; Das, A.; Mishra, R.; Mazumder, S.; Chanda, N. Gold Nanoparticle-Assisted Enhancement in the Anti-Cancer Properties of Theaflavin against Human Ovarian Cancer Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109909. [Google Scholar] [CrossRef]
- Subramani, C.; Natesh, R.K. Molecular Mechanisms and Biological Implications of Green Tea Polyphenol, (−)-Epigallocatechin-3-Gallate. J. Pharma Biosci. Technol. 2013, 1, 54–63. [Google Scholar]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial Effects of Green Tea: A Literature Review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Crew, K.D.; Ho, K.A.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Sneige, N.; Hudis, C.; McArthur, H.L.; Chang, J.; et al. Effects of a Green Tea Extract, Polyphenon E, on Systemic Biomarkers of Growth Factor Signalling in Women with Hormone Receptor-Negative Breast Cancer. J. Hum. Nutr. Diet. 2015, 28, 272–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.-J.; Yin, Y.-C.; Wang, J.; Jiang, Y.-F. Green Tea Compounds in Breast Cancer Prevention and Treatment. World J. Clin. Oncol. 2014, 5, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Sakai, H.; Kochi, T.; Seishima, M.; Shimizu, M. Catechins and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 67–90. [Google Scholar] [PubMed]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of Anti-Inflammatory Effects of Green Tea and Black Tea: A Comparative in Vitro Study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [PubMed]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De, F.V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell. Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey through Blood-Brain Barrier towards Neuronal Protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [Green Version]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green Tea Effects on Cognition, Mood and Human Brain Function: A Systematic Review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E.; et al. Acute Neurocognitive Effects of Epigallocatechin Gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef]
- Vauzour, D. Dietary Polyphenols as Modulators of Brain Functions: Biological Actions and Molecular Mechanisms Underpinning Their Beneficial Effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [Green Version]
- Newsome, B.J.; Petriello, M.C.; Han, S.G.; Murphy, M.O.; Eske, K.E.; Sunkara, M.; Morris, A.J.; Hennig, B. Green Tea Diet Decreases PCB 126-Induced Oxidative Stress in Mice by up-Regulating Antioxidant Enzymes. J. Nutr. Biochem. 2014, 25, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-F.; Hsu, Y.-W.; Ting, H.-C.; Huang, C.-F.; Yen, C.-C. The in Vivo Antioxidant and Antifibrotic Properties of Green Tea (Camellia sinensis, Theaceae). Food Chem. 2013, 136, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A. Cardiovascular Effects of Green Tea Catechins: Progress and Promise. Recent Pat. Cardiovasc. Drug Discov. 2012, 7, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Khanna, D. Green Tea Catechins: Defensive Role in Cardiovascular Disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, Endothelial Stress, Inflammation, and the Metabolic Syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Al Qarni, A.; Hawwari, A.; Alghanem, A.F.; Ahmed, G. Metabolic Syndrome, Dyslipidemia and Regulation of Lipoprotein Metabolism. Curr. Diabetes Rev. 2018, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-Y.; Li, Q.-S.; Lin, X.-M.; Qiao, R.-Y.; Yang, R.; Li, X.-M.; Dong, Z.-B.; Xiang, L.-P.; Zheng, X.-Q.; Lu, J.-L.; et al. Antidiabetic Effects of Tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef] [Green Version]
- Munir, K.M.; Chandrasekaran, S.; Gao, F.; Quon, M.J. Mechanisms for Food Polyphenols to Ameliorate Insulin Resistance and Endothelial Dysfunction: Therapeutic Implications for Diabetes and Its Cardiovascular Complications. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E679–E686. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The Anti-Obesity Effects of Green Tea in Human Intervention and Basic Molecular Studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [Green Version]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of Adipose Tissue Inflammation by Bioactive Food Compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-Gallate on Obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef] [Green Version]
- Araghizadeh, A.; Kohanteb, J.; Fani, M.M. Inhibitory Activity of Green Tea (Camellia sinensis) Extract on Some Clinically Isolated Cariogenic and Periodontopathic Bacteria. Med. Princ. Pract. 2013, 22, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Aylıkcı, B.U.; Colak, H. Halitosis: From Diagnosis to Management. J. Nat. Sci. Biol. Med. 2013, 4, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, M.D.R.; Carneiro, J.R.M.; Passos, V.F.; Santiago, S.L. Effect of Green Tea as a Protective Measure against Dental Erosion in Coronary Dentine. Braz. Oral Res. 2016, 30, S1806-83242016000100213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushiyama, M.; Shimazaki, Y.; Murakami, M.; Yamashita, Y. Relationship between Intake of Green Tea and Periodontal Disease. J. Periodontol. 2009, 80, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Narotzki, B.; Levy, Y.; Aizenbud, D.; Reznick, A.Z. Green Tea and Its Major Polyphenol EGCG Increase the Activity of Oral Peroxidases. Adv. Exp. Med. Biol. 2013, 756, 99–104. [Google Scholar] [PubMed]
- Song, M.; Teng, Z.; Li, M.; Niu, X.; Wang, J.; Deng, X. Epigallocatechin Gallate Inhibits Streptococcus Pneumoniae Virulence by Simultaneously Targeting Pneumolysin and Sortase A. J. Cell. Mol. Med. 2017, 21, 2586–2598. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.-H.; Chun, S. Bactericidal Activity of Green Tea Extracts: The Importance of Catechin Containing Nano Particles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular Responses and Proteomic Analysis of Escherichia coli Exposed to Green Tea Polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular Binding of Catechins to Biomembranes: Relationship to Biological Activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular Dynamics Study on the Biophysical Interactions of Seven Green Tea Catechins with Lipid Bilayers of Cell Membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Horiuchi, T.; Suganuma, M.; Nishiwaki, S.; Yatsunami, J.; Okabe, S.; Okuda, T.; Muto, Y.; Frenkel, K.; Troll, W.; et al. Penta-O-Galloyl-β-D-Glucose and (−)-Epigallocatechin Gallate. In Phenolic Compounds in Food and Their Effects on Health II; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 507, pp. 316–325. ISBN 9780841224766. [Google Scholar]
- Ann Beltz, L.; Kay Bayer, D.; Lynn Moss, A.; Mitchell Simet, I. Mechanisms of Cancer Prevention by Green and Black Tea Polyphenols. Anticancer Agents Med. Chem. 2006, 6, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Suematsu, S.; Hisanobu, Y.; Saigo, H.; Matsuda, R.; Hara, K. Effects of PH and Temperature on Reaction Kinetics of Catechins in Green Tea Infusion. Biosci. Biotechnol. Biochem. 1993, 57, 907–910. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic Enhancement of Anticancer Effects on Numerous Human Cancer Cell Lines Treated with the Combination of EGCG, Other Green Tea Catechins, and Anticancer Compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef]
- Vidak, M.; Rozman, D.; Komel, R. Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells. Molecules 2015, 20, 19406–19432. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The Antioxidant and Pro-Oxidant Activities of Green Tea Polyphenols: A Role in Cancer Prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Zaveri, N.T. Green Tea and Its Polyphenolic Catechins: Medicinal Uses in Cancer and Noncancer Applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid. Based. Complement. Alternat. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary Phytochemicals as Epigenetic Modifiers in Cancer: Promise and Challenges. Semin. Cancer Biol. 2016, 40–41, 82–99. [Google Scholar] [CrossRef] [Green Version]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Tse, L.A.; Chan, W.-C.; Kwok, C.-H.; Leung, S.-L.; Wu, C.; Yu, W.-C.; Yu, I.T.-S.; Yu, C.H.-T.; Wang, F.; et al. Evaluation of Breast Cancer Risk Associated with Tea Consumption by Menopausal and Estrogen Receptor Status among Chinese Women in Hong Kong. Cancer Epidemiol. 2016, 40, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-M.; Sun, C.; Butler, L.M. Tea and Cancer Prevention: Epidemiological Studies. Pharmacol. Res. 2011, 64, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Li, J.-P.; Zhang, C. Green Tea Consumption and Breast Cancer Risk: Three Recent Meta-Analyses. Breast Cancer Res. Treat. 2011, 127, 581–583. [Google Scholar] [CrossRef]
- Rathore, K.; Choudhary, S.; Odoi, A.; Wang, H.-C.R. Green Tea Catechin Intervention of Reactive Oxygen Species-Mediated ERK Pathway Activation and Chronically Induced Breast Cell Carcinogenesis. Carcinogenesis 2012, 33, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of Cytotoxicity and Inhibition of Intercellular Communication by Antioxidant Catechins Isolated from Chinese Green Tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-Gallate Promotes Apoptosis in Human Breast Cancer T47D Cells through down-Regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG Promoting Apoptosis of MCF-7 Cell Line in Human Breast Cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, Y.-Y.; Meeran, S.M.; Tollefsbol, T.O. Synergistic Epigenetic Reactivation of Estrogen Receptor-α (ERα) by Combined Green Tea Polyphenol and Histone Deacetylase Inhibitor in ERα-Negative Breast Cancer Cells. Mol. Cancer 2010, 9, 274. [Google Scholar] [CrossRef] [Green Version]
- Farabegoli, F.; Govoni, M.; Spisni, E.; Papi, A. EGFR Inhibition by (−)-Epigallocatechin-3-Gallate and IIF Treatments Reduces Breast Cancer Cell Invasion. Biosci. Rep. 2017, 37, BSR20170168. [Google Scholar] [CrossRef] [Green Version]
- Aller, G.S.V.; Carson, J.D.; Tang, W.; Peng, H.; Zhao, L. Biochemical and Biophysical Research Communications Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem. Biophys. Res. Commun. 2011, 406, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.M.; Bauer, A.C. Green Tea Catechin, EGCG, Suppresses PCB 102-Induced Proliferation in Estrogen-Sensitive Breast Cancer Cells. Int. J. Breast Cancer 2015, 2015, 163591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Liu, J.; Xu, Y. The Inhibitory Effect of (−)-Epigallocatechin-3-Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic Induction of Tissue Inhibitor of Matrix Metalloproteinase-3 by Green Tea Polyphenols in Breast Cancer Cells. Mol. Carcinog. 2015, 54, 485–499. [Google Scholar] [CrossRef]
- De Amicis, F.; Russo, A.; Avena, P.; Santoro, M.; Vivacqua, A.; Bonofiglio, D.; Mauro, L.; Aquila, S.; Tramontano, D.; Fuqua, S.A.W.; et al. In Vitro Mechanism for Downregulation of ER-α Expression by Epigallocatechin Gallate in ER+/PR+ Human Breast Cancer Cells. Mol. Nutr. Food Res. 2013, 57, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.-Y.; Lee, J.-K.; Jeon, Y.-K.; Kim, C.-W. Exosome Derived from Epigallocatechin Gallate Treated Breast Cancer Cells Suppresses Tumor Growth by Inhibiting Tumor-Associated Macrophage Infiltration and M2 Polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, M.; Sun, H.; Zhu, S.; Jin, J. Synergetic Effect of EP1 Receptor Antagonist and (−)-Epigallocatechin-3-Gallate in Hepatocellular Carcinoma. Pharmacology 2019, 104, 267–275. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Kochi, T.; Ideta, T.; Miyazaki, T.; Moriwaki, H. Chemopreventive Potential of Green Tea Catechins in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2015, 16, 6124–6139. [Google Scholar] [CrossRef] [Green Version]
- Larsen, C.A.; Dashwood, R.H.; Bisson, W.H. Tea Catechins as Inhibitors of Receptor Tyrosine Kinases: Mechanistic Insights and Human Relevance. Pharmacol. Res. 2010, 62, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Pal, D.; Mandal, S.; Roy, A.; Panda, C.K. Tea Polyphenols Epigallocatechin Gallete and Theaflavin Restrict Mouse Liver Carcinogenesis through Modulation of Self-Renewal Wnt and Hedgehog Pathways. J. Nutr. Biochem. 2016, 27, 32–42. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (−)-Epigallocatechin Gallate Suppresses Azoxymethane-Induced Colonic Premalignant Lesions in Male C57BL/KsJ-Db/Db Mice. Cancer Prev. Res. 2008, 1, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakami, Y.; Shimizu, M.; Adachi, S.; Sakai, H.; Nakagawa, T.; Yasuda, Y.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin Gallate Suppresses the Growth of Human Hepatocellular Carcinoma Cells by Inhibiting Activation of the Vascular Endothelial Growth Factor-Vascular Endothelial Growth Factor Receptor Axis. Cancer Sci. 2009, 100, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Iranikhah, M.; Stricker, S.; Freeman, M.K. Future of Bisphosphonates and Denosumab for Men with Advanced Prostate Cancer. Cancer Manag. Res. 2014, 6, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLarty, J.; Bigelow, R.L.H.; Smith, M.; Elmajian, D.; Ankem, M.; Cardelli, J.A. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. 2009, 2, 673–682. [Google Scholar]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, A.C.; Xiao, H.; Zhu, Z.; Li, Q.; Bai, Q.; Wakefield, M.R.; Mann, J.D.; Fang, Y. A Potential Role for Green Tea as a Radiation Sensitizer for Prostate Cancer. Pathol. Oncol. Res. 2019, 25, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.-P.; Chen, R.-Y.; Kokontis, J.M.; Hiipakka, R.A.; Liao, S. Suppression of Androgen Receptor Signaling and Prostate Specific Antigen Expression by (−)-Epigallocatechin-3-Gallate in Different Progression Stages of LNCaP Prostate Cancer Cells. Cancer Lett. 2009, 275, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Mukhtar, H. Tea and Health: Studies in Humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Ahmad, N.; Nieminen, A.L.; Mukhtar, H. Growth Inhibition, Cell-Cycle Dysregulation, and Induction of Apoptosis by Green Tea Constituent (−)-Epigallocatechin-3-Gallate in Androgen-Sensitive and Androgen-Insensitive Human Prostate Carcinoma Cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef]
- Duhon, D.; Bigelow, R.L.H.; Coleman, D.T.; Steffan, J.J.; Yu, C.; Langston, W.; Kevil, C.G.; Cardelli, J.A. The Polyphenol Epigallocatechin-3-Gallate Affects Lipid Rafts to Block Activation of the c-Met Receptor in Prostate Cancer Cells. Mol. Carcinog. 2010, 49, 739–749. [Google Scholar] [CrossRef]
- Hagen, R.M.; Chedea, V.S.; Mintoff, C.P.; Bowler, E.; Morse, H.R.; Ladomery, M.R. Epigallocatechin-3-Gallate Promotes Apoptosis and Expression of the Caspase 9a Splice Variant in PC3 Prostate Cancer Cells. Int. J. Oncol. 2013, 43, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastak, K.; Agarwal, M.K.; Mukhtar, H.; Agarwal, M.L. Ablation of Either P21 or Bax Prevents P53-Dependent Apoptosis Induced by Green Tea Polyphenol Epigallocatechin-3-Gallate. FASEB J. 2005, 19, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.L.; Luo, J.-H.; Yu, Y.P. (−)-Epigallocatechin-3-Gallate Induces Du145 Prostate Cancer Cell Death via Downregulation of Inhibitor of DNA Binding 2, a Dominant Negative Helix-Loop-Helix Protein. Cancer Sci. 2010, 101, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.D.; Gilmore, P.E.; Hart, C.A.; Samuel, J.D.; Ramani, V.A.C.; George, N.J.; Clarke, N.W. Characterization of Benign and Malignant Prostate Epithelial Hoechst 33342 Side Populations. Prostate 2007, 67, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; Henry, E.C.; Ricke, W.A.; Gasiewicz, T.A. The Heat Shock Protein 90 Inhibitor, (−)-Epigallocatechin Gallate, Has Anticancer Activity in a Novel Human Prostate Cancer Progression Model. Cancer Prev. Res. 2015, 8, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dizdar, Ö.; Kılıçkap, S. Global Epidemiology of Gastrointestinal Cancers. In Textbook of Gastrointestinal Oncology; Yalcin, S., Philip, P.A., Eds.; Springer International Publishing: Cham, Switwerland, 2019; pp. 1–12. ISBN 9783030188900. [Google Scholar]
- Suganuma, M.; Takahashi, A.; Watanabe, T.; Iida, K.; Matsuzaki, T.; Yoshikawa, H.Y.; Fujiki, H. Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins. Molecules 2016, 21, 1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.-S.; Yang, J.; Fu, Y.-Q.; Huang, T.; Huang, Y.-J.; Li, D. Effects of Green Tea, Black Tea, and Coffee Consumption on the Risk of Esophageal Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Cancer 2013, 65, 1–16. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Lim, J.T.E.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green Tea Polyphenol EGCG Suppresses Wnt/β-Catenin Signaling by Promoting GSK-3β- and PP2A-Independent β-Catenin Phosphorylation/Degradation. Biofactors 2014, 40, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and Alternative P38MAPK Signalling Pathways in Human Colon Cancer Cell Apoptosis Induced by Green Tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef]
- Navarro-Perán, E.; Cabezas-Herrera, J.; Sánchez-del-Campo, L.; García-Cánovas, F.; Rodríguez-López, J.N. The Anti-Inflammatory and Anti-Cancer Properties of Epigallocatechin-3-Gallate Are Mediated by Folate Cycle Disruption, Adenosine Release and NF-ΚB Suppression. Inflamm. Res. 2008, 57, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Involvement of 67-KDa Laminin Receptor-Mediated Myosin Phosphatase Activation in Antiproliferative Effect of Epigallocatechin-3-O-Gallate at a Physiological Concentration on Caco-2 Colon Cancer Cells. Biochem. Biophys. Res. Commun. 2008, 371, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nagao, T.; Ingolfsson, H.I.; Maxfield, F.R.; Andersen, O.S.; Kopelovich, L.; Weinstein, I.B. The Inhibitory Effect of (−)-Epigallocatechin Gallate on Activation of the Epidermal Growth Factor Receptor Is Associated with Altered Lipid Order in HT29 Colon Cancer Cells. Cancer Res. 2007, 67, 6493–6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, S.; Shimizu, M.; Shirakami, Y.; Yamauchi, J.; Natsume, H.; Matsushima-Nishiwaki, R.; To, S.; Weinstein, I.B.; Moriwaki, H.; Kozawa, O. (−)-Epigallocatechin Gallate Downregulates EGF Receptor via Phosphorylation at Ser1046/1047 by P38 MAPK in Colon Cancer Cells. Carcinogenesis 2009, 30, 1544–1552. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ju, Y.; Wang, J.; Zhou, R. Epigallocatechin-3-Gallate Promotes Apoptosis and Reversal of Multidrug Resistance in Esophageal Cancer Cells. Pathol. Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef]
- Zhu, B.-H.; Zhan, W.-H.; Li, Z.-R.; Wang, Z.; He, Y.-L.; Peng, J.-S.; Cai, S.-R.; Ma, J.-P.; Zhang, C.-H. (−)-Epigallocatechin-3-Gallate Inhibits Growth of Gastric Cancer by Reducing VEGF Production and Angiogenesis. World J. Gastroenterol. 2007, 13, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.; Moseley, V.R.; Cabang, A.B.; Coleman, K.; Wei, W.; Garrett-Mayer, E.; Wargovich, M.J. Reduction in Promotor Methylation Utilizing EGCG (Epigallocatechin-3-Gallate) Restores RXRα Expression in Human Colon Cancer Cells. Oncotarget 2016, 7, 35313–35326. [Google Scholar] [CrossRef]
- Li, Z.G.; Shimada, Y.; Sato, F.; Maeda, M.; Itami, A.; Kaganoi, J.; Komoto, I.; Kawabe, A.; Imamura, M. Inhibitory Effects of Epigallocatechin-3-Gallate on N-Nitrosomethylbenzylamine-Induced Esophageal Tumorigenesis in F344 Rats. Int. J. Oncol. 2002, 21, 1275–1283. [Google Scholar] [CrossRef]
- Wu, H.; Xin, Y.; Xiao, Y.; Zhao, J. Low-Dose Docetaxel Combined with (−)-Epigallocatechin-3-Gallate Inhibits Angiogenesis and Tumor Growth in Nude Mice with Gastric Cancer Xenografts. Cancer Biother. Radiopharm. 2012, 27, 204–209. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin Gallate Inhibits Growth and Activation of the VEGF/VEGFR Axis in Human Colorectal Cancer Cells. Chem. Biol. Interact. 2010, 185, 247–252. [Google Scholar] [CrossRef]
- American Cancer Society. Key Statistics for Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 2 January 2023).
- Fu, H.; He, J.; Mei, F.; Zhang, Q.; Hara, Y.; Ryota, S.; Lubet, R.A.; Chen, R.; Chen, D.; You, M. Lung Cancer Inhibitory Effect of Epigallocatechin-3-Gallate Is Dependent on Its Presence in a Complex Mixture (Polyphenon E). Cancer Prev. Res. 2009, 2, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Manna, S.; Mukherjee, S.; Roy, A.; Das, S.; Panda, C.K. Tea Polyphenols Can Restrict Benzo[a]Pyrene-Induced Lung Carcinogenesis by Altered Expression of P53-Associated Genes and H-Ras, c-Myc and Cyclin D1. J. Nutr. Biochem. 2009, 20, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Banerjee, S.; Mukherjee, S.; Das, S.; Panda, C.K. Epigallocatechin Gallate Induced Apoptosis in Sarcoma180 Cells In Vivo: Mediated by P53 Pathway and Inhibition in U1B, U4-U6 UsnRNAs Expression. Apoptosis 2006, 11, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Liao, J.; Yang, G.; Reuhl, K.R.; Hao, X.; Yang, C.S. Inhibition of Adenoma Progression to Adenocarcinoma in a 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone-Induced Lung Tumorigenesis Model in A/J Mice by Tea Polyphenols and Caffeine. Cancer Res. 2006, 66, 11494–11501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.D.; Chen, S.H.; Lin, C.L.; Tsai, S.H.; Liang, Y.C. Inhibition of Melanoma Growth and Metastasis by Combination with (−)-Epigallocatechin-3-Gallate and Dacarbazine in Mice. J. Cell. Biochem. 2001, 83, 631–642. [Google Scholar] [CrossRef]
- Takahashi, A.; Watanabe, T.; Mondal, A.; Suzuki, K.; Kurusu-Kanno, M.; Li, Z.; Yamazaki, T.; Fujiki, H.; Suganuma, M. Mechanism-Based Inhibition of Cancer Metastasis with (−)-Epigallocatechin Gallate. Biochem. Biophys. Res. Commun. 2014, 443, 1–6. [Google Scholar] [CrossRef]
- Takahashi, N.; Kobayashi, M.; Ogura, J.; Yamaguchi, H.; Satoh, T.; Watanabe, K.; Iseki, K. Immunoprotective Effect of Epigallocatechin-3-Gallate on Oral Anticancer Drug-Induced α-Defensin Reduction in Caco-2 Cells. Biol. Pharm. Bull. 2014, 37, 490–492. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-X.; Chen, Y.-K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.-J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-Oxidative Activities and Dose–Response Relationship of (−)-Epigallocatechin-3-Gallate in the Inhibition of Lung Cancer Cell Growth: A Comparative Study in Vivo and in Vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.-H.; Su, Z.-Y.; Chae, J.-I.; Kim, D.J.; Zhu, F.; Ma, W.-Y.; Bode, A.M.; Yang, C.S.; Dong, Z. Epigallocatechin Gallate Suppresses Lung Cancer Cell Growth through Ras-GTPase-Activating Protein SH3 Domain-Binding Protein 1. Cancer Prev. Res. 2010, 3, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Milligan, S.A.; Burke, P.; Coleman, D.T.; Bigelow, R.L.; Steffan, J.J.; Carroll, J.L.; Williams, B.J.; Cardelli, J.A. The Green Tea Polyphenol EGCG Potentiates the Antiproliferative Activity of C-Met and Epidermal Growth Factor Receptor Inhibitors in Non-Small Cell Lung Cancer Cells. Clin. Cancer Res. 2009, 15, 4885–4894. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Liu, F.; Zhang, W.; Liu, X.; Lin, B.; Tang, X. Epigallocatechin-3-Gallate Inhibits Nicotine-Induced Migration and Invasion by the Suppression of Angiogenesis and Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer Cells. Oncol. Rep. 2015, 33, 2972–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadava, D.; Whitlock, E.; Kane, S.E. The Green Tea Polyphenol, Epigallocatechin-3-Gallate Inhibits Telomerase and Induces Apoptosis in Drug-Resistant Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2007, 360, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.-W.; Lee, J.H.; Kim, Y.-J.; Hwang, G.S.; Kim, S.-N.; Kwak, J.H.; Cheon, G.J.; Kim, K.H.; Jang, H.-J.; Ham, J.; et al. Synergistic Effect of Curcumin on Epigallocatechin Gallate-Induced Anticancer Action in PC3 Prostate Cancer Cells. BMB Rep. 2015, 48, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafif, A.; Schantz, S.P.; Chou, T.C.; Edelstein, D.; Sacks, P.G. Quantitation of Chemopreventive Synergism between (−)-Epigallocatechin-3-Gallate and Curcumin in Normal, Premalignant and Malignant Human Oral Epithelial Cells. Carcinogenesis 1998, 19, 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The Combination of Epigallocatechin Gallate and Curcumin Suppresses ER Alpha-Breast Cancer Cell Growth in Vitro and in Vivo. Int. J. Cancer 2008, 122, 1966–1971. [Google Scholar] [CrossRef]
- Wang, S.; Chen, R.; Zhong, Z.; Shi, Z.; Chen, M.; Wang, Y. Epigallocatechin-3-Gallate Potentiates the Effect of Curcumin in Inducing Growth Inhibition and Apoptosis of Resistant Breast Cancer Cells. Am. J. Chin. Med. 2014, 42, 1279–1300. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, G.-H.; Guan, B.-X.; Xu, X.-C. Suppression of Esophageal Cancer Cell Growth Using Curcumin, (−)-Epigallocatechin-3-Gallate and Lovastatin. World J. Gastroenterol. 2012, 18, 126–135. [Google Scholar] [CrossRef]
- Zhou, D.-H.; Wang, X.; Yang, M.; Shi, X.; Huang, W.; Feng, Q. Combination of Low Concentration of (−)-Epigallocatechin Gallate (EGCG) and Curcumin Strongly Suppresses the Growth of Non-Small Cell Lung Cancer in Vitro and in Vivo through Causing Cell Cycle Arrest. Int. J. Mol. Sci. 2013, 14, 12023–12036. [Google Scholar] [CrossRef] [Green Version]
- Du, G.-J.; Wang, C.-Z.; Qi, L.-W.; Zhang, Z.-Y.; Calway, T.; He, T.-C.; Du, W.; Yuan, C.-S. The Synergistic Apoptotic Interaction of Panaxadiol and Epigallocatechin Gallate in Human Colorectal Cancer Cells. Phytother. Res. 2013, 27, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Kostin, S.F.; McDonald, D.E.; McFadden, D.W. Inhibitory Effects of (−)-Epigallocatechin-3-Gallate and Pterostilbene on Pancreatic Cancer Growth in Vitro. J. Surg. Res. 2012, 177, 255–262. [Google Scholar] [CrossRef]
- Nair, S.; Barve, A.; Khor, T.-O.; Shen, G.-X.; Lin, W.; Chan, J.Y.; Cai, L.; Kong, A.-N. Regulation of Nrf2- and AP-1-Mediated Gene Expression by Epigallocatechin-3-Gallate and Sulforaphane in Prostate of Nrf2-Knockout or C57BL/6J Mice and PC-3 AP-1 Human Prostate Cancer Cells. Acta Pharmacol. Sin. 2010, 31, 1223–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.A.; Makpol, S.; Jamal, R.; Harun, R.; Mokhtar, N.; Ngah, W.Z.W. Tocotrienol-Rich Fraction, [6]-Gingerol and Epigallocatechin Gallate Inhibit Proliferation and Induce Apoptosis of Glioma Cancer Cells. Molecules 2014, 19, 14528–14541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.D.; Hong, J.; Kim, D.H.; Mishin, V.M.; Yang, C.S. Piperine Enhances the Bioavailability of the Tea Polyphenol (−)-Epigallocatechin-3-Gallate in Mice. J. Nutr. 2004, 134, 1948–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.D.; Kwon, S.-J.; Ju, J.; Bose, M.; Lee, M.-J.; Hong, J.; Hao, X.; Yang, C.S. Effect of Genistein on the Bioavailability and Intestinal Cancer Chemopreventive Activity of (−)-Epigallocatechin-3-Gallate. Carcinogenesis 2008, 29, 2019–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with Ascorbic Acid and Sucrose Modulates Catechin Bioavailability from Green Tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.-M.; Yoo, S.-H.; Ra, C.-S.; Kim, Y.-K.; Chung, J.-O.; Lee, S.-J. Digestive Stability and Absorption of Green Tea Polyphenols: Influence of Acid and Xylitol Addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Amin, A.R.M.R.; Wang, D.; Zhang, H.; Peng, S.; Shin, H.J.C.; Brandes, J.C.; Tighiouart, M.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Enhanced Anti-Tumor Activity by the Combination of the Natural Compounds (−)-Epigallocatechin-3-Gallate and Luteolin: Potential Role of P53. J. Biol. Chem. 2010, 285, 34557–34565. [Google Scholar] [CrossRef] [Green Version]
- Papi, A.; Farabegoli, F.; Iori, R.; Orlandi, M.; De Nicola, G.R.; Bagatta, M.; Angelino, D.; Gennari, L.; Ninfali, P. Vitexin-2-O-Xyloside, Raphasatin and (−)-Epigallocatechin-3-Gallate Synergistically Affect Cell Growth and Apoptosis of Colon Cancer Cells. Food Chem. 2013, 138, 1521–1530. [Google Scholar] [CrossRef]
- Mirzaaghaei, S.; Foroughmand, A.M.; Saki, G.; Shafiei, M. Combination of Epigallocatechin-3-Gallate and Silibinin: A Novel Approach for Targeting Both Tumor and Endothelial Cells. ACS Omega 2019, 4, 8421–8430. [Google Scholar] [CrossRef]
- Papież, M.A.; Krzyściak, W. Epicatechin Acts Synergistically with Curcumin-Induced Cytogenotoxic Effect in Acute Promyelocytic Leukemia HL-60 Cell Line. J. Unexplored Med. Data 2017, 2, 52. [Google Scholar] [CrossRef]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (−)-Epigallocatechin Gallate Sensitizes Breast Cancer Cells to Paclitaxel in a Murine Model of Breast Carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stearns, M.E.; Wang, M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-Gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, M.; Du, G.-J.; Wang, C.-Z.; Yuan, C.-S. Letter to the Editor: Panaxadiol’s Anticancer Activity Is Enhanced by Epicatechin. Am. J. Chin. Med. 2010, 38, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.; Wagner, A.; Neureiter, D.; Pichler, M.; Jakab, M.; Illig, R.; Berr, F.; Kiesslich, T. The Green Tea Catechin Epigallocatechin Gallate Induces Cell Cycle Arrest and Shows Potential Synergism with Cisplatin in Biliary Tract Cancer Cells. BMC Complement. Altern. Med. 2015, 15, 194. [Google Scholar] [CrossRef] [Green Version]
- Shervington, A.; Pawar, V.; Menon, S.; Thakkar, D.; Patel, R. The Sensitization of Glioma Cells to Cisplatin and Tamoxifen by the Use of Catechin. Mol. Biol. Rep. 2009, 36, 1181–1186. [Google Scholar] [CrossRef]
- Qian, F.; Ye, C.L.; Wei, D.Z.; Lu, Y.H.; Yang, S.L. In Vitro and in Vivo Reversal of Cancer Cell Multidrug Resistance by 2′,4′-Dihydroxy-6′-Methoxy-3′,5′-Dimethylchalcone. J. Chemother. 2005, 17, 309–314. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, D.; Liu, J. In Vivo Reversal of Doxorubicin Resistance by (−)-Epigallocatechin Gallate in a Solid Human Carcinoma Xenograft. Cancer Lett. 2004, 208, 179–186. [Google Scholar] [CrossRef]
- Liang, G.; Tang, A.; Lin, X.; Li, L.; Zhang, S.; Huang, Z.; Tang, H.; Li, Q.Q. Green Tea Catechins Augment the Antitumor Activity of Doxorubicin in an in Vivo Mouse Model for Chemoresistant Liver Cancer. Int. J. Oncol. 2010, 37, 111–123. [Google Scholar]
- Farhan, M.; Zafar, A.; Chibber, S.; Khan, H.Y.; Arif, H.; Hadi, S.M. Mobilization of copper ions in human peripheral lymphocytes by catechins leading to oxidative DNA breakage: A structure activity study. Arch. Biochem. Biophys. 2015, 580, 31–40. [Google Scholar] [CrossRef]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins 2016, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef] [Green Version]
- Jigisha, A.; Nishant, R.; Navin, K. Green tea: A magical herb with miraculous outcomes. Int. Res. J. Pharm. 2012, 3, 139–148. [Google Scholar]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Marzec, Z.; Szwerc, W.; Głowniak, K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, D.S. Green tea polyphenolic compounds and human health. J. Verbr. Lebensm. 2007, 2, 407–413. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Young, W.; Shahidi, F. Epigallocatechin (EGC) esters as potential sources of antioxidants. Food Chem. 2020, 309, 125609. [Google Scholar] [CrossRef] [PubMed]
- Lawless, M.W.; O’Byrne, K.J.; Gray, S.G. Targeting oxidative stress in cancer. Expert Opin. Ther. Targets 2010, 14, 1225–1245. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.J.; Graf, T.N.; Paine, M.F.; McCune, J.S.; Kvalheim, O.M.; Oberlies, N.H.; Cech, N.B. Comparison of Metabolomics Approaches for Evaluating the Variability of Complex Botanical Preparations: Green Tea (Camellia sinensis) as a Case Study. J. Nat. Prod. 2017, 80, 1457–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Matcha green tea (MGT) inhibits the propagation of cancer stem cells (CSCs), by targeting mitochondrial metabolism, glycolysis, and multiple cell signaling pathways. Aging 2018, 10, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Schulze, J.; Melzer, L.; Smith, L.; Teschke, R. Green Tea and Its Extracts in Cancer Prevention and Treatment. Beverages 2017, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. A putative mechanism for anticancer and apoptosis inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef] [PubMed]

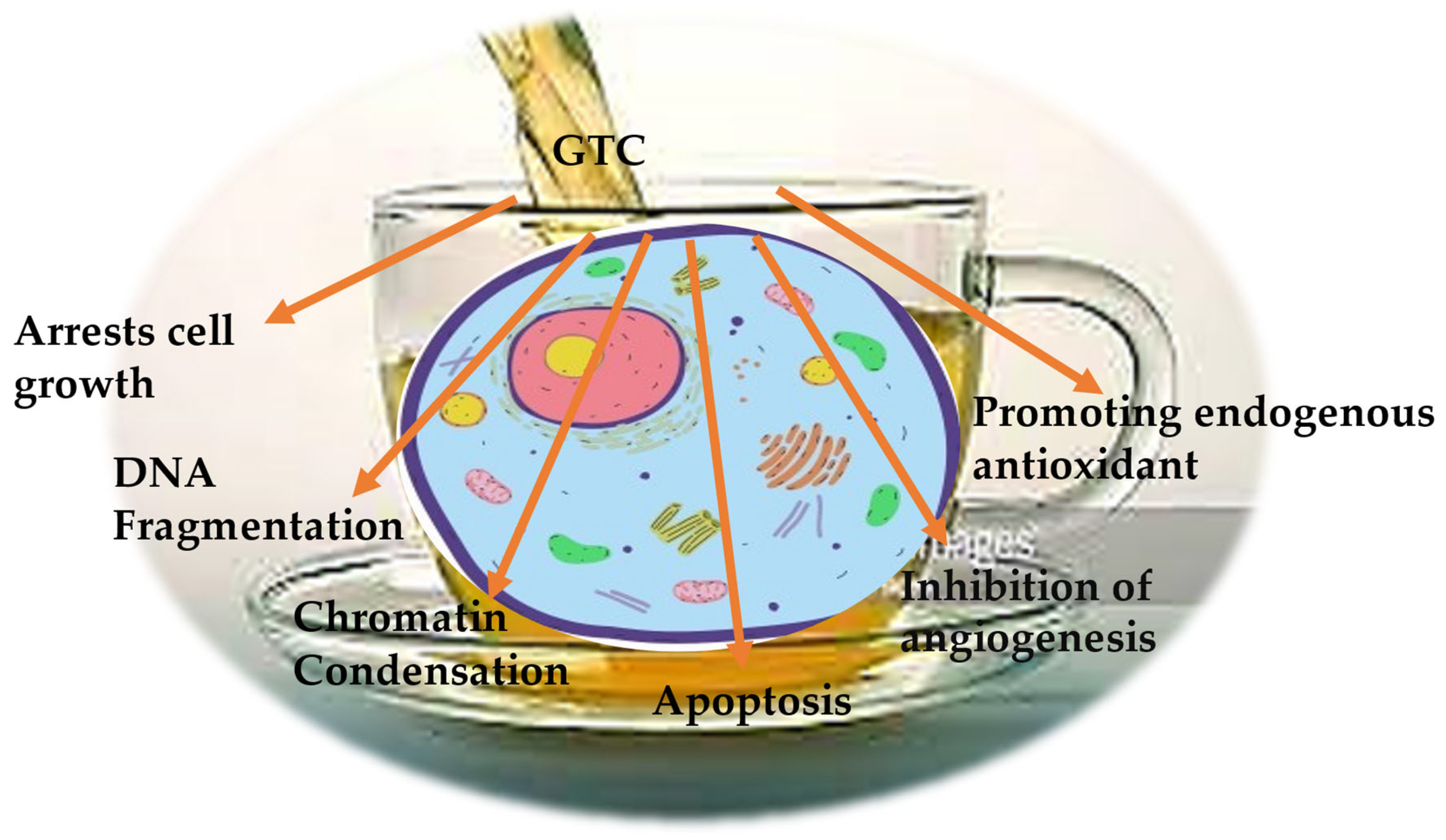

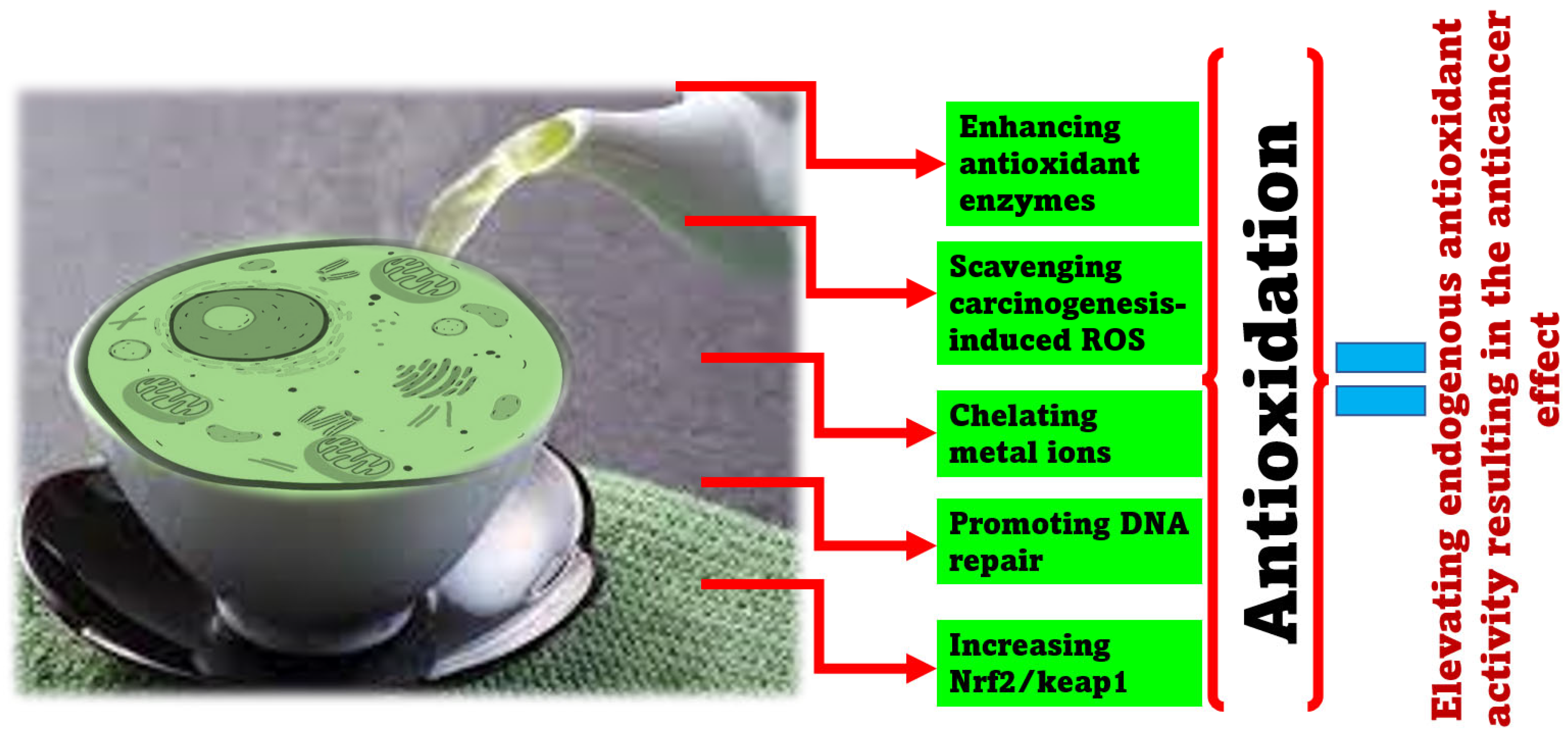
| GTC Component | Natural Compounds | Cancer Type | Anticarcinogenic Activity | Molecular Mechanism | References |
|---|---|---|---|---|---|
| EGCG | Curcumin | MCF-7 breast cancer | Inhibition of cancer cell growth and induction of apoptosis | Activation of caspase-dependent apoptosis, inhibition of P-gP pump function | [160] |
| EGCG | Curcumin | MDA-MB-231 breast cancer | Inhibition of cancer cell growth | G(2)/M-phase arrest. Decreased VEGFR-1 protein expression in tumours | [159] |
| Epicatechin | Curcumin | HL-60 myeloid leukaemia | Inhibition of cancer cell growth | Cell cycle arrest at S phase | [174] |
| EGCG | Curcumin | Normal, premalignant and malignant oral cells | Inhibition of cancer cell growth | Cell cycle arrest at G1 phase and S/G2M | [158] |
| EGCG | Curcumin | A549 and NCI-H460 lung cancer | Inhibition of cancer cell and tumour growth | Cell cycle arrest at G1 and S/G2 phases via cyclin D1 and cyclin B1 inhibition | [162] |
| EGCG | Curcumin | PC3—human prostate cancer | Inhibition of cancer cell and tumour growth | Arrests S and G2/M phases by upregulated expression of p-21 | [157] |
| EGCG | Curcumin and lovastatin | SKGT-4 and TE-8 esophageal cancer | Inhibition of cancer cell and tumour growth | Suppression of mitotic signal transduction pathway through Phosphorylation/dephosporylation of /Erk1/2, c-Jun and COX-2 | [161] |
| EGCG | Silibinin | Non-small-cell lung cancer cells | Inhibition of angiogenesis and cell migration of endothelial and lung tumour cells | Antiangiogenic activity via VEGF, VEGFR2, and miR-17–92 cluster and miR-19b cluster | [173] |
| EGCG | Luteolin | esophageal cancer cell lines—TE-8 and SKGT-4 | Inhibition of growth and induction of apoptosis of cancer cells and tumour | induction of p53-dependent apoptotic pathways | [171] |
| EGCG | 6-Gingerol | 1321 N1 and LN18 glioma cells | Inhibition of cancer cell growth and induce apoptosis | Apoptosis induction by activated Caspase-3 and Annexin-V FITC/PI | [166] |
| EGCG | Panaxadiol (PD) | HCT-116 and SW-480 human colorectal cancers | Inhibits cancer cell growth and induces apoptosis | Cell cycle arrest at G1 and G2/M | [163] |
| EGCG | Sulforaphane (SFN) | PC3 prostate cancer | Arrests cancer cells | Inhibition of genes in AP-1 pathway inhibits cell proliferation, differentiation, apoptosis, angiogenesis and tumour invasion | [165] |
| EGCG | Pterostilbene | MIA PaCa-2 and PANC-1 Pancreatic cancer cells | Inhibits growth of cancer cells and induces apoptosis | Induction of cell cycle arrest and cell apoptosis | [164] |
| EGCG | Paclitaxel (Taxol) | Breast cancer cells (4T1, MCF-7, and MDA-MB231) | Inhibits growth of cancer cells and induces apoptosis | JNK phosphorylation and cell death | [175] |
| EGCG | taxane (i.e., paclitaxel and docetaxel) | Human Prostrate cancer cells PC-3ML cells | Inhibition of growth and induction of apoptosis | Induction of cell cycle arrest and cell apoptosis | [176] |
| EC epicatechin | Panaxadio | HCT-116 human colorectal cancer cells | Inhibition of cancer cell growth | Mechanism not reported | [177] |
| EGCG | Cisplatin/ tamoxifen | 1321N1 cells and U87-MG cells/ biliary tract cancer cells | Cytotoxicity on Cancer cells | Inhibition of telomerase and induction of cell cycle arrest | [178,179] |
| EGCG | doxorubicin | Drug-resistant KB-A1 cells | Cytotoxicity on Cancer cells | Modulating P-glycoprotein efflux pump | [180] |
| EGCG | doxorubicin (DOX) | human oral epidermoid carcinoma (KB-A-1) | In vivo reversal of doxorubicin resistanceby (-)-epigallocatechin gallate in a solid human carcinoma | Induction of cell apoptosis | [181] |
| EGCG | Vitexin-2-o- xyloside, Raphasatin | Colon cancer cells—LoVo and CaCo-2; Breast cancer cells—MDA-MB-231 and MCF-7 | Inhibition of cancer cell growth and induction of apoptosis | Arrests Cell cycle at G0/G1 phases and activating ROS- mediated mitochondrial apoptotic pathways | [172] |
| EGCG and ECG | DOX | Chemoresistant hepatocellular carcinoma (HCC) cell line BEL-7404 | Inhibition of cancer cell and tumour growth. | Inhibition of P-glycoprotein efflux pump | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-W.; Muthu, M.; Pushparaj, S.S.C.; Gopal, J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules 2023, 28, 2151. https://doi.org/10.3390/molecules28052151
Oh J-W, Muthu M, Pushparaj SSC, Gopal J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules. 2023; 28(5):2151. https://doi.org/10.3390/molecules28052151
Chicago/Turabian StyleOh, Jae-Wook, Manikandan Muthu, Suraj Shiv Charan Pushparaj, and Judy Gopal. 2023. "Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components" Molecules 28, no. 5: 2151. https://doi.org/10.3390/molecules28052151
APA StyleOh, J.-W., Muthu, M., Pushparaj, S. S. C., & Gopal, J. (2023). Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules, 28(5), 2151. https://doi.org/10.3390/molecules28052151





