Abstract
Terpenes and essential oils are materials of great commercial use due to their broad spectra of antibacterial, antifungal, membrane permeation enhancement and antioxidant biological properties, as well as for their use as flavors and fragrances. Yeast particles (YPs) are 3–5 µm hollow and porous microspheres, a byproduct of some food-grade yeast (Saccharomyces cerevisiae) extract manufacturing processes, that have been used for the encapsulation of terpenes and essential oils with high payload loading capacity (up to 500% weight) and efficiency, providing stability and sustained-release properties. This review focuses on encapsulation approaches for the preparation of YP–terpene and essential oil materials that have a wide range of potential agricultural, food and pharmaceutical applications.
1. Introduction
Essential oils are concentrated, complex mixtures of volatile and non-volatile compounds obtained from plants. Terpenes are organic compounds that constitute the main component of essential oils and can be obtained as highly purified (>90%) materials extracted from natural sources or that are chemically synthesized. Both essential oils and pure terpenes are of great commercial interest due to their broad range of functional properties. These materials are used as permeation enhancers and antioxidants in cosmetics, as flavors in foods or as additives in food packaging to prevent microbial spoilage oxidation, as naturally derived pesticides in agricultural products, and as bioactive compounds or excipients in nutraceuticals and pharmaceuticals [1,2,3,4,5]. The interest in the use of terpenes will continue increasing in response to consumer trends favoring the use of natural compounds in food, agricultural, consumer, and pharmaceutical products.
However, despite their many applications, the use of terpenes and essential oils in commercial products presents a few challenges due to terpene’s low water solubility, volatility, and chemical decomposition when exposed to air, heat, light and moisture. There are also challenges in some applications due to safety limits and the need for targeted sustained delivery of the active compounds or marked organoleptic effects when used as food additives. These challenges are generally overcome by encapsulation of terpenes or essential oil compositions in delivery systems. Encapsulation improves the stability of terpene by reducing loss of terpene content due to volatility or by chemical degradation. It also masks undesirable flavors associated with some terpenes utilized as additives to prevent food spoilage and increases shelf-life storage, and depending on the encapsulation material, it can provide for sustained terpene release required in some applications such as agricultural and pharmaceutical products [6,7,8,9,10].
Encapsulation techniques commonly used in the preparation of terpene formulations include emulsification, spray drying, liposomes, molecular complexation, complex coacervation and nanoparticles [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. An effective terpene encapsulation process should produce terpene compositions with (1) high payload encapsulation efficiency, (2) high terpene loading capacity, (3) homogenous payload distribution in the matrix carrier, (4) improved terpene protection, (5) retention of the biological activity of terpenes, and (6) sustained terpene release characteristics. In addition to these properties, the process of encapsulation should be inexpensive, work under mild processing conditions, and the preferred materials for terpene encapsulation should have mechanical strength, controlled release properties, and should not pose any safety risk. Examples of common encapsulation techniques currently used in the formulation of terpene products are presented in Table 1.

Table 1.
Encapsulation methods that can be used to prepare terpene and essential oil formulations.
We have developed alternative methods using yeast particles (YPs) to efficiently encapsulate high levels of terpenes and essential oils. In this review, we present these different approaches to produce YP–terpene compositions with higher terpene payload capacity and encapsulation efficiency as well as and the methods to achieve controlled release.
2. Yeast Particles
Yeast particles (YPs) are 3–5 µm hollow and porous microspheres, a byproduct of some food-grade Baker’s yeast (Saccharomyces cerevisiae) extract manufacturing processes. The hollow cavity of YPs can be used for encapsulation of a broad range of hydrophilic and hydrophobic macromolecules and small molecules [37,38,39,40,41,42,43,44,45]. YPs offer several potential advantages as delivery carriers, such as high payload capacity, payload protection from external environmental stresses, possibility of controlled payload release, biocompatibility, and biodegradability.
Some work in the literature describing the use of YPs for encapsulation of bioactives has reported a few challenges using YPs due to the difficulty of loading bioactive substances, resulting in low encapsulation efficiency, low payload capacity, and aggregation of payload on the outer surface YP wall rather than efficiently encapsulated within the hollow cavity of YPs [46,47,48,49,50]. These encapsulation challenges are significantly diminished by careful control of terpene:water:YP weight ratios during the loading process to maximize terpene diffusion into YPs. In this review, we report different methods for the encapsulation of terpenes and essential oils in YPs that result in the production of YP–terpene formulations with high payload capacity (up to 5:1 w/w terpene:YP ratio), high encapsulation efficiency (>95%), homogenous terpene loading inside the hollow cavity of YPs, and sustained terpene release.
3. First Generation YP–Terpenes—Diffusion-Based Loading and Release
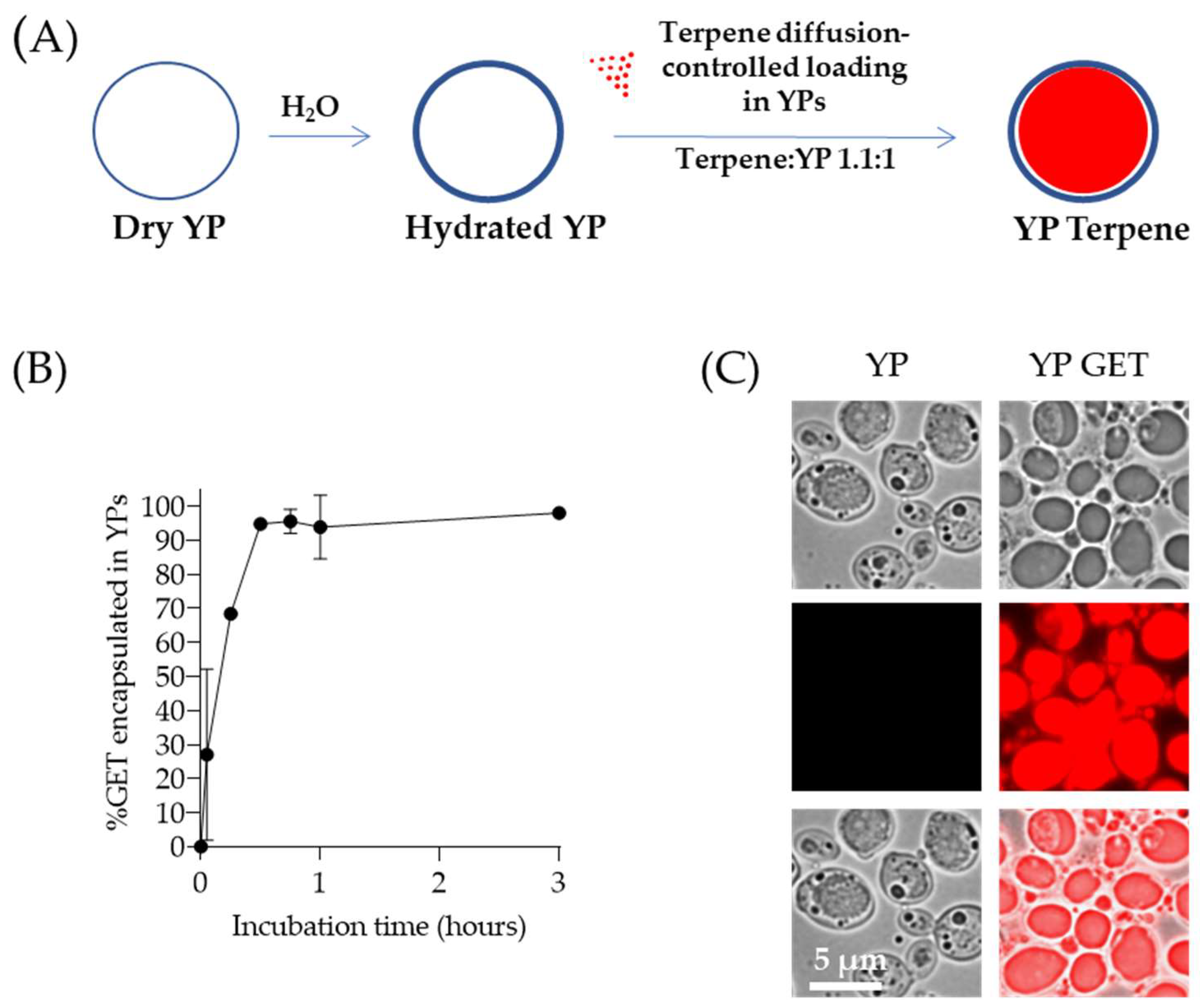
Terpene encapsulation in YPs is based on the loading of terpenes inside the hydrophobic YP cavity by the passive diffusion of terpenes through the porous cell walls in a homogenized aqueous suspension of YPs, as illustrated in Figure 1A. This YP–terpene loading method is a low-cost, easily scaled approach that produces stable YP–terpene suspensions up to a 2:1 w/w terpene:YP ratio, without the use of surfactants or alcohols to stabilize the terpene in the formulation. A mixture of geraniol (G), eugenol (E), and thymol (T) at a composition of 2:1:2 G:E:T weight ratio was used to evaluate the production of YP–terpenes. This GET mixture has been shown to be highly effective in agricultural applications against a broad range of plant pathogens [51]. The passive diffusion of the GET mixture into YPs in a homogenized aqueous YP suspension is a rapid process, and >95% of the GET mixture is encapsulated within one hour in samples prepared at a GET:YP weight ratio of 1.1:1 as shown by HPLC quantification in Figure 1B. The loading of terpenes inside the hollow cavity of YPs can be visualized by microscopy as a clear terpene droplet in the brightfield images and by using Nile red dye to stain terpenes as shown in Figure 1C. Nile red is a lipophilic stain; it does not fluoresce in a polar (water) solvent, but it is highly fluorescent in a hydrophobic (terpene) environment. These formulations are prepared and stored as homogenized stable, aqueous YP GET suspensions at 150 g YP/L, 165 g GET/L. At these high concentrations, the terpenes remain >95% encapsulated within the hollow hydrophobic cavity of the particles. Sustained terpene release is the reverse diffusion process that occurs upon sample dilution and is a function of terpene water solubility, which can be modified by the addition of surfactants or water-miscible organic solvents.
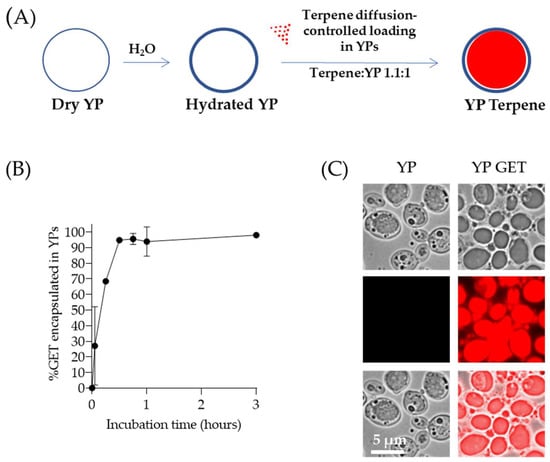
Figure 1.
(A) Schematics of diffusion-controlled terpene loading in YPs, (B) kinetics of 2:1:2 GET loading in YPs quantified by HPLC of samples prepared at a GET:YP ratio of 1.1:1 in a homogenized YP suspension (final concentration of 150 g YP/L, 16.5% GET), and (C) microscopy images of Nile red-stained YP control (t = 0) and YPs loaded with GET after 1-h incubation. Brightfield images show a droplet inside YP GET, and Nile red staining of hydrophobic terpene confirms this droplet corresponds to a terpene droplet loaded in YPs. Figures adapted from Reference [40] and reproduced with permission from MDPI.
This first-generation YP–terpene encapsulation approach has been effectively implemented to develop and commercialize YP–terpene-based fungicide and nematicide products for agricultural applications [51,52,53,54,55,56]. This approach has also been used in the encapsulation of essential oils and fragrances to develop YP-based odor neutralizers, head-lice lotions, and household insecticidal sprays effective against fleas, dust mites, and bed bugs.
In addition to the development of YP–terpenes for agricultural and consumer care products, we have evaluated YP–terpenes in studies focused on the potential development of YP–terpene formulations for pharmaceutical applications, specifically in the possible use of YP–terpenes for the treatment of gastrointestinal parasitic nematode (e.g., roundworm, hookworm, whipworm) infections. Terpenes have a long history of use as anthelmintics, and thymol was successfully employed to eradicate hookworm infections in the United States in the early 20th century [57]. Although effective, the medical application of thymol and other terpenes has long been discontinued due to the need of using large doses of terpenes, as >90% of orally administered terpene is rapidly absorbed in the stomach [58], resulting in toxic side effects and less than 10% of terpenes reaching the target sites in the intestine where parasitic nematodes reside. Currently, parasitic nematode infections affect ~1 billion people in developing countries [59], and the current anthelmintic drugs (benzimidazoles) employed in mass drug administration programs do not have broad specificity, and their application is at risk by the emergence of drug-resistant nematodes [60,61].
The encapsulation method developed for YP GET formulations was evaluated with 17 commercially available terpenes and three essential oils (lavender, tea tree, and peppermint oil) to produce YP–terpene (or essential oil) compositions at 1.1:1 w/w terpene:YP ratio [38]. All YP–terpene samples were produced with >95% encapsulation efficiency. These YP–terpenes were evaluated for their in vitro anthelmintic activity on hookworms (Ancylostoma ceylanicum, Nippostronglyus brasiliensis) and whipworms (Trichuris muris). The results showed that YP–terpenes are broad-acting anthelmintics [38]. The use of YPs as a terpene delivery carrier is a promising approach for the development of low-cost anthelmintics with broad specificity and with the potential to overcome parasite resistance.
Terpene release from this first-generation YP–terpene compounds is based on passive diffusion of terpenes through the porous YP cell walls and is a function of terpene water solubility. This release process can pose limitations in the application of YP–terpene materials, for example, for agricultural applications (rapid elution of terpenes from YP–terpene in diluted samples) or in the development of YP–terpenes as anthelmintics (dilution in the digestive tract leading to burst release before payload reaching target pathogens in the intestine). The following sections describe encapsulation approaches to produce YP–terpene formulations with better control of terpene release.
4. YP Encapsulation Methods to Extend Duration of Terpene Release
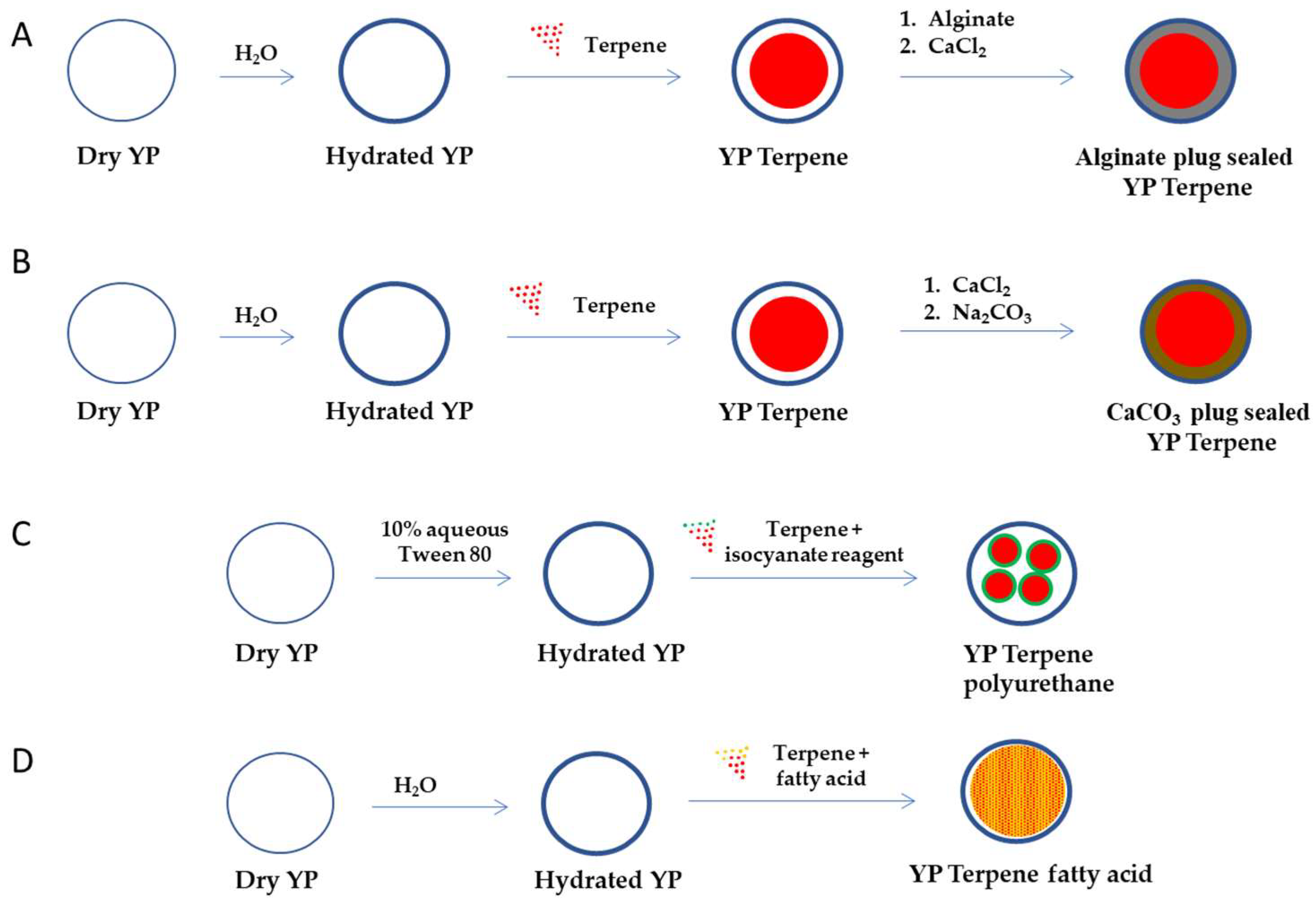
To develop YP–terpene formulations with better control of terpene release, we evaluated alternative YP encapsulation strategies that prevent rapid burst release of terpene upon dilution of YP–terpenes below the solubility of the encapsulated terpene in water. These YP–terpene encapsulation approaches incorporate some aspects of other methods for terpene delivery and include the plug seal of YP–terpene samples using hydrocolloid crosslinked gels, encapsulation of terpenes in nanoparticles generated in situ in YPs, and co-loading of terpene with a hydrophobic sequestering agent. The steps to prepare these materials are schematically depicted in Figure 2.
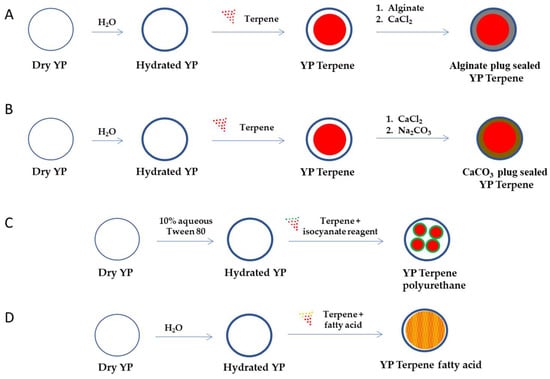
Figure 2.
Schematic representation of steps required to encapsulate terpenes in YPs using (A) an alginate plug seal, (B) calcium carbonate plug seal, (C) encapsulation of terpenes in polyurethane nanoparticles, and (D) co-encapsulation with fatty acids (YP–terpene fatty acid can also be prepared by sequential loading of fatty acid and then the terpene).
4.1. YP–Terpene with a Hydrocolloid Plug Seal
Hydrogels are high-water content materials prepared by crosslinking polymers and have applications in encapsulation technologies due to their ability to trap and slowly release the target payload [62,63]. Several non-toxic, biocompatible, and biodegradable compounds such as the natural polysaccharides chitosan and alginate have been extensively studied in drug encapsulation. The use of hydrocolloids can be applied in YPs by loading of the hydrocolloid polymer precursor and crosslinking to form a shell embedding the payload and plug sealing the pores of YPs. This approach has been previously demonstrated by plug-sealing yeast particles encapsulating the TB drug rifampicin using alginate–calcium and chitosan hydrocolloids [64].
Hydrocolloid plug-sealed YP–terpene samples have been prepared using calcium crosslinked alginate. Alginate plug-sealed YP–terpene samples extend the duration of terpene release for a few hours compared to YP–terpenes. Limitations of this approach are the need for a rapid crosslinking reaction to plug seal the YP pores and prevent dissolution of the polymer matrix in the terpene payload.italics
4.2. YP–Terpenes with a Calcium Carbonate Plug Seal
The use of inorganic amorphous insoluble materials prepared in situ in YPs is an approach, similar to the use of hydrocolloids in which the YP pores are plug sealed by the inorganic matrix slowing diffusion of terpene release. Calcium carbonate can be formed in situ in YPs by absorption of calcium chloride and subsequent reaction with sodium carbonate or sodium bicarbonate at pH 9–10. This encapsulation method also extends the duration of terpene release for a few hours compared to YP–terpenes. The short extension of terpene release with hydrocolloid or carbonate plug seal methods over the first-generation YP–terpene technology limits their application. Encapsulation approaches with better control of payload release and that significantly extend the duration of terpene release from the particles are described in the following sections.
4.3. YP In Situ Encapsulation of Terpenes in Polyurethane/Polyurea Nanoparticles
We have previously shown that nanoparticles can be used in combination with YPs by either physisorption or chemical absorption by non-covalent or covalent binding of large nanoparticles (average diameter > 40 nm) to the outer surface of YPs, encapsulation of small nanoparticles (average diameter < 30 nm) inside the hollow cavity of YPs, or by in situ formation of nanoparticles in YPs [43,45,65]. Polyurethane/polyurea nanoparticle drug delivery systems are formed by the reaction of isocyanates and diols on the surface of an emulsion containing target drug [66,67].
YP–terpene polyurethane nanoparticles can be formed in situ in YPs by co-loading of terpene with the polyol/polyamine precursor and then crosslinking with an isocyanate reagent (e.g., isophorone diisocyanate, toluene diisocyanate).
This YP–terpene encapsulation approach achieves high encapsulation efficiency, similar to YP–terpenes 1.1:1, and significantly decreases the rate of terpene release (Figure 3B). However, the approach has limitations due to the need to use toxic isocyanate crosslinkers, possible side crosslinking reactions of polyurethane with terpenes containing hydroxyl groups, and regulatory concerns with the application of polyurethane materials in agricultural products or for direct human health applications. However, this YP–terpene encapsulation approach has potential if developed using green materials such as non-isocyanate polyurethane nanoparticles [68].
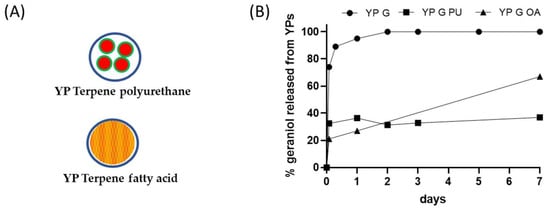
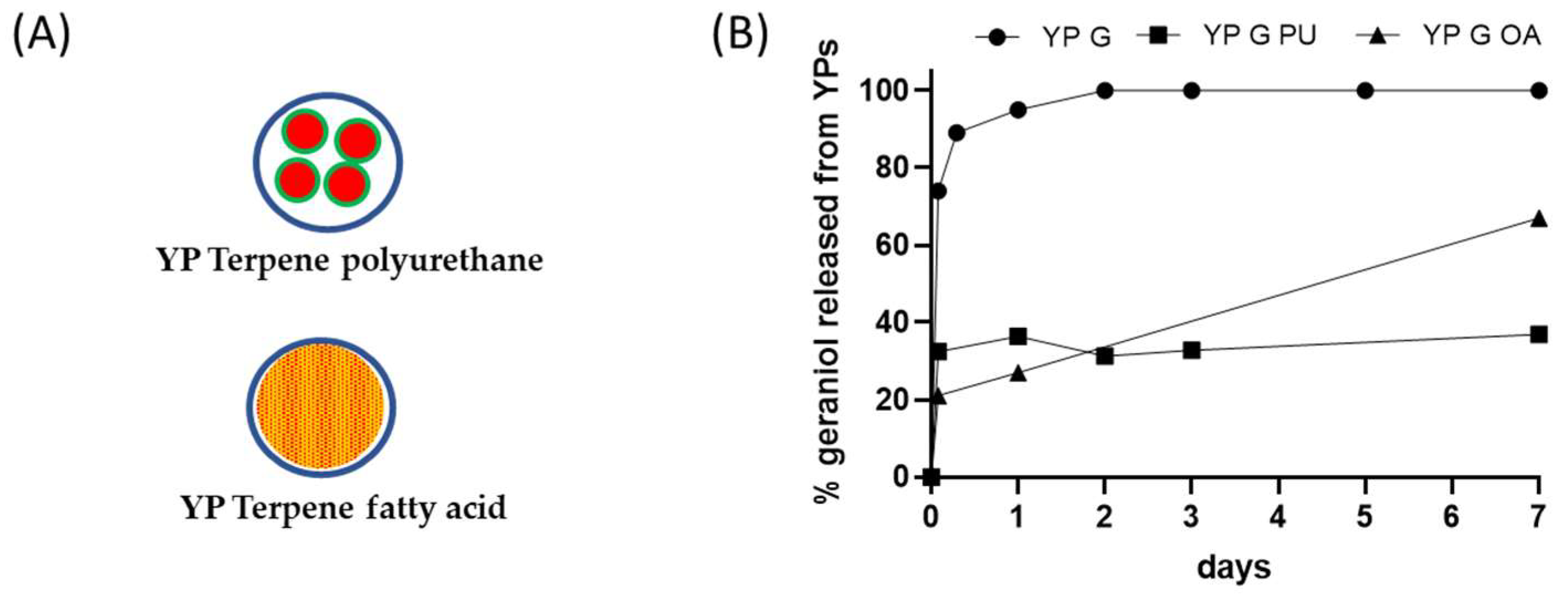
Figure 3.
(A) Schematics of YP–terpene formulations prepared by encapsulation of terpene in polyurethane or co-loading of terpene with a fatty acid, and (B) kinetics of geraniol release from YP samples at 25 °C in YP–terpene suspensions at total geraniol concentration of 1 mg/mL (YP G: YP geraniol, YP G PU: YP geraniol polyurethane, YP G OA: YP geraniol oleic acid).
4.4. YP Lipid Terpenes
Fatty acids can be used as sequestering agents of terpenes inside YPs. The rationale in this approach is to use fatty acids that (1) dissolve the target terpene compound and (2) are more hydrophobic than the terpene. The materials can be co-loaded in a single step or sequentially loaded in YPs. This approach generally requires loading of fatty acid and terpenes at a >1:1 fatty acid:terpene weight ratio to maximize terpene retention in the hydrophobic lipid environment. The selection of lipids with melting point above room temperature improves the stability of the YP–terpene lipid material by forming a solid matrix inside the cavity of YPs. The results in Figure 3B show significant reduction in the release of geraniol from a YP sample containing geraniol and oleic acid (1:1 oleic acid:geraniol) compared to the YP geraniol control. The geraniol release from YP–geraniol–oleic acid shows a burst release (~30% geraniol) between 0 and 4 h, and the remaining geraniol remains stably encapsulated up to 7 days (Figure 2B). These YP lipid terpene materials have potential use for long-term sustained terpene-release applications.
4.5. Hyper-Loaded YP–Terpenes
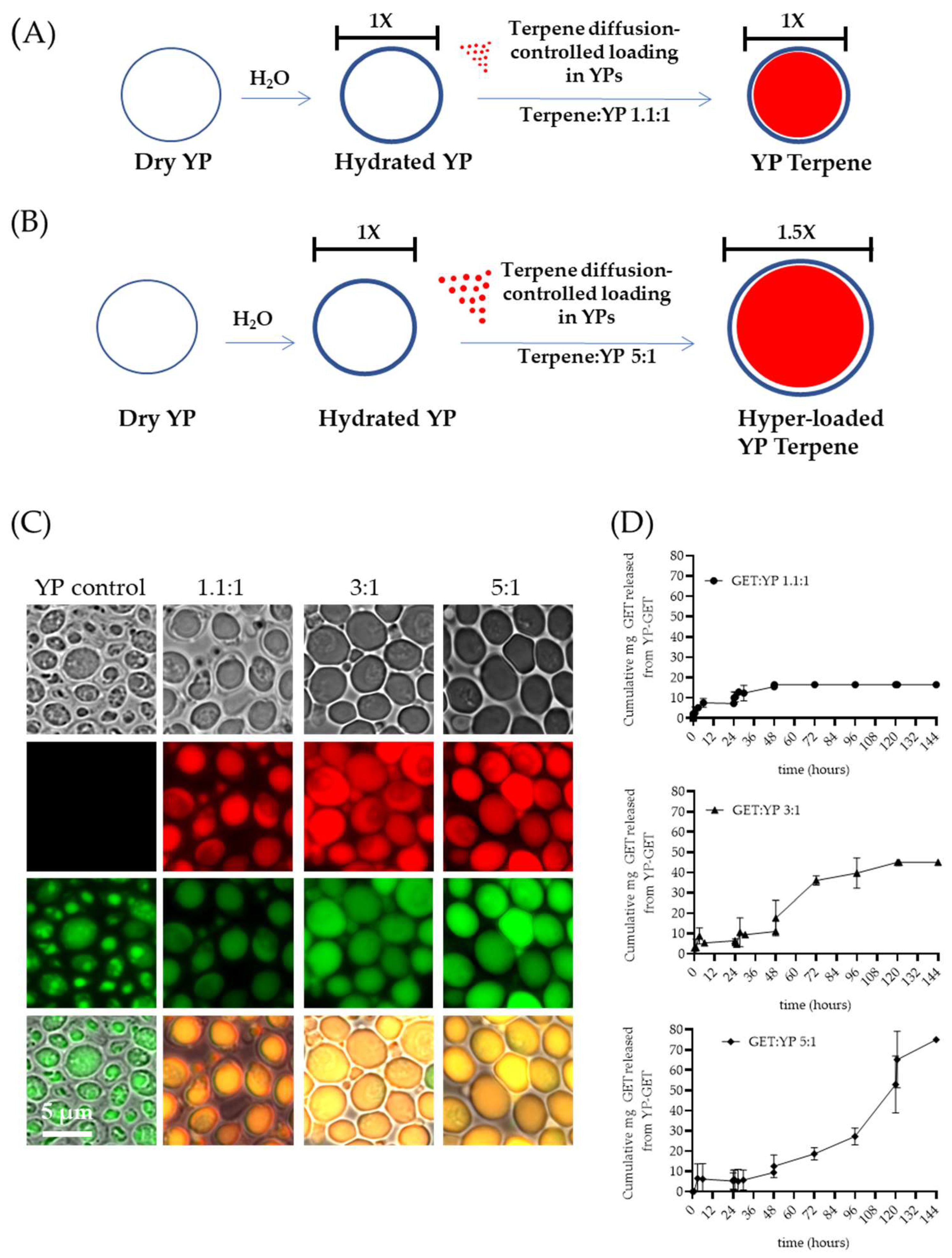
The first-generation YP–terpene formulations were developed to encapsulate up to a 2:1 w/w terpene:YP ratio. Recently, methods to increase terpene loading capacity in YPs up to 5:1 w/w were reported in the literature [40]. The preparation of hyper-loaded YP–terpenes is possible by carefully controlling the ratio of water to YP to minimally hydrate the porous shell, allowing for slow diffusion of terpene payload. The hyper-loading of terpenes in YPs leads to an increase in YP diameter from 5.4 µm (empty YPs) to 7.7 µm (YP–terpene 5:1) to accommodate the large terpene droplet at higher payload levels in the swollen hollow cavity of the particles (Figure 4A–C). In addition to increasing efficient payload loading capacity, these hyper-loaded YP–terpene samples provide for enhanced sustained terpene release, up to three-fold compared to the previously developed YP–terpene 1.1:1 formulation (Figure 4D) and show higher antimicrobial potency than unencapsulated terpenes. The original work also described methods to improve thermal terpene storage encapsulation stability by producing hyper-loaded YP–terpene samples in a solvent mixture of 70% water–30% glycerin at 100 g YP/L and water-suspended terpene concentrations from 300 to 450 g/L.
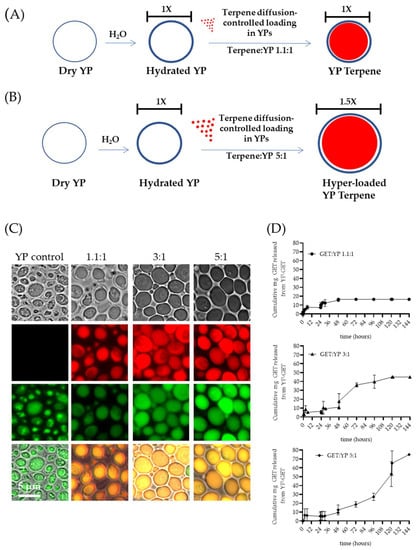
Figure 4.
Schematics of diffusion-controlled terpene loading in YPs at terpene:YP ratios of (A) 1.1:1 and (B) 5:1. (C) Microscopy images showing FITC–concanavalin A stained YPs and Nile red stained terpenes in empty YP control and YP GETs loaded at GET:YP ratios of 1.1:1, 3:1, and 5.1. (D) Cumulative GET release from YPs showing extension of wetting/terpene release cycles in YP GET 1.1:1 and hyper-loaded YP GET at ratios of 3:1 and 5:1 GET:YP. Figures adapted from Reference [40] and reproduced with permission from MDPI.
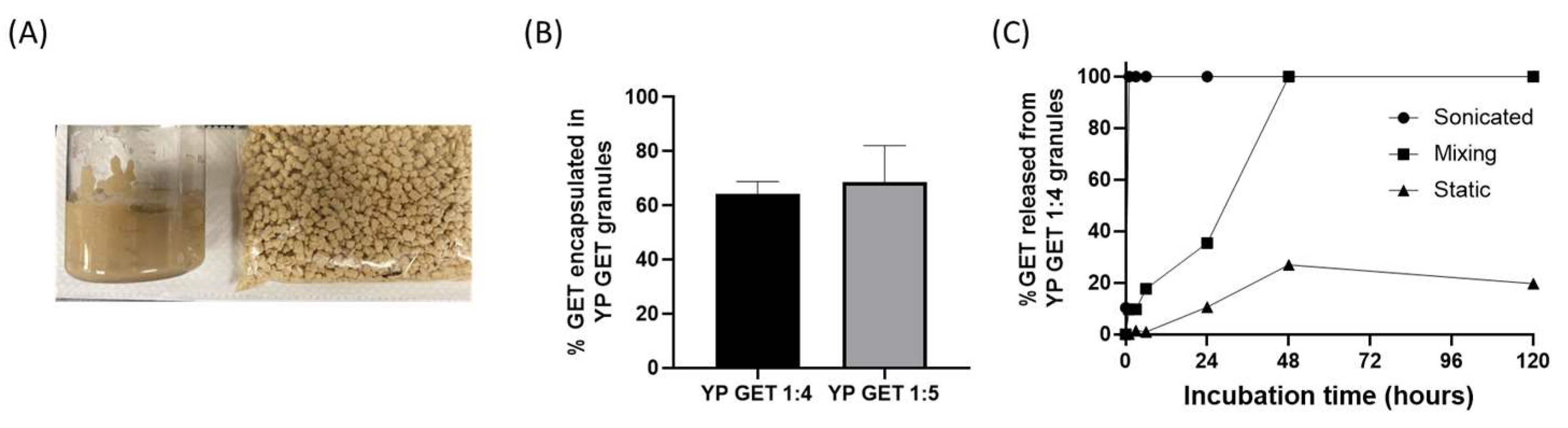
The hyper-loaded YP–terpenes previously reported in the literature were prepared as homogenized liquid suspensions. It is also possible to extrude the hyper-loaded YP–terpene suspension to produce dry hyper-loaded YP–terpene granules (Figure 5A). Samples of YP GET suspensions hyper-loaded at GET:YP weight ratios of 4:1 and 5:1 were extruded to form YP GET granules. The dry granules were characterized for encapsulation efficiency and kinetics of terpene release. The results show that ~60–70% of GET was retained inside YPs during the extrusion and drying steps (Figure 5B). The kinetics of release from YP GET 1:5 granules was evaluated under three conditions: (1) granules that were suspended in water and sonicated, resulting in rapid disaggregation of granules into single YPs, (2) granules suspended in water and mixed with gentle agitation, and (3) granules suspended in water and not mixed (static). The results in Figure 4C show the effect of mixing on the release of GET from YP GET granules, samples that were sonicated showed burst GET release, YP GET granules that were mixed with mild agitation released payload in ~48 h, and samples that were not mixed only released up to 20% GET after 5 days of incubation in water at a target GET concentration of 1 mg/mL.
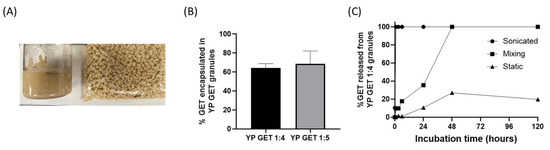
Figure 5.
(A) Picture of homogenized aqueous suspension of YP GET 1:4 and YP GET 1:4 granules, (B) encapsulation efficiency of GET 2:1:2 in YP GET granules, and (C) GET release from YP GET 1:4 granules suspended in water at 1 mg GET/mL at room temperature under three incubation conditions.
The development of hyper-loaded YP–terpenes has a wide range of potential agricultural and pharmaceutical applications that could benefit from a delivery system with a high payload loading capacity combined with increased payload stability and sustained release properties.
4.6. YP–Terpene with a pH-Sensitive Eudragit® Coating
Eudragit® is a brand name of polymethacrylate-based copolymers. These copolymers are composed of methacrylic acid and methacrylic/acrylic esters and are modified to include anionic, cationic and/or neutral charge. The polymers are responsive under different pH conditions and are extensively used in pharmaceutical coatings for taste-masking and targeted delivery [69,70].
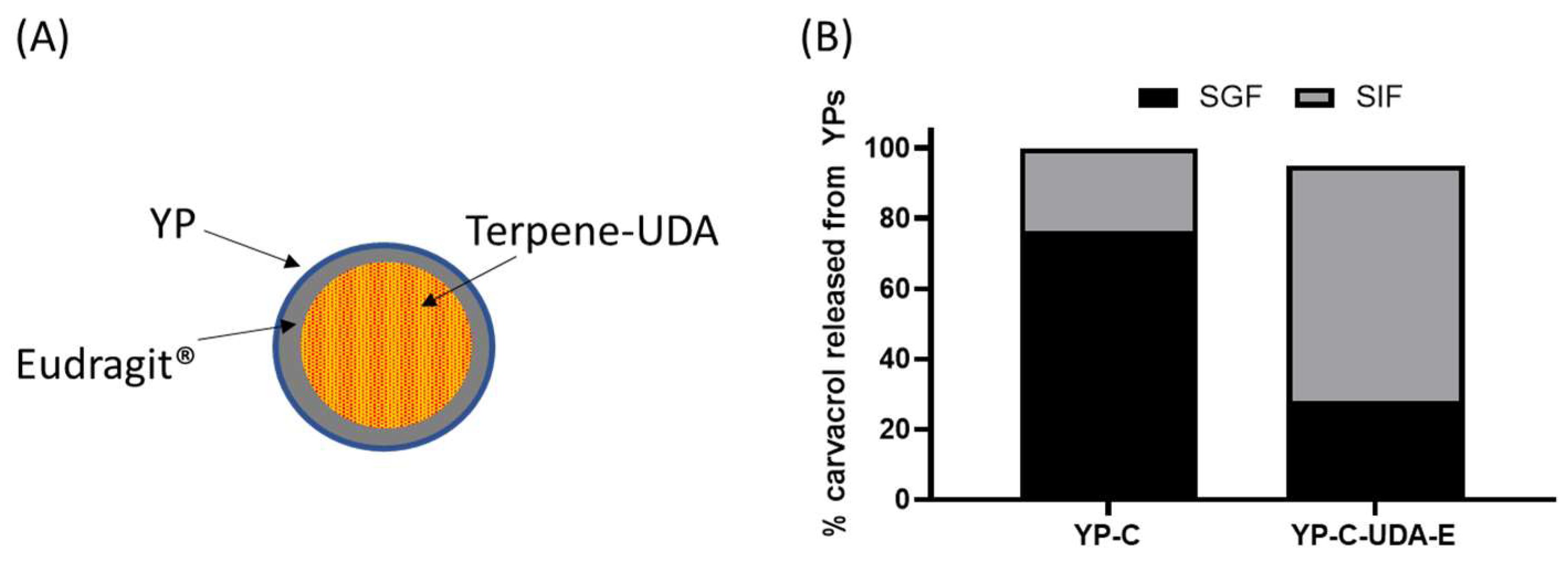
We evaluated the coating of YP carvacrol with Eudragit® L-100, a polymer that forms an insoluble coat below pH < ~5.5 and dissolves at pH > 5.5 and is used in pharmaceutical coating of pills for oral delivery of drugs to the intestine. It was not possible to efficiently coat YP carvacrol or other terpenes with Eudragit® L-100 or other Eudragit® polymers, as the terpene dissolves the coat regardless of pH. This limitation was overcome by coating a mixture of carvacrol with undecanoic acid (UDA), as the fatty acid acts as a sequestering agent of carvacrol, preventing the terpene from dissolving the coat. Samples of YP carvacrol–UDA–Eudragit® L-100 and YP carvacrol control were incubated in simulated digestion conditions: first, the samples were incubated in simulated gastric fluid (SGF, pH 1.5) with pepsin for 2 hours at 37 °C; the samples were centrifuged, and the SGF was collected for HPLC analysis of released carvacrol; the YP pellets were suspended in simulated intestinal fluid (SIF, pH 6.8) with pancreatin at 37 °C; and the SIF was also analyzed for released carvacrol after 2 h incubation. The results in Figure 6B show that coated samples were effective at reducing carvacrol release in SGF, and they released the payload in SIF after dissolution of the Eudragit® coat. These results are encouraging, as this approach offers the possibility of selectively targeting terpenes to the intestine. However, this approach has the limitation that the use of fatty acid and Eudragit® L-100 reduces the loading capacity of terpenes in YPs.
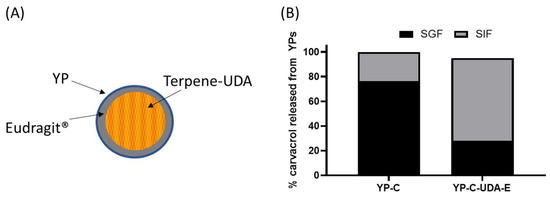
Figure 6.
(A) Schematic of a YP–terpene–UDA particle coated with Eudragit® polymer and (B) carvacrol released from YPs in YP carvacrol (YP-C) control sample and YP carvacrol co-loaded with UDA and coated with Eudragit® L-100 (YP-C-UDA-E). The release assay was performed by sequential incubation of YP samples in simulated gastric fluid (SGF) at 37 °C for 2 h followed by incubation in simulated intestinal fluid (SIF) at 37 °C for 2 h. Carvacrol concentration during simulated digestion was 1 mg/mL.
4.7. YP Pro-Terpene with Stimuli-Controlled Terpene Release
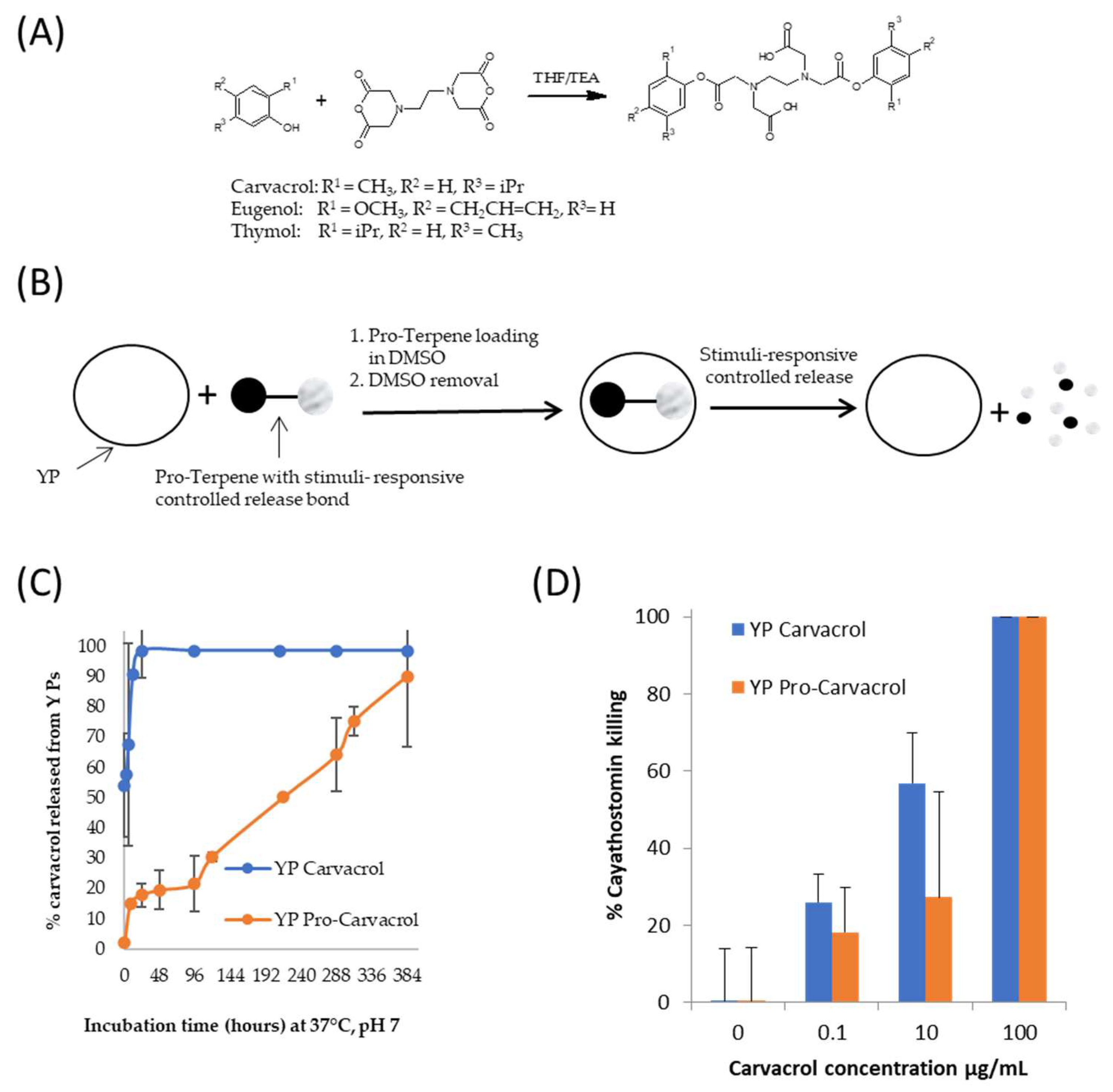
A method to use YP for delivery of terpenes with stimuli-responsive control of terpene release was recently developed [37,39]. Biodegradable pro-terpene compounds were synthesized (Figure 7A) and encapsulated in YPs (Figure 7B) at a target weight ratio of 1:1 terpene:YP (~1.7–1.9 pro-terpene:YP weight ratio). In one pro-carvacrol-YP example formulation (Figure 7C), the kinetics of carvacrol release at pH 7, 37 °C, in suspensions at 1 mg carvacrol/mL show that it takes up to 16 days for YP pro-carvacrol to completely release its carvacrol content. The YP carvacrol control completely released carvacrol in < 1 d. The kinetics of terpene release from YP pro-carvacrol increased at higher pH (complete release in < 2 h at pH 10) or at acidic or neutral pH in the presence of esterases that hydrolyze the scaffolded terpene compound. In addition to the stimuli-responsive release of terpenes, the YP pro-terpenes show higher encapsulation stability than YP–terpenes due to pro-terpenes being non-volatile solids at room temperature.
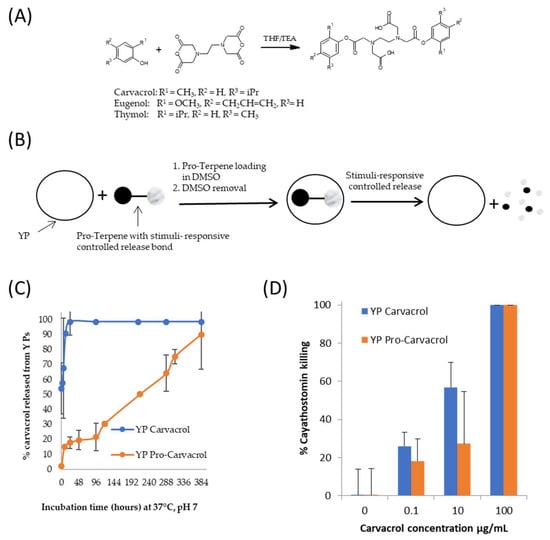
Figure 7.
(A) Synthesis of pro-terpenes from parent terpene compounds (eugenol, carvacrol, thymol) and EDTA dianhydride, (B) schematics of pro-terpene loading in YPs and stimuli-controlled terpene release, (C) kinetics of carvacrol release from YP carvacrol and YP pro-carvacrol in 0.1 M phosphate buffer saline (PBS, pH 7) at 37 °C at a concentration of 1 mg carvacrol/mL, and (D) in vitro activity of carvacrol samples in cyathostomin egg-to-larvae assay. Figures adapted from Reference [39] and reproduced with permission from MDPI.
The YP pro-terpenes retained the full biological activity of the parent terpene compound in antibacterial, antifungal, and anthelmintic assays. The results in Figure 7D are an example of retention of biological in vitro activity showing that YP pro-carvacrol had similar efficacy as YP carvacrol in a cyathostomin egg-to-larvae assay. These YP pro-terpene samples have potential for development in pharmaceutical applications due to their improved stability over YP–terpenes and stimuli-responsive controlled release.
Yeast particles can be used for the encapsulation of terpenes and essential oils with high payload capacity and efficiency. The use of YPs allows for encapsulation levels up to 5:1 terpene:YP weight ratio; this high encapsulation capacity is achieved without the use of alcohols and potentially toxic surfactants typically required to stabilize terpenes in different formulations. The key attributes and limitations of the various terpene encapsulation approaches are summarized in Table 1.
5. Summary and Future Perspectives
The broad applications for terpenes range from antioxidants, flavors and odors in foods and cosmetics, to biocides, insecticides, and pesticides in agricultural and consumer products, to bioactive compounds or permeation enhancing excipients in nutraceuticals and pharmaceuticals. These broad applications of terpenes as natural compounds in food, agricultural, consumer, and pharmaceutical products will continue to grow if the limitations of terpenes are addressed, by encapsulation of terpenes or essential oil compositions in appropriate delivery systems. Encapsulation improves the stability of terpene by reducing loss of terpene content due to volatility or by chemical degradation. It also masks undesirable flavors associated with some terpenes utilized as additives to prevent food spoilage, increases shelf-life storage, and depending on the encapsulation material, it can provide for sustained terpene release required in some applications such as agricultural and pharmaceutical products [6,7,8,9,10].
A number of different formulation strategies have been employed to overcome the limitations of terpenes. Most approaches only address some of these limitations of terpene’s poor water solubility, volatility, organoleptic properties and stability to air, heat, light and moisture, as summarized in Table 1.
We have developed the use of yeast particles for the encapsulation and delivery of terpenes and essential oils in the hollow cavity of YPs based on the passive diffusion of payloads through the porous yeast cell walls. YP–terpene compositions can be prepared with high loading capacity, up to 5:1 terpene:YP ratio (which is significantly higher than other available encapsulation methods for terpenes), high encapsulation efficiency, enhanced terpene stability, and sustained release properties. The first-generation approach to prepare YP–terpenes was successfully implemented to develop and commercialize YP–terpene-based fungicide and nematicide products for agricultural applications. Further advancement of YP-encapsulated terpenes has focused on developing materials with improved controlled of terpene release by methods that reduce the rate of diffusion-based release of terpene from YPs or the use of YP–terpene compositions with stimuli-response (pH, enzyme) release. The development of these different approaches for encapsulation of YP–terpenes has the potential of enabling the use of terpenes for a wide range of agricultural, food and pharmaceutical applications.
Author Contributions
Methodology, E.R.S., F.R. and Z.M.; resources, G.R.O.; writing—original draft preparation, E.R.S.; writing—review and editing, G.R.O.; funding acquisition, G.R.O. All authors have read and agreed to the published version of the manuscript.
Funding
Portions of this work were funded by a Sponsored Research Agreement from Eden Research plc and funding from UMass Chan Medical School.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
G.R.O. has licensed the YP–terpene 1.1:1 technology to Eden Research plc. G.R.O., E.R.S. and F.R. are co-inventors of U.S. Patent App. 63/346,012, Hyperloaded Yeast Cell Wall Particle and Uses Thereof; G.R.O. and E.R.S. are inventors of U.S. Patent App. 16/981,072 Yeast Cell Wall Particle Encapsulation of Biodegradable Pro-Payloads. These patents are assigned to UMass Chan Medical School and managed by the Office of Technology Management and the Conflicts of Interest policies of the University of Massachusetts. The other authors declare no conflict of interest.
References
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Salha, G.B.; Abderrabba, M.; Labidi, J. A Status Review of Terpenes and Their Separation Methods. Rev. Chem. Eng. 2019, 37, 433–447. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; Loffredo, M.R.; Del Plato, C.; Cammarone, S.; Casciaro, B.; Quaglio, D.; Mangoni, M.L.; Botta, B.; Ghirga, F. The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics 2020, 9, 325. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.; Ferdowsi, R.; Rahman, M.; Van Vuong, Q. Micro and Nano Encapsulation, Retention and Controlled Release of Flavor and Aroma Compounds: A Critical Review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Bhalerao, Y.P.; Wagh, S.J. A Review on Thymol Encapsulation and its Controlled Release through Biodegradable Polymer Shells. Int. J. Pharm. Sci. 2018, 2, 4522–4532. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; De Lima, A.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: A review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Plati, F.; Paraskevopoulou, A. Micro- and Nano-encapsulation as Tools for Essential Oils Advantages’ Exploitation in Food Applications: The Case of Oregano Essential Oil. Food Bioprocess Technol. 2022, 15, 949–977. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Felipe, L.D.O.; Bicas, J.L.; Moreira, R.C.; Alexandrino, T.D.; Changwatchai, T.; Nakajima, M.; Neves, M.A. Elaboration and Properties of an Oil-in-Water Nanoemulsion Loaded with a Terpene-Enriched Oil Mixture Obtained Biotechnologically. ACS Agric. Sci. Technol. 2021, 1, 631–639. [Google Scholar] [CrossRef]
- Huang, K.; Liu, R.; Zhang, Y.; Guan, X. Characteristics of two cedarwood essential oil emulsions and their antioxidant and antibacterial activities. Food Chem. 2021, 346, 128970. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, J.; Yu, F.; Wang, H.; Kou, X.; Ma, C.; Zhu, S. The antioxidant, antibacterial, antibiofilm activity of essential oil from Citrus medica L. var. sarcodactylis and its nanoemulsion. LWT 2017, 80, 371–377. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Jobdeedamrong, A.; Jenjob, R.; Crespy, D. Encapsulation and Release of Essential Oils in Functional Silica Nanocontainers. Langmuir 2018, 34, 13235–13243. [Google Scholar] [CrossRef]
- Palmas, L.; Aroffu, M.; Petretto, G.L.; Escribano-Ferrer, E.; Diez-Sales, O.; Usach, I.; Peris, J.E.; Marongiu, F.; Ghavam, M.; Fais, S.; et al. Entrapment of citrus limon var. Pompia essential oil or pure citral in liposomes tailored as mouthwash for the treatment of oral cavity diseases. Pharmaceuticals 2020, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Barbosa Souto, E.M.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.; Cortes, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nga, P.T.T.; Nguyen, H.P.N.; Nguyen, X.T.; Pham, T.P.M.; Le, P.T.K. A Mini-review on Essential oils, Chitosan, and Their Application in Preserving Fruits and Vegetables. Chem. Eng. Trans. 2022, 97, 109–114. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; de Mello, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef]
- Lima, P.S.S.; Lucchese, A.M.; Araujo-Filho, H.G.; Menezes, P.P.; Araujo, A.A.S.; Quintans-Junior, L.J.; Quintans, J.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization, and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X.; Li, Y.; Zhang, S. Microencapsulation of essential oils by complex coacervation method: Preparation, thermal stability, release properties and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Dos Santos Da Veiga, R.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavije, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Altay, O.; Koprualan, O.; Ilter, I.; Koc, M.; Ertekin, F.K.; Jafari, S.M. Spray drying encapsulation of essential oils; process efficiency, formulation strategies, and applications. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Soto, E.R.; Rus, F.; Ostroff, G.R. Yeast Cell Wall Particle Encapsulation of Pro-Terpene Payloads. 2019. Available online: https://briefs.techconnect.org/papers/yeast-cell-wall-particle-encapsulation-of-pro-terpene-payloads/ (accessed on 5 November 2021).
- Mirza, Z.; Soto, E.R.; Hu, Y.; Nguyen, T.T.; Koch, D.; Aroian, R.V.; Ostroff, G.R. Anthelmintic Activity of Yeast Particle-Encapsulated Terpenes. Molecules 2020, 25, 2958. [Google Scholar] [CrossRef]
- Soto, E.R.; Rus, F.; Li, H.; Garceau, C.; Chicca, J.; Elfawal, M.; Gazzola, D.; Nielsen, M.K.; Urban, J.F., Jr.; Aroian, R.V.; et al. Yeast Particle Encapsulation of Scaffolded Terpene Compounds for Controlled Terpene Release. Foods 2021, 10, 1207. [Google Scholar] [CrossRef]
- Soto, E.R.; Rus, F.; Ostroff, G.R. Yeast Particles Hyper-Loaded with Terpenes for Biocide Applications. Molecules 2022, 27, 3580. [Google Scholar] [CrossRef]
- Soto, E.R.; Ostroff, G.R. Characterization of Multilayered Nanoparticles Encapsulated in Yeast Cell Wall Particles for DNA delivery. Bioconjug. Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.R.; Ostroff, G.R.; Czech, M.P. Orally Delivered siRNA Targeting Macrophage Map4k4 Suppresses Systemic Inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.R.; Ostroff, G.R. Glucan Particles as Carriers of Nanoparticles for Macrophage-Targeted Delivery. In Nanomaterials for Biomecidine; American Chemical Society: Washington, DC, USA, 2012; pp. 57–79. [Google Scholar] [CrossRef]
- Mirza, Z.; Soto, E.R.; Dikengil, F.; Levitz, S.M.; Ostroff, G.R. Beta-Glucan Particles as Vaccine Adjuvant Carriers. In Vaccines for Invasive Fungal Infections; Humana Press: New York, NY, USA, 2017; pp. 143–157. [Google Scholar] [CrossRef]
- Soto, E.R.; O’Connell, O.; Dikengil, F.; Peters, P.J.; Clapham, P.R.; Ostroff, G.R. Targeted Delivery of Glucan Particle Encapsulated Gallium Nanoparticles Inhibits HIV Growth in Human Macrophages. J. Drug Deliv. 2016, 2016, 8520629. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Huang, M.; McClements, D.J.; Sun, B.; Wang, J. Yeast cell-derived delivery systems for bioactives. Trends Food Sci. Technol. 2021, 118, 362–373. [Google Scholar] [CrossRef]
- Kavetsou, E.; Koutsoukos, S.; Daferera, D.; Polissiou, M.G.; Karagiannis, D.; Perdikis, D.C.; Detsi, A. Encapsulation of Mentha pulegium Essential Oil in Yeast Cell Microcarriers: An Approach to Environmentally Friendly Pesticides. J. Agric. Food Chem. 2019, 67, 4746–4753. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Miyamoto, A.; Hy, Q.L.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Microencapsulation of flavors by spray drying using Saccharomyces cerevisiae. J. Food Eng. 2017, 199, 36–41. [Google Scholar] [CrossRef]
- Jilani, H.; Cilla, A.; Barbera, R.; Hamdi, M. Biosorption of green and black tea polyphenols into Saccharomyces cerevisiae improves their bioaccessibility. J. Funct. Foods 2015, 17, 11–21. [Google Scholar] [CrossRef]
- Dardelle, G.; Normand, V.; Steenhoudt, M.; Bouquerand, P.E.; Chevalier, M.; Baumgartner, P. Flavour-encapsulation and flavour-release performances of a commercial yeast-based delivery system. Food Hydrocoll. 2007, 21, 953–960. [Google Scholar] [CrossRef]
- Franklin, L.; Ostroff, G.; Harman, G. Terpene-Containing Compositions and Methods of Making and Using Them. U.S. Patent 10,667,512, 2 June 2020. [Google Scholar]
- Franklin, L.; Ostroff, G. Compositions and Methods Comprising Terpenes or Terpene Mixtures Selected from Thymol, Eugenol, Geraniol, Citral, and L-Carvone. U.S. Patent 9,439,416, 13 September 2016. [Google Scholar]
- Franklin, L.; Ostroff, G. Nematicidal Compositions and Methods of Using Them. U.S. Patent 9,655,360, 23 May 2017. [Google Scholar]
- Franklin, L.; Ostroff, G. Nematicidal Compositions and Methods of Using Them. U.S. Patent 10,004,229, 26 June 2018. [Google Scholar]
- Franklin, L.; Ostroff, G. Compositions and Methods Comprising Terpenes or Terpene Mixtures Selected from Thymol, Eugenol, Geraniol, Citral and L-Carvone. U.S. Patent 10,258,033, 26 April 2019. [Google Scholar]
- Franklin, L.; Ostroff, G. Compositions Containing a Hollow Glucan Particle or a Cell Wall Particle Encapsulating a Terpene Component, Methods of Making and Using Them. U.S. Patent 10,638,750, 5 May 2020. [Google Scholar]
- Ferrell, J.A. Hookworm disease: Its ravages, prevention and cure. J. Am. Med. Assoc. 1914, 52, 1937–1944. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J. Efficacy of Current Drugs Against Soil-Transmitted Helminth Infections. JAMA 2008, 299, 1937–1948. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J. The Drugs We Have and the Drugs We Need Against Major Helminth Infections. In Advances in Parasitology; Elsevier Ltd.: London, UK, 2010; Volume 73, pp. 197–230. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current status of alginate in drug delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Soto, E.R.; Kim, Y.S.; Lee, J.; Kornfeld, H.; Ostroff, G.R. Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers 2010, 2, 681–689. [Google Scholar] [CrossRef]
- Soto, E.R.; Caras, A.C.; Kut, L.; Castle, M.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524. [Google Scholar] [CrossRef] [PubMed]
- Morral-Ruíz, G.; Solans, C.; García, M.L.; García-Celma, M.J. Formation of pegylated polyurethane and lysine-coated polyurea nanoparticles obtained from O/W nano-emulsions. Langmuir 2012, 28, 6256–6264. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef]
- Bossion, A.; Jones, G.O.; Taton, D.; Mecerreyes, D.; Hedrick, J.L.; Ong, Z.Y.; Yang, Y.Y.; Sardon, H. Non-Isocyanate Polyurethane Soft Nanoparticles Obtained by Surfactant-Assisted Interfacial Polymerization. Langmuir 2017, 33, 1959–1968. [Google Scholar] [CrossRef]
- Patel, I.; Ajaykumar, T.; Srivastav, B. Eudragit a versatile Polymer: A Review. Int. J. Chem. Pharm. Sci. 2011, 1, 152–164. Available online: http://www.pharmaresearchlibrary.com/wp-content/uploads/2013/08/PRL2013-IJCPS-1783.pdf (accessed on 22 January 2023).
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).