Cytotoxicity and Autophagy Induced by Ivermectin via AMPK/mTOR Signaling Pathway in RAW264.7 Cells
Abstract
1. Introduction
2. Results
2.1. Colony Formation and LDH Activity
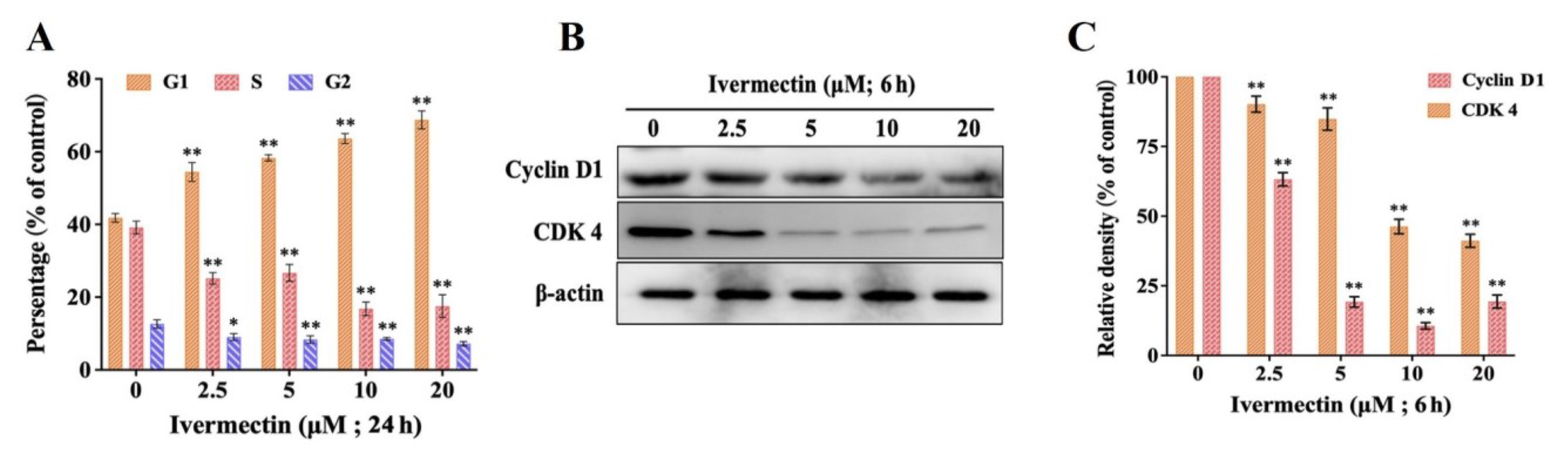
2.2. IVM Induced Cell Cycle Arrest
2.3. IVM Induced Autophagy
2.4. IVM Induced Mitochondrial Damage and Mitophagy
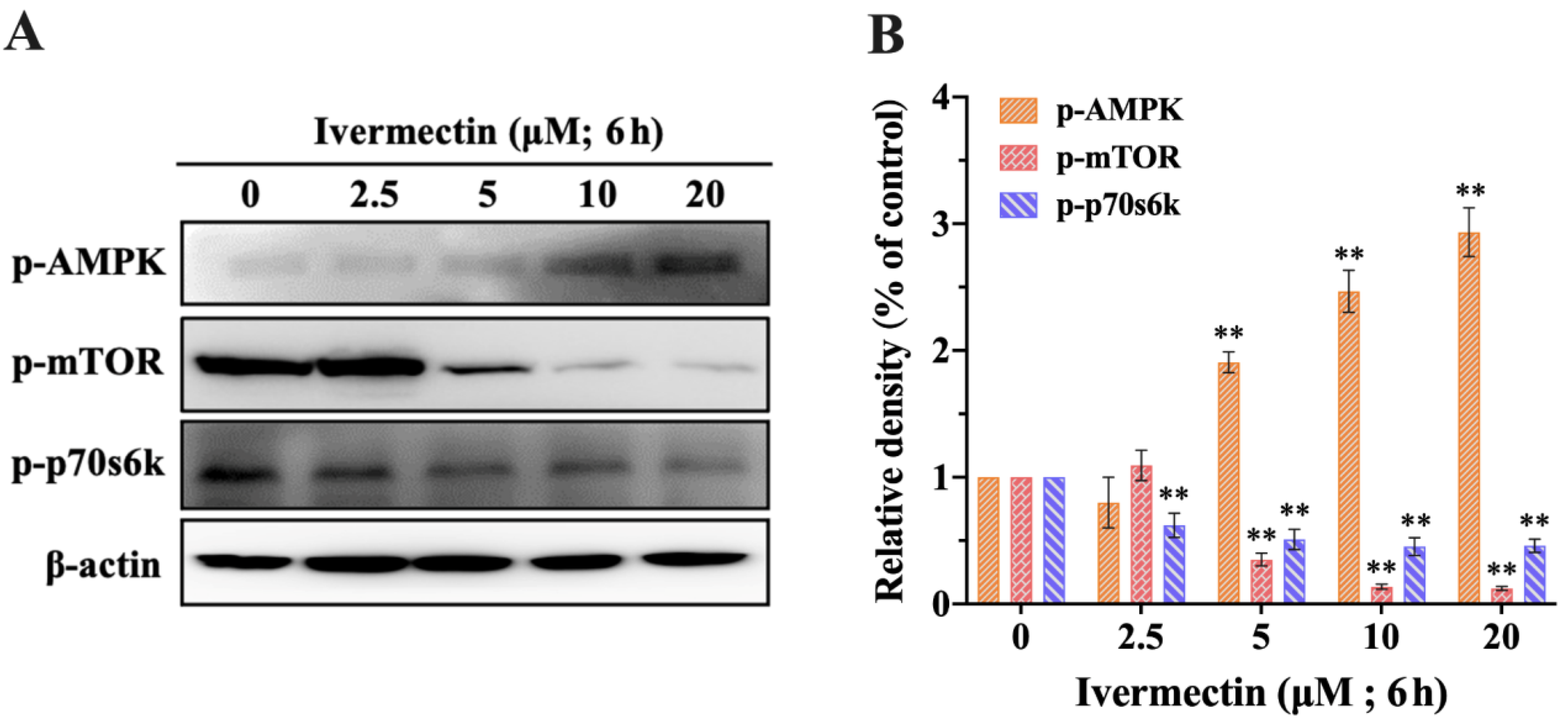
2.5. IVM Activated the AMPK/mTOR Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and IVM Treatments
4.2. Colony Formation Assay
4.3. Lactate Dehydrogenase (LDH) Assay
4.4. Cell Cycle Assay
4.5. Monodansylcadaverine (MDC) Staining
4.6. Colocalization of Mitochondria and Lysosomes
4.7. Mitochondrial Permeability Transition Pore (mPTP) Assay
4.8. Intracellular ATP Assay
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lee, J.Y.; Lim, W.; Ham, J.; Kim, J.; You, S.; Song, G. Ivermectin induces apoptosis of porcine trophectoderm and uterine luminal epithelial cells through loss of mitochondrial membrane potential, mitochondrial calcium ion overload, and reactive oxygen species generation. Pestic. Biochem. Physiol. 2019, 159, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Giordano, J.E.; Conte, S.; Levin, M.; Kaplan, D.L. Ivermectin Promotes Peripheral Nerve Regeneration during Wound Healing. ACS Omega 2018, 3, 12392–12402. [Google Scholar] [CrossRef] [PubMed]
- González Canga, A.; Sahagún Prieto, A.M.; José Diez Liébana, M.; Martínez, N.F.; Vega, M.S.; Vieitez, J.J. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 2009, 179, 25–37. [Google Scholar] [CrossRef]
- Lifschitz, A.; Ballent, M.; Virkel, G.; Sallovitz, J.; Lanusse, C. Sex-related differences in the gastrointestinal disposition of ivermectin in the rat: P-glycoprotein involvement and itraconazole modulation. J. Pharm. Pharmacol. 2006, 58, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, X.; Wu, H.; Li, C.; Zhong, P.; Liu, Z.; Ma, C.; Liu, W.; Wang, C.; Zhang, Y.; et al. Ivermectin contributes to attenuating the severity of acute lung injury in mice. Biomed. Pharmacother. 2022, 155, 113706. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Marsico, E.T.; Conte-Junior, C.A.; Furtado, L.d.A.; Brasil, T.F.; Pereira Netto, A.D. Macrocyclic lactone residues in butter from Brazilian markets. J. Dairy Sci. 2015, 98, 3695–3700. [Google Scholar] [CrossRef]
- Mahdjoub, H.; Blanckenhorn, W.U.; Luepold, S.; Roy, J.; Gourgoulianni, N.; Khelifa, R. Fitness consequences of the combined effects of veterinary and agricultural pesticides on a non-target insect. Chemosphere 2020, 250, 126271. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, M.; Stuchlikova, L.R.; Skalova, L.; Szotakova, B.; Langhansova, L.; Podlipna, R. Pharmaceuticals in environment: The effect of ivermectin on ribwort plantain (Plantago lanceolata L.). Environ. Sci. Pollut. Res. 2020, 27, 31202–31210. [Google Scholar] [CrossRef]
- Mesa, L.; Gutierrez, F.; Montalto, L.; Perez, V.; Lifschitz, A. Concentration and environmental fate of ivermectin in floodplain wetlands: An ecosystem approach. Sci. Total Environ. 2019, 706, 135692. [Google Scholar] [CrossRef]
- Foerster, B.; Boxall, A.; Coors, A.; Jensen, J.; Liebig, M.; Pope, L.; Moser, T.; Roembke, J. Fate and effects of ivermectin on soil invertebrates in terrestrial model ecosystems. Ecotoxicology 2011, 20, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, B.; Li, S.; Du, X.; Cao, Y.; Lu, T. Assessment of the fate and effect of ivermectin in a simulated aquaculture ecosystem. Aquac. Res. 2019, 51, 535–541. [Google Scholar] [CrossRef]
- Yu, L. A special review collection on autophagy. Cell Res. 2020, 30, 553. [Google Scholar] [CrossRef]
- Cavalli, G.; Cenci, S. Autophagy and Protein Secretion. J. Mol. Biol. 2020, 432, 2525–2545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.-W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Espert, L.; Biard-Piechaczyk, M.; Codogno, P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 2008, 90, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T. p62: A versatile multitasker takes on cancer. Trends Biochem. Sci. 2012, 37, 230–236. [Google Scholar] [CrossRef]
- Schmitz, K.J.; Ademi, C.; Bertram, S.; Schmid, K.W.; Baba, H.A. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J. Surg. Oncol. 2016, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, F.; Chen, X.; Wu, X.; Zhu, J. Ginsenoside Rg3 attenuates ovariectomy-induced osteoporosis viaAMPK/mTORsignaling pathway. Drug Dev. Res. 2020, 81, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Tao, W.; Zhang, F.; Shen, W.; Tan, J.; Li, L.; Meng, Q.; Chen, Y.; Yang, Y.; Cheng, H. Trifolirhizin induces autophagy-dependent apoptosis in colon cancer via AMPK/mTOR signaling. Signal Transduct. Target. Ther. 2020, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Xue, W.; Han, B.; Yang, F.; Yin, Y.; Hu, C. Acetaminophen aggravates fat accumulation in NAFLD by inhibiting autophagy via the AMPK/mTOR pathway. Eur. J. Pharmacol. 2019, 850, 15–22. [Google Scholar] [CrossRef]
- Antonangeli, F.; Grimsholm, O.; Rossi, M.N.; Velotti, F. Editorial: Cellular Stress and Inflammation: How the Immune System Drives Tissue Homeostasis. Front. Immunol. 2021, 12, 668876. [Google Scholar] [CrossRef] [PubMed]
- Huntington, N.D.; Gray, D.H.D. Immune homeostasis in health and disease. Immunol. Cell Biol. 2018, 96, 451–452. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Xu, W.; Cheng, J.; Zhang, C.; Gao, J.; Li, Z.; Tao, L.; Zhang, Y. Immunotoxicity induced by Ivermectin is associated with NF-kappa B signaling pathway on macrophages. Chemosphere 2022, 289, 133087. [Google Scholar] [CrossRef]
- Steuerman, Y.; Gat-Viks, I. Exploiting Gene-Expression Deconvolution to Probe the Genetics of the Immune System. PLoS Comput. Biol. 2016, 12, e1004856. [Google Scholar] [CrossRef]
- Escribano, M.; San Andrés, M.I.; de Lucas, J.J.; González-Canga, A. Ivermectin residue depletion in food producing species and its presence in animal foodstuffs with a view to human safety. Curr. Pharm. Biotechnol. 2012, 13, 987–998. [Google Scholar] [CrossRef]
- Davis, W.C.; Hamilton, M.J. Comparison of the unique characteristics of the immune system in different species of mammals. Vet. Immunol. Immunopathol. 1998, 63, 7–13. [Google Scholar] [CrossRef]
- Kaja, S.; Payne, A.J.; Naumchuk, Y.; Koulen, P. Quantification of Lactate Dehydrogenase for Cell Viability Testing Using Cell Lines and Primary Cultured Astrocytes. Curr. Protoc. Toxicol. 2017, 72, 2–26. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Tang, N.; Zhang, C.; Cheng, J.; Zhang, Z.; Wang, S.; Wu, C.; Zhang, L.; Tao, L.; Li, Z.; et al. Avermectin induced DNA damage to the apoptosis and autophagy in human lung epithelial A549 cells. Ecotoxicol. Environ. Saf. 2021, 215, 112129. [Google Scholar] [CrossRef]
- Qu, J.; Li, M.; Zhao, F.; Liu, C.; Zhang, Z.; Xu, S.; Li, S. Autophagy is upregulated in brain tissues of pigeons exposed to avermectin. Ecotoxicol. Environ. Saf. 2015, 113, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Liu, C.; Khoso, P.A.; Zheng, W.; Li, M.; Li, S. Autophagy response in the liver of pigeon exposed to avermectin. Environ. Sci. Pollut. Res. Int. 2017, 24, 12767–12777. [Google Scholar] [CrossRef]
- Dou, Q.; Chen, H.N.; Wang, K.; Yuan, K.; Lei, Y.; Li, K.; Lan, J.; Chen, Y.; Huang, Z.; Xie, N.; et al. Ivermectin Induces Cytostatic Autophagy by Blocking the PAK1/Akt Axis in Breast Cancer. Cancer Res. 2016, 76, 4457–4469. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Chen, C.; Wang, X.; Qu, F.; Wang, H.; Yang, K.; Wang, Q.; Zhao, N.; Meng, J.; et al. Ivermectin induces autophagy-mediated cell death through the AKT/mTOR signaling pathway in glioma cells. Biosci. Rep. 2019, 39, BSR20192489. [Google Scholar] [CrossRef]
- Wang, K.; Gao, W.; Dou, Q.; Chen, H.; Li, Q.; Nice, E.C.; Huang, C. Ivermectin induces PAK1-mediated cytostatic autophagy in breast cancer. Autophagy 2016, 12, 2498–2499. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Li, M.; Lin, Y.; Liu, Y.; Tang, S.; Dai, C. Ivermectin-Induced Apoptotic Cell Death in Human SH-SY5Y Cells Involves the Activation of Oxidative Stress and Mitochondrial Pathway and Akt/mTOR-Pathway-Mediated Autophagy. Antioxidants 2022, 11, 908. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Pedro, J.M.B.-S.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Isabel Colombo, M.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S. The autophagy process. Oncotarget 2017, 8, 18623. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Gomez-Suaga, P.; Paillusson, S.; Miller, C.C.J. ER-mitochondria signaling regulates autophagy. Autophagy 2017, 13, 1250–1251. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, B.; Li, P.; Wen, X.; Yang, J. Maslinic Acid Inhibits Colon Tumorigenesis by the AMPK-mTOR Signaling Pathway. J. Agric. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef]
- Lai, S.-L.; Mustafa, M.R.; Wong, P.-F. Panduratin A induces protective autophagy in melanoma via the AMPK and mTOR pathway. Phytomedicine 2018, 42, 144–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Xu, W.; Yang, M.; Wang, B.; Gao, J.; Li, Y.; Tao, L. Avermectin Confers Its Cytotoxic Effects by Inducing DNA Damage and Mitochondria-Associated Apoptosis. J. Agric. Food Chem. 2016, 64, 6895–6902. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Liu, K.; Liu, B.; Xu, W.; Gao, J.; Ding, L.; Tao, L. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019, 52, e12543. [Google Scholar] [CrossRef]
- Hao, Y.; Xu, W.; Gao, J.; Zhang, Y.; Yang, Y.; Tao, L. Roundup-Induced AMPK/mTOR-Mediated Autophagy in Human A549 Cells. J. Agric. Food Chem. 2019, 67, 11364–11372. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, J.; Zhang, P.; Zhang, C.; Wang, W.; Wu, M.; Xu, W.; Tao, L.; Li, Z.; Zhang, Y. Cytotoxicity and Autophagy Induced by Ivermectin via AMPK/mTOR Signaling Pathway in RAW264.7 Cells. Molecules 2023, 28, 2201. https://doi.org/10.3390/molecules28052201
Wang X, Wang J, Zhang P, Zhang C, Wang W, Wu M, Xu W, Tao L, Li Z, Zhang Y. Cytotoxicity and Autophagy Induced by Ivermectin via AMPK/mTOR Signaling Pathway in RAW264.7 Cells. Molecules. 2023; 28(5):2201. https://doi.org/10.3390/molecules28052201
Chicago/Turabian StyleWang, Xiang, Jian Wang, Ping Zhang, Cheng Zhang, Weiguo Wang, Mengqi Wu, Wenping Xu, Liming Tao, Zhong Li, and Yang Zhang. 2023. "Cytotoxicity and Autophagy Induced by Ivermectin via AMPK/mTOR Signaling Pathway in RAW264.7 Cells" Molecules 28, no. 5: 2201. https://doi.org/10.3390/molecules28052201
APA StyleWang, X., Wang, J., Zhang, P., Zhang, C., Wang, W., Wu, M., Xu, W., Tao, L., Li, Z., & Zhang, Y. (2023). Cytotoxicity and Autophagy Induced by Ivermectin via AMPK/mTOR Signaling Pathway in RAW264.7 Cells. Molecules, 28(5), 2201. https://doi.org/10.3390/molecules28052201






