The Study of Optimal Adsorption Conditions of Phosphate on Fe-Modified Biochar by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phosphorus Removal Efficiency of Fe-B
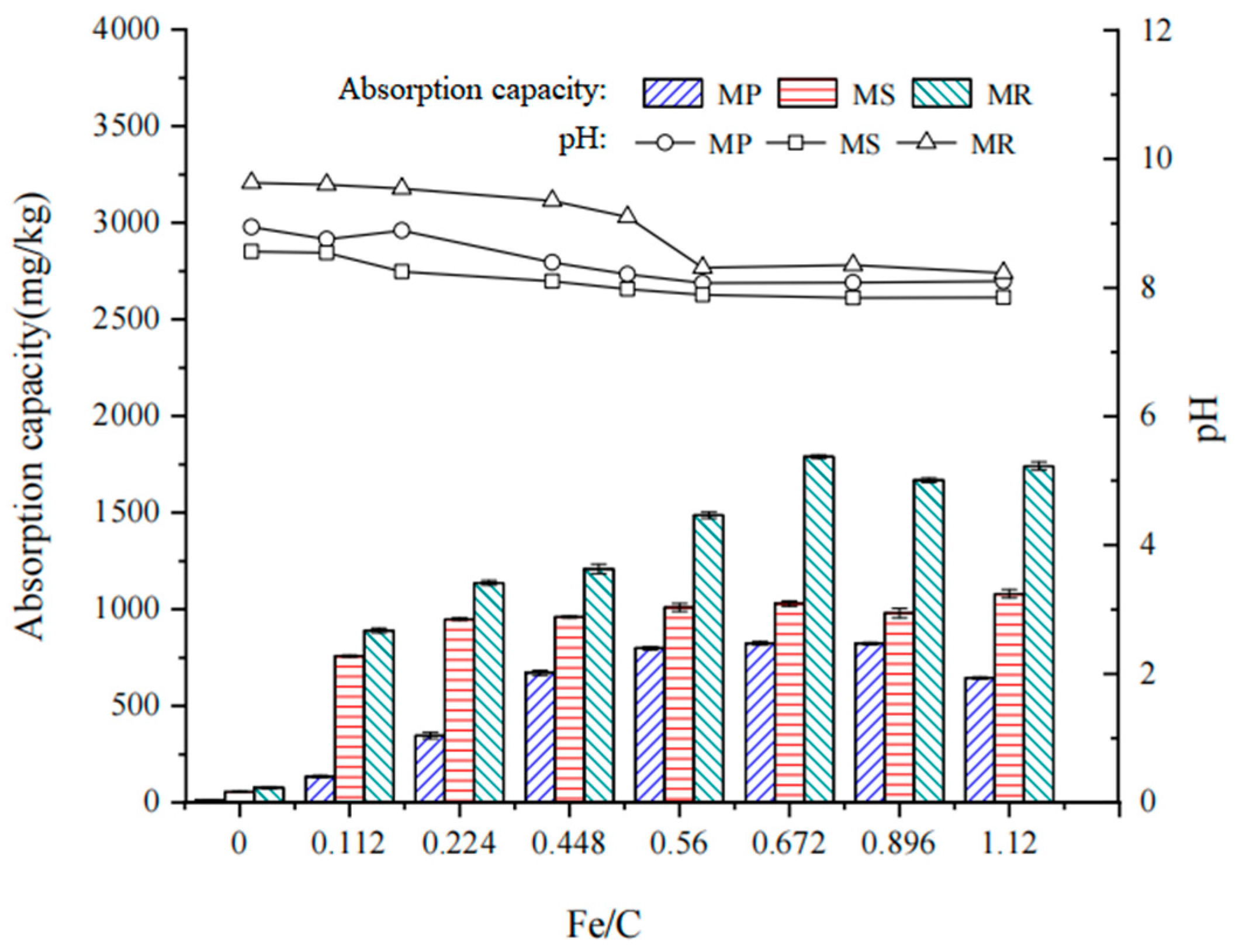
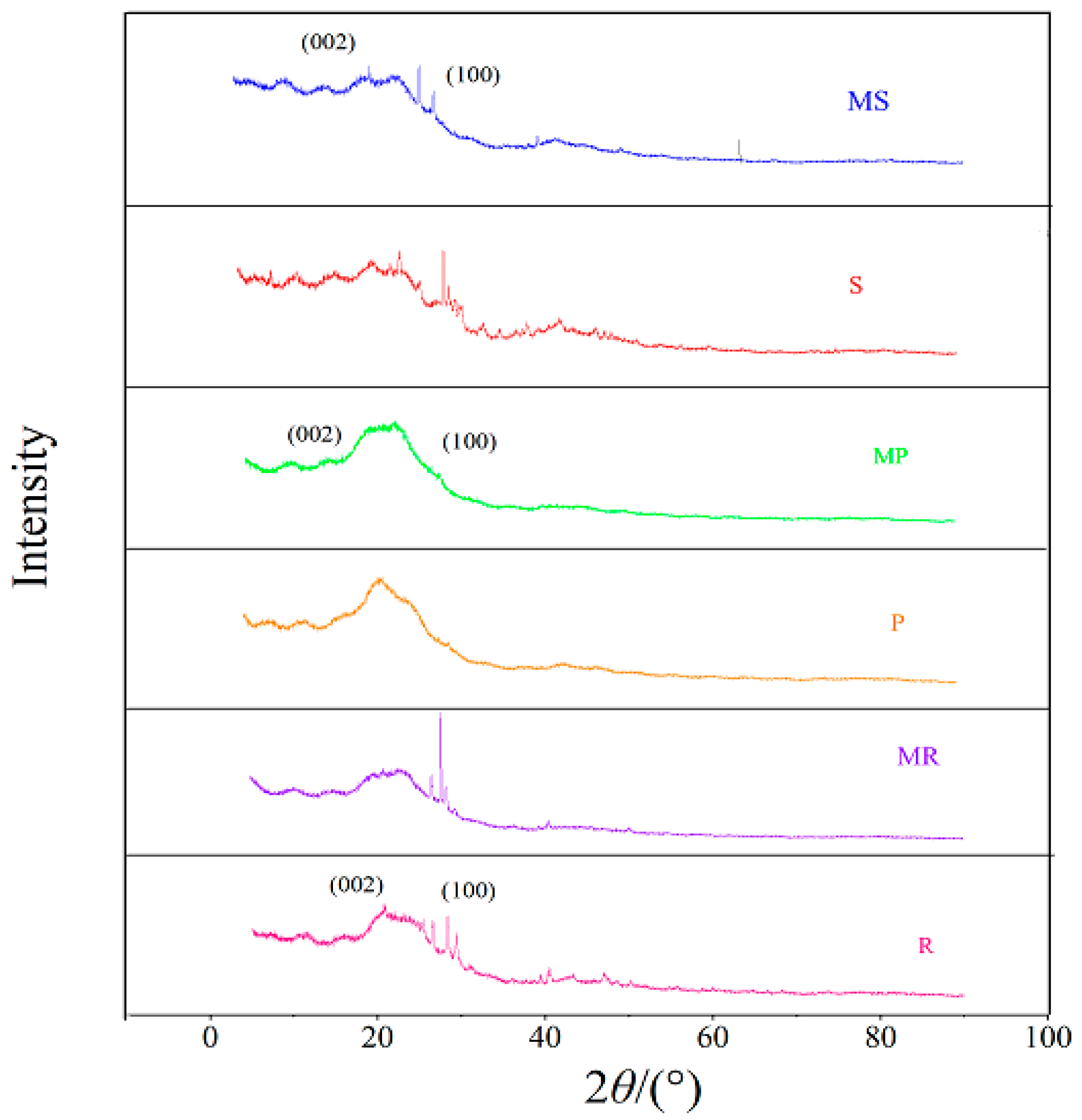
2.2. Properties of Fe-B Composites
2.3. Optimization of P Removal Efficiency
2.4. Influence of Environmental Factors on Phosphorus Removal Efficiency
2.4.1. Effect of pH on Phosphorus Removal Efficiency
2.4.2. Effect of Initial Phosphate Concentration on Phosphorus Removal Efficiency
2.4.3. Effect of Ambient Temperature on P Removal efficiency
2.5. Optimization of Fe-B via RSM
2.6. Adsorption Isotherm
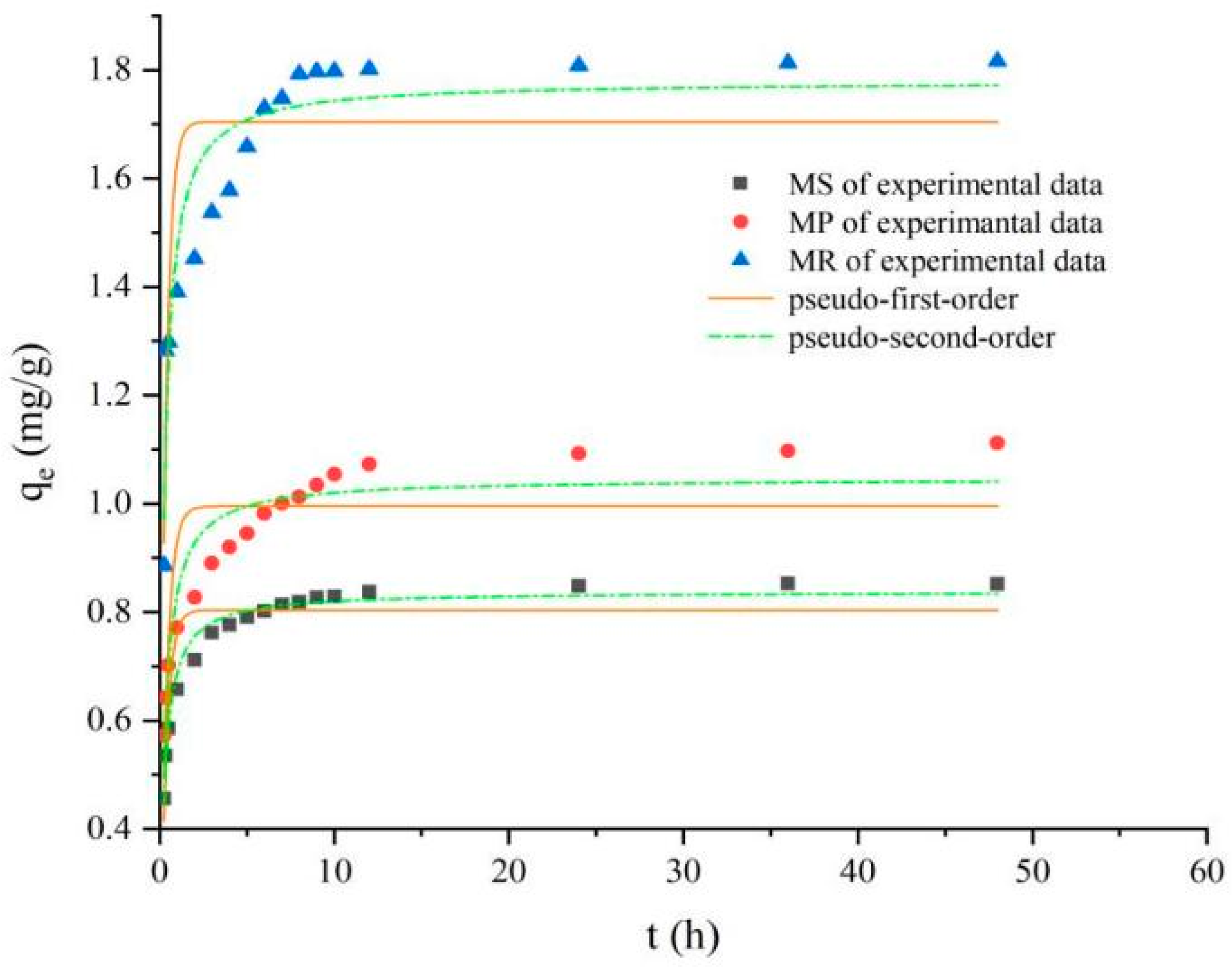
2.7. Phosphorus Adsorption Kinetics
2.8. Mechanism for Phosphorus Adsorption
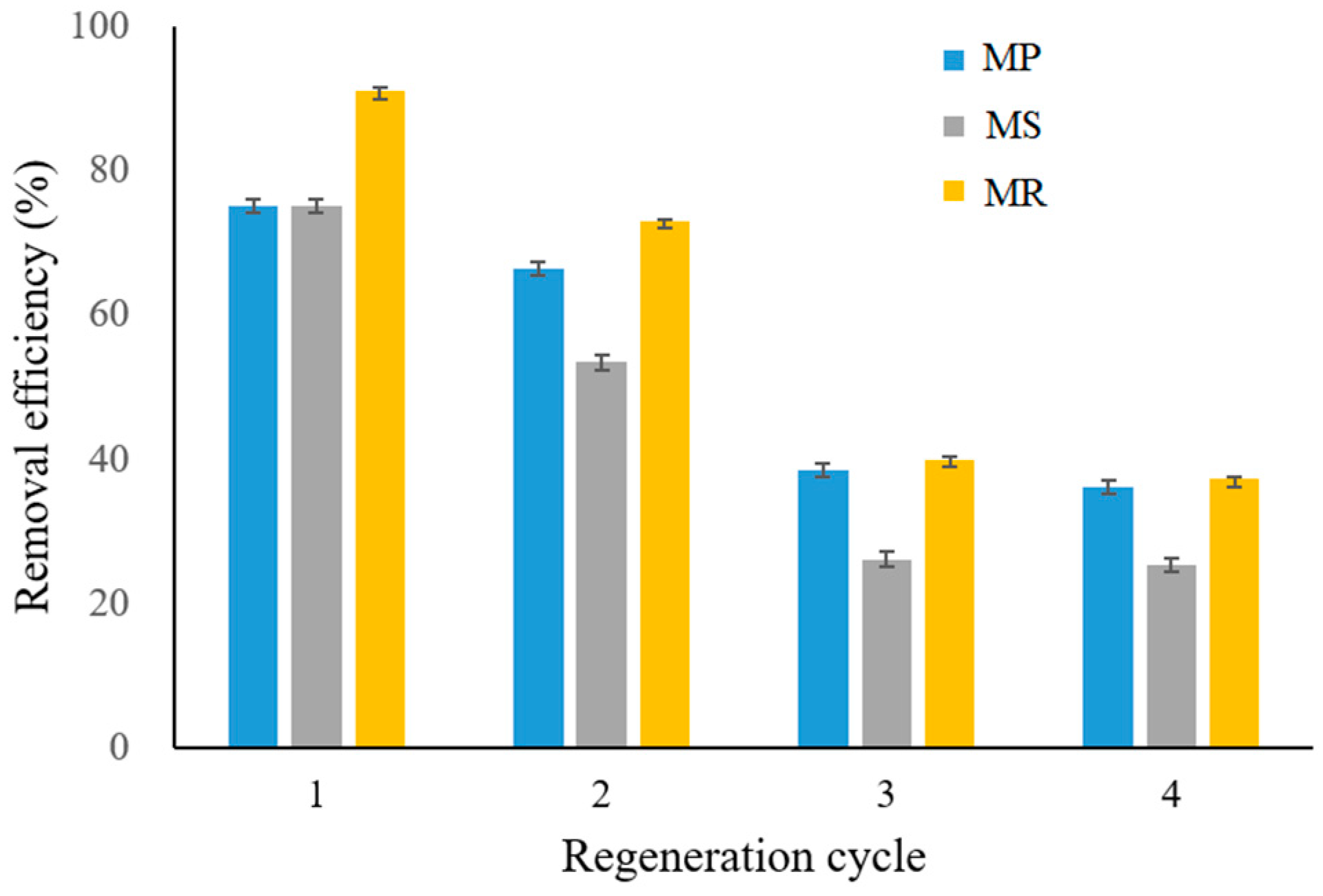
2.9. Desorption and Regeneration
3. Materials and Methods
3.1. Preparation and Characterization of the Biochars
3.1.1. Reagents and Chemicals
3.1.2. Instruments
3.2. Batch Adsorption Experiments
3.2.1. Phosphate Adsorption Experiments
3.2.2. Isotherm Study
3.2.3. Kinetic Study
3.3. Experimental Design Using RSM
3.4. Recyclability of Different Modified Biochars
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Lei, Y.; Zhan, Z.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J. Electrochemical recovery of phosphorus from acidic cheese wastewater: Feasibility, quality of products, and comparison with chemical precipitation. ACS EST Water 2021, 1, 1002–1013. [Google Scholar] [CrossRef]
- Wong, P.Y.; Ginige, M.P.; Kaksonen, A.H.; Cord-Ruwisch, R.; Sutton, D.C.; Cheng, K.Y. Simultaneous phosphorus uptake and denitrification by EBPR-r biofilm under aerobic conditions: Effect of dissolved oxygen. Water Sci. Technol. 2015, 72, 1147–1154. [Google Scholar] [CrossRef]
- Kiani, M.; Tammeorg, P.; Niemistö, J.; Simojoki, A.; Tammeorg, O. Internal phosphorus loading in a small shallow lake: Response after sediment removal. Sci. Total. Environ. 2020, 725, 138279. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, S.; Song, J.; Zhao, Y.; Yang, F. Activation of porous magnetized biochar by artificial humic acid for effective removal of lead ions. J. Hazard. Mater. 2020, 389, 122115. [Google Scholar] [CrossRef]
- Zhou, C.; Heal, K.; Tigabu, M.; Xia, L.; Hu, H.; Yin, D.; Ma, X. Biochar addition to forest plantation soil enhances phosphorus availability and soil bacterial community diversity. Forest Ecol. Manag. 2020, 455, 117635. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Tsang, D.C.; Li, G. Effects of external additives: Biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 2020, 248, 125927. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total. Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.Z.; Deng, R.; Xue, W.J.; Gong, X.M.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Chu, C.; Yang, J.; Huang, D.; Li, J.; Wang, A.; Alvarez, P.J.; Kim, J.H. Cooperative pollutant adsorption and persulfate-driven oxidation on hierarchically ordered porous carbon. Environ. Sci. Technol. 2019, 53, 10352–10360. [Google Scholar] [CrossRef]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Baghernejad, M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J. Therm. Anal. Calorim. 2019, 138, 331–342. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Abubakari, A.H.; Quainoo, A.K.; Amadu, Y. Review of biochar properties and remediation of metal pollution of water and soil. J. Health. Pollut. 2020, 10, 200902. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Q.; Liu, Y.; Zhu, J.; Deng, Y.; Fu, Q.; Hu, H. Co-pyrolysis biochar derived from rape straw and phosphate rock: Carbon retention, aromaticity, and Pb removal capacity. Energy Fuels 2019, 33, 413–419. [Google Scholar] [CrossRef]
- Widmer, T.L.; Costa, J.M. Impact of the United States Department of Agriculture, Agricultural Research Service on Plant Pathology: 2015–2020. Phytopathology 2021, 111, 1265–1276. [Google Scholar] [CrossRef]
- Xiong, J.; Hassan, M.; Wang, W.; Ding, W. Methane enhancement by the co-digestion of soybean straw and farm wastewater under different thermo-chemical pretreatments. Renew. Energy 2020, 145, 116–123. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D.A.; van Leeuwen, J.H. Sustainable pyrolytic production of zerovalent iron. ACS Sustain. Chem. Eng. 2016, 5, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, B.; Huang, H.M.; Zhao, N.; Zhang, M.G.; Cao, L. Investigation into lanthanum-coated biochar obtained from urban dewatered sewage sludge for enhanced phosphate adsorption. Sci. Total. Environ. 2020, 714, 136839. [Google Scholar] [CrossRef]
- Yin, Q.Q.; Liu, M.T.; Li, Y.H.; Li, H.P.; Wen, Z.C. Computation study of phosphate adsorption on Mg/Ca modified biochar structure in aqueous solution. Chemosphere 2021, 269, 129374. [Google Scholar] [CrossRef]
- Li, Z.W.; Liu, X.J.; Wang, Y. Modification of sludge-based biochar and its application to phosphorus adsorption from aqueous solution. J. Mater. Cycles Waste Manag. 2020, 22, 123–132. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Singh, R.K.; Chakraborty, J.P.; Sarkar, A. Optimizing the torrefaction of pigeon pea stalk (cajanus cajan) using response surface methodology (RSM) and characterization of solid, liquid and gaseous products. Renew. Energ. 2020, 155, 677–690. [Google Scholar] [CrossRef]
- Ramola, S.; Belwal, T.; Li, C.J.; Wang, Y.Y.; Lu, H.H.; Yang, S.M.; Zhou, C.H. Improved lead removal from aqueous solution using novel porous bentonite-and calcite-biochar composite. Sci. Total Environ. 2020, 709, 136171. [Google Scholar] [CrossRef]
- Iberahim, N.; Sethupathi, S.; Bashir, M.J. Optimization of palm oil mill sludge biochar preparation for sulfur dioxide removal. Environ. Sci. Pollut. Res. 2018, 25, 25702–25714. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Jiao, G.J.; Ma, J.; Li, Y.; Jin, D.; Ali, Z.; Zhou, J.; Sun, R. Recent advances and challenges on removal and recycling of phosphate from wastewater using biomass-derived adsorbents. Chemosphere 2021, 278, 130377. [Google Scholar] [CrossRef]
- Mohammadi, R.; Hezarjaribi, M.; Ramasamy, D.L.; Sillanpää, M.; Pihlajamäki, A. Application of a novel biochar adsorbent and membrane to the selective separation of phosphate from phosphate-rich wastewaters. Chem. Eng. J. 2021, 407, 126494. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Essayem, N.; Tuel, A.; Clacens, J.M.; Taarit, Y.B. Studies on MeAPSO-5: An investigation of physicochemical and acidic properties. Catal. Today 2008, 133, 56–62. [Google Scholar] [CrossRef]
- Strehlau, J.H.; Toner, B.M.; Arnold, W.A.; Penn, R.L. Accessible reactive surface area and abiotic redox reactivity of iron oxyhydroxides in acidic brines. Geochim. Cosmochim. Acta 2017, 197, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Tong, L.; Zhao, N.; Li, J.; Lv, Y. Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 2019, 219, 493–503. [Google Scholar] [CrossRef]
- Hua, L.L.; Huang, X.H.; Qian, J.; Wang, K. CHaracteristics pf iron modified biochar for nitrogen adsorption in water(In Chinese). China Water Wastewater 2022, 38, 61–68. [Google Scholar]
- Arivuselvan, N.; Radhiga, M.; Anantharaman, P. In vitro antioxidant and anticoagulant activities of sulphated polysaccharides from brown seaweed (Turbinaria ornata)(Turner). J. Inflamm. Asian J. Pharm. Biol. Res. 2011, 14, 16. [Google Scholar]
- Qian, L.; Zhang, W.; Yan, J.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr (VI) removal. Environ. Pollut. 2016, 223, 153–160. [Google Scholar] [CrossRef]
- Lim, S.F.; Zheng, Y.M.; Chen, J.P. Organic arsenic adsorption onto a magnetic sorbent. Langmuir 2009, 25, 4973–4978. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Ma, J. Novel synthesis of carbon spheres supported nanoscale zero-valent iron for removal of metronidazole. Appl. Surf. Sci. 2016, 390, 50–59. [Google Scholar] [CrossRef]
- Agwupuye, J.A.; Louis, H.; Enudi, O.C.; Unimuke, T.O.; Edim, M.M. Theoretical insight into electronic and molecular properties of halogenated (F, Cl, Br) and hetero-atom (N, O, S) doped cyclooctane. Mater. Chem. Phys. 2022, 275, 125239. [Google Scholar] [CrossRef]
- Segal, L.G.J.M.A.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Wu, S.; Uddin, M.A.; Sasaoka, E. Characteristics of the removal of mercury vapor in coal derived fuel gas over iron oxide sorbents. Fuel 2006, 85, 213–218. [Google Scholar] [CrossRef]
- Fan, R.; Chen, C.L.; Lin, J.Y.; Tzeng, J.H.; Huang, C.P.; Dong, C.; Huang, C.P. Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour. Technol. 2019, 272, 465–472. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.X.; Liu, D.Q.; Ren, Y.Z.; Li, D.S. Preferable phosphate removal by nano-La (III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, G.; Mo, W.; Zhang, C.; Huang, H.; Li, S.G.; Gao, M.; Liu, X.J.; Zhang, B.Q.; Zhu, H.P. Enhanced phosphate sequestration by Fe (iii) modified biochar derived from coconut shell. RSC Adv. 2019, 9, 10425–10436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.B.; Chen, F.; Tao, Z.L.; Huang, X.D. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Pan, B.; Xing, B. Norfloxacin sorption and its thermodynamics on surface-modified carbon nanotubes. Environ. Sci. Technol. 2010, 44, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.; Chen, Q. Optimizing the synthesis of Fe/Al (Hydr) oxides-Biochars to maximize phosphate removal via response surface model. J. Clean. Prod. 2019, 237, 117770. [Google Scholar] [CrossRef]
- Saadat, S.; Raei, E.; Talebbeydokhti, N. Enhanced removal of phosphate from aqueous solutions using a modified sludge derived biochar: Comparative study of various modifying cations and RSM based optimization of pyrolysis parameters. J. Environ. Manag. 2018, 225, 75–83. [Google Scholar] [CrossRef]
- Oubagaranadin, J.U.K.; Murthy, Z.V.P. Adsorption of divalent lead on a montmorilloniteIllite type of clay. Ind. Eng. Chem. Res. 2009, 48, 10627–10636. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Ren, Z.; Shao, L.; Zhang, G. Adsorption of phosphate from aqueous solution using an iron–zirconium binary oxide sorbent. Water Air Soil Pollut. 2012, 223, 4221–4231. [Google Scholar] [CrossRef]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Henriksen, S.W.; Andersen, M.N. Mechanism of orthophosphate (PO4-P) adsorption onto different biochars. Environ. Technol. Innov. 2020, 17, 100572. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q. Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J. Clean. Prod. 2016, 112, 3927–3934. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Wu, S.; Ajmal, Z.; Lv, T.; Dong, R. Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration. J. Environ. Manag. 2017, 201, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Sui, F.; Jiao, M.; Kang, Y.; Joseph, S.; Li, L.; Bian, R.J.; Munroe, P.; Mitchell, D.R.G.; Pan, G.X. Investigating the cadmium adsorption capacities of crop straw biochars produced using various feedstocks and pyrolysis temperatures. Environ. Sci. Pollut. Res. 2021, 28, 21516–21527. [Google Scholar] [CrossRef]



| Properties | S | P | R | MS | MP | MR |
|---|---|---|---|---|---|---|
| pH | 8.44 ± 0.03 | 8.83 ± 0.1 | 9.56 ± 0.3 | 7.89 ± 0.07 | 7.89 ± 0.2 | 8.64 ± 0.3 |
| C% | 51.19 ± 1.5 | 59.19 ± 1.2 | 77.17 ± 0.6 | 52.45 ± 1.2 | 58.77 ± 1.2 | 65.56 ± 2.0 |
| H% | 1.78 ± 1.0 | 1.44 ± 2.1 | 1.87 ± 1.5 | 1.59 ± 1.2 | 1.36 ± 1.4 | 1.51 ± 1.4 |
| O% | 44.66 ± 1.2 | 39.4 ± 1.4 | 31.92 ± 2.1 | 46.20 ± 1.5 | 30.07 ± 2.2 | 20.68 ± 1.7 |
| H/C | 0.08 | 0.07 | 0.03 | 0.03 | 0.02 | 0.02 |
| O/C | 0.85 | 0.67 | 0.49 | 0.90 | 0.51 | 0.27 |
| Specific surface areas (g/m2) | 3.02 | 7.44 | 3.05 | 14.19 | 16.14 | 9.43 |
| Fe | - | - | - | 0.10 | 0.14 | 0.27 |
| Sl.no. | X1 Initial Concentration (mg L−1) | X2 pH | X3 Ambient Temperature (°C) | YMS (%) Experimental | YMS’ (%) Fitted by Model | YMP (%) Experimental | YMP’ (%) Fitted by Model | YMR (%) Experimental | YMR’ (%) Fitted by Model |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 7 | 30 | 94.86 ± 0.05 | 94.64 | 94.58 ± 0.04 | 94.05 | 93.81 ± 0.06 | 93.02 |
| 2 | 100 | 3 | 20 | 95.48 ± 0.02 | 94.84 | 93.39 ± 0.05 | 92.83 | 94.15 ± 0.07 | 93.79 |
| 3 | 100 | 11 | 40 | 97.39 ± 0.04 | 96.76 | 96.47 ± 1.01 | 95.92 | 93.44 ± 0.08 | 92.79 |
| 4 | 50 | 11 | 30 | 87.72 ± 0.11 | 86.55 | 86.26 ± 0.04 | 85.90 | 85.16 ± 0.03 | 85.51 |
| 5 | 150 | 3 | 30 | 97.15 ± 0.07 | 97.12 | 96.40 ± 0.08 | 95.65 | 96.47 ± 0.13 | 95.33 |
| 6 | 100 | 7 | 30 | 94.86 ± 0.10 | 94.64 | 94.59 ± 1.02 | 94.05 | 92.19 ± 0.11 | 92.79 |
| 7 | 50 | 3 | 30 | 87.12 ± 0.05 | 87.09 | 85.54 ± 1.05 | 85.16 | 93.19 ± 0.06 | 92.79 |
| 8 | 100 | 11 | 20 | 94.29 ± 0.06 | 93.35 | 93.02 ± 0.07 | 92.29 | 83.17 ± 0.14 | 82.51 |
| 9 | 100 | 3 | 40 | 96.83 ± 1.12 | 96.64 | 95.70 ± 0.06 | 95.32 | 90.17 ± 0.06 | 91.30 |
| 10 | 50 | 7 | 40 | 90.28 ± 0.10 | 89.45 | 89.35 ± 0.05 | 88.95 | 83.18 ± 0.07 | 82.71 |
| 11 | 100 | 7 | 30 | 94.86 ± 0.05 | 94.64 | 94.58 ± 0.05 | 94.05 | 92.78 ± 0.08 | 92.79 |
| 12 | 100 | 7 | 30 | 95.78 ± 0.07 | 94.64 | 94.58 ± 0.07 | 94.05 | 93.17 ± 0.13 | 92.86 |
| 13 | 150 | 11 | 30 | 97.46 ± 0.04 | 96.29 | 95.69 ± 0.05 | 94.96 | 94.16 ± 0.06 | 94.82 |
| 14 | 50 | 7 | 20 | 84.58 ± 0.05 | 84.42 | 83.60 ± 0.01 | 83.43 | 90.16 ± 0.09 | 90.47 |
| 15 | 100 | 7 | 30 | 95.47 ± 1.02 | 94.64 | 94.58 ± 0.06 | 94.05 | 92.16 ± 0.07 | 92.63 |
| 16 | 150 | 7 | 20 | 96.94 ± 0.07 | 96.73 | 96.38 ± 1.02 | 95.66 | 86.36 ± 0.07 | 87.14 |
| 17 | 150 | 7 | 40 | 97.80 ± 0.06 | 96.91 | 97.20 ± 0.07 | 96.26 | 92.37 ± 0.08 | 92.79 |
| Isotherms | Parameters | MS | MP | MR |
|---|---|---|---|---|
| Langmuir | KL (L mg−1) | 0.03736 | 0.0429 | 0.11275 |
| Qm (mg kg−1) | 3807.99 | 4560.34 | 5110.81 | |
| R2 | 0.9919 | 0.95325 | 0.97762 | |
| Freundlich | KF | 248.83883 | 378.87182 | 853.17842 |
| 1/n | 0.61823 | 0.55680 | 0.46379 | |
| R2 | 0.99624 | 0.98104 | 0.98646 | |
| Temkin | A | 0.68666 | 2.70744 | 1.93992 |
| B | 630.22108 | 502.93638 | 925.75533 | |
| R2 | 0.93611 | 0.74858 | 0.94703 | |
| Kinetics parameters | Qe (mg kg−1) | 803.86 | 995.17 | 1704.66 |
| Pseudo-first-order | K1 (L mg−1) | 2.913 | 2.733 | 3.140 |
| R2 | 0.85 | 0.72 | 0.75 | |
| Pseudo-second-order | Qe (mg kg−1) | 837.95 | 1046.44 | 1779.2479 |
| K2 (L mg−1) | 0.005 | 0.004 | 0.003 | |
| R2 | 0.97 | 0.89 | 0.88 |
| Variable | Symbols | −1 | 0 | +1 |
|---|---|---|---|---|
| Initial concentration (mg L−1) | X1 | 50 | 100 | 150 |
| pH | X2 | 3 | 7 | 11 |
| Ambient temperature (°C) | X3 | 20 | 30 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.; Zhou, X.; Cai, Q.; Zhao, J.; Huang, X. The Study of Optimal Adsorption Conditions of Phosphate on Fe-Modified Biochar by Response Surface Methodology. Molecules 2023, 28, 2323. https://doi.org/10.3390/molecules28052323
Qian J, Zhou X, Cai Q, Zhao J, Huang X. The Study of Optimal Adsorption Conditions of Phosphate on Fe-Modified Biochar by Response Surface Methodology. Molecules. 2023; 28(5):2323. https://doi.org/10.3390/molecules28052323
Chicago/Turabian StyleQian, Jing, Xiaoyu Zhou, Qingsong Cai, Jinjin Zhao, and Xianhuai Huang. 2023. "The Study of Optimal Adsorption Conditions of Phosphate on Fe-Modified Biochar by Response Surface Methodology" Molecules 28, no. 5: 2323. https://doi.org/10.3390/molecules28052323
APA StyleQian, J., Zhou, X., Cai, Q., Zhao, J., & Huang, X. (2023). The Study of Optimal Adsorption Conditions of Phosphate on Fe-Modified Biochar by Response Surface Methodology. Molecules, 28(5), 2323. https://doi.org/10.3390/molecules28052323





