Cortisol Monitoring Devices toward Implementation for Clinically Relevant Biosensing In Vivo
Abstract
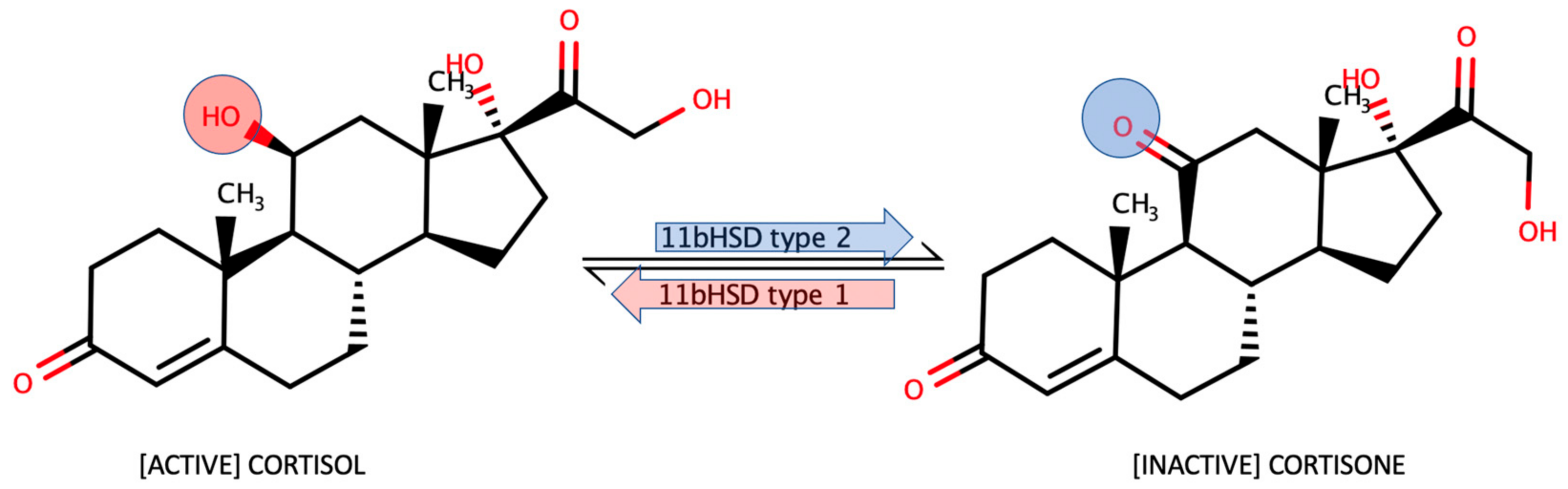
:1. Introduction
2. Continuous Monitoring Device
2.1. Example of the Clinically Relevant, FDA-Approved Long-Term Implanted Concenration Monitoring Medical Device: Continuous Glucose Monitoring (CGM) Eversense
2.2. Demands of the Continuous Cortisol Monitoring Device in Clinics, Point of Care, and Drug Development
3. Perspective Cortisol Biosensors Developed towards Continuous Monitoring: Recognition Elements, Physico-Chemical Phenomena for Signal Generation and Analytical Strategies
3.1. Electrochemical Cortisol Sensing
3.2. Immunosensors for CCM
3.3. Molecularly Imprinted Polymer Based Cortisol Biorecognition
3.4. Alternative Protein Scaffolds for CCM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bhake, R.C.; Kluckner, V.; Stassen, H.; Russell, G.M.; Leendertz, J.; Stevens, K.; Linthorst, A.C.E.; Lightman, S.L. Continuous Free Cortisol Profiles—Circadian Rhythms in Healthy Men. J. Clin. Endocrinol. Metab. 2019, 104, 5935–5947. [Google Scholar] [CrossRef]
- Chen, L.-S.; Singh, R.J. Niche Point-of-Care Endocrine Testing – Reviews of Intraoperative Parathyroid Hormone and Cortisol Monitoring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 115–128. [Google Scholar] [CrossRef]
- Lee, M.A.; Bakh, N.; Bisker, G.; Brown, E.N.; Strano, M.S. A Pharmacokinetic Model of a Tissue Implantable Cortisol Sensor. Adv. Healthc. Mater. 2016, 5, 3004–3015. [Google Scholar] [CrossRef]
- Boolani, A.; Channaveerappa, D.; Dupree, E.J.; Jayathirtha, M.; Aslebagh, R.; Grobe, S.; Wilkinson, T.; Darie, C.C. Trends in Analysis of Cortisol and Its Derivatives. In Advancements of Mass Spectrometry in Biomedical Research; Woods, A.G., Darie, C.C., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1140, pp. 649–664. ISBN 978-3-030-15949-8. [Google Scholar]
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO Nanorod Integrated Flexible Carbon Fibers for Sweat Cortisol Detection. ACS Appl. Electron. Mater. 2020, 2, 499–509. [Google Scholar] [CrossRef]
- Ganguly, A.; Lin, K.C.; Muthukumar, S.; Prasad, S. Autonomous, Real-Time Monitoring Electrochemical Aptasensor for Circadian Tracking of Cortisol Hormone in Sub-Microliter Volumes of Passively Eluted Human Sweat. ACS Sens. 2021, 6, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, D.; Ghanta, R.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Portable Biosensor for Monitoring Cortisol in Low-Volume Perspired Human Sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef] [Green Version]
- Persu, A. 11β-Hydroxysteroid Deshydrogenase: A Multi-Faceted Enzyme. J. Hypertens. 2005, 23, 29–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, A.; El Gierari, E.T.M.; Nally, L.M.; Sturmer, L.R.; Dodd, D.; Shi, R.-Z. Clinical Utility of an Ultrasensitive Urinary Free Cortisol Assay by Tandem Mass Spectrometry. Steroids 2019, 146, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Harno, E.; White, A. Will Treating Diabetes with 11β-HSD1 Inhibitors Affect the HPA Axis? Trends Endocrinol. Metab. 2010, 21, 619–627. [Google Scholar] [CrossRef]
- Markey, K.; Mitchell, J.; Botfield, H.; Ottridge, R.S.; Matthews, T.; Krishnan, A.; Woolley, R.; Westgate, C.; Yiangou, A.; Alimajstorovic, Z.; et al. 11β-Hydroxysteroid Dehydrogenase Type 1 Inhibition in Idiopathic Intracranial Hypertension: A Double-Blind Randomized Controlled Trial. Brain Commun. 2020, 2, fcz050. [Google Scholar] [CrossRef] [Green Version]
- Molenaar, N.; Groeneveld, A.B.J.; de Jong, M.F.C. Three Calculations of Free Cortisol versus Measured Values in the Critically Ill. Clin. Biochem. 2015, 48, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, P.K.; Murai, J.T.; Raymoure, W.J.; Kuhn, R.W.; Hammond, G.L.; Nisker, J.A. The Serum Transport of Steroid Hormones. In Proceedings of the 1981 Laurentian Hormone Conference; Elsevier: Amsterdam, The Netherlands, 1982; pp. 457–510. ISBN 978-0-12-571138-8. [Google Scholar]
- Cameron, A.; Henley, D.; Carrell, R.; Zhou, A.; Clarke, A.; Lightman, S. Temperature-Responsive Release of Cortisol from Its Binding Globulin: A Protein Thermocouple. J. Clin. Endocrinol. Metab. 2010, 95, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Dhillo, W.; Kong, W.; Le Roux, C.; Alaghband-Zadeh, J.; Jones, J.; Carter, G.; Mendoza, N.; Meeran, K.; O’Shea, D. Cortisol-Binding Globulin Is Important in the Interpretation of Dynamic Tests of the Hypothalamic--Pituitary--Adrenal Axis. Eur. J. Endocrinol. 2002, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, E.; Nenke, M.; Rankin, W.; Lewis, J.; Torpy, D. Corticosteroid-Binding Globulin: A Review of Basic and Clinical Advances. Horm. Metab. Res. 2016, 48, 359–371. [Google Scholar] [CrossRef]
- Lee, J.H.; Meyer, E.J.; Nenke, M.A.; Falhammar, H.; Torpy, D.J. Corticosteroid-Binding Globulin (CBG): Spatiotemporal Distribution of Cortisol in Sepsis. Trends Endocrinol. Metab. 2023, S1043276023000139. [Google Scholar] [CrossRef]
- Fusani, L. Field Techniques in Hormones and Behavior. In Encyclopedia of Animal Behavior; Elsevier: Amsterdam, The Netherlands, 2019; pp. 488–494. ISBN 978-0-12-813252-4. [Google Scholar]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring Cortisol in Serum, Urine and Saliva – Are Our Assays Good Enough? Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 308–322. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Central Effects of Stress Hormones in Health and Disease: Understanding the Protective and Damaging Effects of Stress and Stress Mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Jafri, R.Z.; Balliro, C.A.; El-Khatib, F.; Maheno, M.M.; Hillard, M.A.; O’Donovan, A.; Selagamsetty, R.; Zheng, H.; Damiano, E.R.; Russell, S.J. A Three-Way Accuracy Comparison of the Dexcom G5, Abbott Freestyle Libre Pro, and Senseonics Eversense Continuous Glucose Monitoring Devices in a Home-Use Study of Subjects with Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 846–852. [Google Scholar] [CrossRef]
- Colvin, A.E.; Jiang, H. Increased in Vivo Stability and Functional Lifetime of an Implantable Glucose Sensor through Platinum Catalysis. J. Biomed. Mater. Res. A 2013, 101A, 1274–1282. [Google Scholar] [CrossRef]
- Mortellaro, M.; DeHennis, A. Performance Characterization of an Abiotic and Fluorescent-Based Continuous Glucose Monitoring System in Patients with Type 1 Diabetes. Biosens. Bioelectron. 2014, 61, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Irace, C.; Cutruzzolà, A.; Tweden, K.; Kaufman, F.R. Device Profile of the Eversense Continuous Glucose Monitoring System for Glycemic Control in Type-1 Diabetes: Overview of Its Safety and Efficacy. Expert Rev. Med. Devices 2021, 18, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Parlak, O. Portable and Wearable Real-Time Stress Monitoring: A Critical Review. Sens. Actuators Rep. 2021, 3, 100036. [Google Scholar] [CrossRef]
- Riedemann, T.; Patchev, A.V.; Cho, K.; Almeida, O.F. Corticosteroids: Way Upstream. Mol. Brain 2010, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Son, G.H.; Kim, K. Circadian Rhythm of Adrenal Glucocorticoid: Its Regulation and Clinical Implications. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2011, 1812, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, R. Systemic Side Effects of Inhaled Corticosteroids in Patients with Asthma. Respir. Med. 2006, 100, 1307–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourakis, S.; Timpani, C.A.; Campelj, D.G.; Hafner, P.; Gueven, N.; Fischer, D.; Rybalka, E. Standard of Care versus New-Wave Corticosteroids in the Treatment of Duchenne Muscular Dystrophy: Can We Do Better? Orphanet J. Rare Dis. 2021, 16, 117. [Google Scholar] [CrossRef]
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef]
- Buttgereit, F.; Doering, G.; Schaeffler, A.; Witte, S.; Sierakowski, S.; Gromnica-Ihle, E.; Jeka, S.; Krueger, K.; Szechinski, J.; Alten, R. Targeting Pathophysiological Rhythms: Prednisone Chronotherapy Shows Sustained Efficacy in Rheumatoid Arthritis. Ann. Rheum. Dis. 2010, 69, 1275–1280. [Google Scholar] [CrossRef]
- Rensen, N.; Gemke, R.J.; van Dalen, E.C.; Rotteveel, J.; Kaspers, G.J. Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression after Treatment with Glucocorticoid Therapy for Childhood Acute Lymphoblastic Leukaemia. Cochrane Database Syst. Rev. 2017, 11, 1465–1858. [Google Scholar] [CrossRef] [Green Version]
- Chen Cardenas, S.M.; Santhanam, P.; Morris-Wiseman, L.; Salvatori, R.; Hamrahian, A.H. Perioperative Evaluation and Management of Patients on Glucocorticoids. J. Endocr. Soc. 2022, 7, bvac185. [Google Scholar] [CrossRef]
- Rison, S.; Rajeev, R.; Bhat, V.S.; Mathews, A.T.; Varghese, A.; Hegde, G. Non-Enzymatic Electrochemical Determination of Salivary Cortisol Using ZnO-Graphene Nanocomposites. RSC Adv. 2021, 11, 37877–37885. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Rana, A.R.S. A Comparison of Edge- and Basal-Plane Pyrolytic Graphite Electrodes towards the Sensitive Determination of Hydrocortisone. Talanta 2010, 83, 149–155. [Google Scholar] [CrossRef]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. A Contemporary Approach for Design and Characterization of Fiber-Optic-Cortisol Sensor Tailoring LMR and ZnO/PPY Molecularly Imprinted Film. Biosens. Bioelectron. 2017, 87, 178–186. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; DeBrosse, M.; Brothers, M.; Kim, S.; Sereda, A.; Ivanov, N.V.; Hussain, S.; Heikenfeld, J. Oil-Membrane Protection of Electrochemical Sensors for Fouling- and PH-Insensitive Detection of Lipophilic Analytes. ACS Appl. Mater. Interfaces 2021, 13, 53553–53563. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.M.; Kusov, P.A.; Kosolobov, S.S.; Borzenkova, O.V.; Khakimov, A.V.; Kotelevtsev, Y.V.; Drachev, V.P. Surface-Specific Washing-Free Immunosensor for Time-Resolved Cortisol Monitoring. Talanta 2021, 225, 122070. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.-J. Label-Free Sensitive Detection of Steroid Hormone Cortisol Based on Target-Induced Fluorescence Quenching of Quantum Dots. Langmuir 2020, 36, 7781–7788. [Google Scholar] [CrossRef]
- Hassan, H.; Bang, O.; Janting, J. Polymer Optical Fiber Tip Mass Production Etch Mechanism to Achieve CPC Shape for Improved Biosensor Performance. Sensors 2019, 19, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Zhou, M.; Li, Y.; Liang, Z.; Li, Y.; Yu, L.; Liu, Y.; Liang, Y.; Chen, L.; Yang, C. A New Optical Fiber Probe-Based Quantum Dots Immunofluorescence Biosensors in the Detection of Staphylococcus Aureus. Front. Cell. Infect. Microbiol. 2021, 11, 665241. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, L.; Saris, A.; Sergelen, K.; de Jong, A.M.; Yan, J.; Prins, M.W.J. Reversible Immunosensor for the Continuous Monitoring of Cortisol in Blood Plasma Sampled with Microdialysis. ACS Sens. 2022, 7, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.W.A.; Miladinovic, J.; Milstein, J.N. An Ultra-Stable and Dense Single-Molecule Click Platform for Sensing Protein-DNA Interactions. bioRxiv 2020, 2020.11.25.397737. [Google Scholar]
- An, J.E.; Kim, K.H.; Park, S.J.; Seo, S.E.; Kim, J.; Ha, S.; Bae, J.; Kwon, O.S. Wearable Cortisol Aptasensor for Simple and Rapid Real-Time Monitoring. ACS Sens. 2022, 7, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon Fiber Based Electrochemical Sensor for Sweat Cortisol Measurement. Sci. Rep. 2019, 9, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennazza, G.; Santonico, M.; Vollero, L.; Zompanti, A.; Sabatini, A.; Kumar, N.; Pini, I.; Quiros Solano, W.F.; Sarro, L.; D’Amico, A. Advances in the Electronics for Cyclic Voltammetry: The Case of Gas Detection by Using Microfabricated Electrodes. Front. Chem. 2018, 6, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheibani, S.; Capua, L.; Kamaei, S.; Akbari, S.S.A.; Zhang, J.; Guerin, H.; Ionescu, A.M. Extended Gate Field-Effect-Transistor for Sensing Cortisol Stress Hormone. Commun. Mater. 2021, 2, 10. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, Y. A Disposable Printed Liquid Gate Graphene Field Effect Transistor for a Salivary Cortisol Test. ACS Sens. 2021, 6, 3024–3031. [Google Scholar] [CrossRef]
- Villa, J.E.L.; Garcia, I.; Jimenez de Aberasturi, D.; Pavlov, V.; Sotomayor, M.D.P.T.; Liz-Marzán, L.M. SERS-Based Immunoassay for Monitoring Cortisol-Related Disorders. Biosens. Bioelectron. 2020, 165, 112418. [Google Scholar] [CrossRef]
- Upasham, S.; Tanak, A.; Jagannath, B.; Prasad, S. Development of Ultra-Low Volume, Multi-Bio Fluid, Cortisol Sensing Platform. Sci. Rep. 2018, 8, 16745. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Lee, K.; Jung, H.; Kang, H.K.; Jo, J.; Park, I.-K.; Lee, H.H. Direct Immune-Detection of Cortisol by Chemiresistor Graphene Oxide Sensor. Biosens. Bioelectron. 2017, 98, 473–477. [Google Scholar] [CrossRef]
- Venugopal, M.; Arya, S.K.; Chornokur, G.; Bhansali, S. A Realtime and Continuous Assessment of Cortisol in ISF Using Electrochemical Impedance Spectroscopy. Sens. Actuators Phys. 2011, 172, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Rice, P.; Upasham, S.; Jagannath, B.; Manuel, R.; Pali, M.; Prasad, S. CortiWatch: Watch-Based Cortisol Tracker. Future Sci. OA 2019, 5, FSO416. [Google Scholar] [CrossRef] [Green Version]
- Ben Halima, H.; Bellagambi, F.G.; Brunon, F.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Hangouët, M.; Zine, N.; Bausells, J.; Errachid, A. Immuno Field-Effect Transistor (ImmunoFET) for Detection of Salivary Cortisol Using Potentiometric and Impedance Spectroscopy for Monitoring Heart Failure. Talanta 2022, 123802. [Google Scholar] [CrossRef]
- Ben Halima, H.; Zine, N.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis. Micromachines 2022, 13, 2235. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Guzmán, M.; Eguílaz, M.; Campuzano, S.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Disposable Immunosensor for Cortisol Using Functionalized Magnetic Particles. The Analyst 2010, 135, 1926. [Google Scholar] [CrossRef]
- Fernandez, R.E.; Umasankar, Y.; Manickam, P.; Nickel, J.C.; Iwasaki, L.R.; Kawamoto, B.K.; Todoki, K.C.; Scott, J.M.; Bhansali, S. Disposable Aptamer-Sensor Aided by Magnetic Nanoparticle Enrichment for Detection of Salivary Cortisol Variations in Obstructive Sleep Apnea Patients. Sci. Rep. 2017, 7, 17992. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; Wang, S.; Jin, X.; Bakh, N.A.; Nguyen, F.T.; Dong, J.; Silmore, K.S.; Gong, X.; Pham, C.; Jones, K.K.; et al. Implantable Nanosensors for Human Steroid Hormone Sensing In Vivo Using a Self-Templating Corona Phase Molecular Recognition. Adv. Healthc. Mater. 2020, 9, 2000429. [Google Scholar] [CrossRef]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly Selective Nanoporous Membrane-Based Wearable Organic Electrochemical Device for Noninvasive Cortisol Sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugo, S.M.; Alberkant, J. Flexible Molecularly Imprinted Electrochemical Sensor for Cortisol Monitoring in Sweat. Anal. Bioanal. Chem. 2020, 412, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Ertuğrul Uygun, H.D.; Uygun, Z.O.; Canbay, E.; Girgin Sağın, F.; Sezer, E. Non-Invasive Cortisol Detection in Saliva by Using Molecularly Cortisol Imprinted Fullerene-Acrylamide Modified Screen Printed Electrodes. Talanta 2020, 206, 120225. [Google Scholar] [CrossRef]
- Kim, S.B.; Sato, M.; Tao, H. Genetically Encoded Bioluminescent Indicators for Stress Hormones. Anal. Chem. 2009, 81, 3760–3768. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Paulmurugan, R. Bioluminescent Imaging Systems for Assay Developments. Anal. Sci. 2021, 37, 233–247. [Google Scholar] [CrossRef]
- Kim, S.B.; Takenaka, Y.; Torimura, M. A Bioluminescent Probe for Salivary Cortisol. Bioconjug. Chem. 2011, 22, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, K.R. Glucocorticoid Receptor Control of Transcription: Precision and Plasticity via Allostery. Nat. Rev. Mol. Cell Biol. 2017, 18, 159–174. [Google Scholar] [CrossRef]
- Laudat, M.H.; Cerdas, S.; Fournier, C.; Guiban, D.; Guilhaume, B.; Luton, J.P. Salivary Cortisol Measurement: A Practical Approach to Assess Pituitary-Adrenal Function. J. Clin. Endocrinol. Metab. 1988, 66, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Plasma Steroid-Binding Proteins: Primary Gatekeepers of Steroid Hormone Action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maganzini, N.; Thompson, I.; Wilson, B.; Soh, H.T. Pre-Equilibrium Biosensors as an Approach towards Rapid and Continuous Molecular Measurements. Nat. Commun. 2022, 13, 7072. [Google Scholar] [CrossRef] [PubMed]
- Lubken, R.M.; Bergkamp, M.H.; de Jong, A.M.; Prins, M.W.J. Sensing Methodology for the Rapid Monitoring of Biomolecules at Low Concentrations over Long Time Spans. ACS Sens. 2021, 6, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Loyez, M.; Lobry, M.; Hassan, E.M.; DeRosa, M.C.; Caucheteur, C.; Wattiez, R. HER2 Breast Cancer Biomarker Detection Using a Sandwich Optical Fiber Assay. Talanta 2021, 221, 121452. [Google Scholar] [CrossRef]











| Sample Source | Linear Range, LOD | Signal Generation | Reference | Applicability In Vivo |
|---|---|---|---|---|
| Saliva | 0.001 ➔ to 104 nM LOD (–) | Field effect transistorm (Liquid Gate, Graphene) | [49] | No |
| Urine and Serum | 1 to 400 ng/mL 2.75 µM–1 mM LOD 3 ng/mL | Magnetically assisted SERS immunoassay (MA-SERSI) | [50] | No |
| Universal | Serum, blood 50–200 ng/mL (137 µM–0.5 mM) Saliva 1–40 ng/mL (2.75 µM–0.1 mM) Sweat 10–150 ng/mL (27 µM–0.4 µM) LOD 1 ng/mL | Non-faradaic electrochemical impedance spectroscopy (EIS) | [51] | No |
| Saliva | 156~10,000 pg/mL (0.4–2.7 µM) LOD 10 pg/mL | Chemiresistor graphene oxide sensor | [52] | No |
| Dialyzed reconstituted Human plasma | 100 nM–10 µM LOD (–) | Particle mobility assay | [43] | Yes; Pre-microdialysis required |
| Extracted ISF | 1 pM to 100 nM LOD (–) | Electrochemical impedance immunoassay | [53] | Yes; ISF extractor required |
| Sweat | 1 pg/mL to 150 ng/mL (2.75 nM–0.04 mM) LOD (–) | Chronoamperometry and non-faradaic electrochemical impedance spectroscopy (EIS) | [54] | No |
| Saliva | 0.2–0.6 ng/mL LOD 0.005 ± 0.002 ng/mL | Field effect transistor | [55] | No |
| Buffer | 2 to 50 ng/mL LOD 0.66 ng/mL | Electrochemical impedance immunoassay | [56] | No |
| Buffer | 0.1 to 20 μg/mL LOD 0.02 μg/mL | Metal-enhanced fluoroimmunoassay | [39] | No |
| Reconstituted Human serum | 5.0 10−3 and 150 ng mL−1 LOD 3.5 pg mL−1 | Magnetic particle-assisted competitive immunoassay with differential pulse voltammetry | [57] | No |
| Preprocessed Saliva | 0.1 ➔ µM to 10 mM LOD 100 pM | Quantum dots fluorescence quenching immunoassay | [40] | No |
| Sample Source | Linear Range, LOD | Signal Generation | Reference | Applicability In Vivo |
|---|---|---|---|---|
| Sweat | 1−256 ng/mL (2.75 µM–138 µM) LOD (–) | Electrochemical ZnO polymer matrix electrode | [6] | No |
| Saliva | 50 to 200 nM LOD 10 pM | Metalloporphyrin based macrocyclic catalyst electrochemical sensor | [58] | No |
| Sweat | 1 pM to 10 μM LOD 10 pM | Liquid-ion gated field-effect transistor (FET) | [48] | No |
| Sample Source | Linear Range, LOD | Signal Generation | Reference | Applicability In Vivo |
|---|---|---|---|---|
| Artificial saliva | 10−12 to 10−6 g/mL (2.75 pM to 2.75 µM) LOD 25.9 fg/mL | Fiber optic Lossy mode resonance ZnO/polypyrrole | [36] | No |
| Sweat | 0.03 to 3.6 × 10−6 g/mL (9.9 µM–82 nM) LOD (–) | Electrochemical transistor | [60] | No |
| Sweat | 10 ng/mL–60 ng/mL 27.5 µM–165 µMLOD 2.0 ng/mL ± 0.4 ng/mL | Electrochemical (PDMS doped with carbon nanotubes-cellulose crystals) | [61] | No |
| ISF | 10 × 10−6 to 100 × 10−6 M (10–100 µM) LOD (–) | Corona phase molecular recognition (CoPhMoRe) | [59] | Yes (concept demonstrated on progesterone) |
| Saliva | 0.5 nM to 64 nM LOD 0.14 nM | Impedimetric sensor | [62] | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusov, P.A.; Kotelevtsev, Y.V.; Drachev, V.P. Cortisol Monitoring Devices toward Implementation for Clinically Relevant Biosensing In Vivo. Molecules 2023, 28, 2353. https://doi.org/10.3390/molecules28052353
Kusov PA, Kotelevtsev YV, Drachev VP. Cortisol Monitoring Devices toward Implementation for Clinically Relevant Biosensing In Vivo. Molecules. 2023; 28(5):2353. https://doi.org/10.3390/molecules28052353
Chicago/Turabian StyleKusov, Pavel A., Yuri V. Kotelevtsev, and Vladimir P. Drachev. 2023. "Cortisol Monitoring Devices toward Implementation for Clinically Relevant Biosensing In Vivo" Molecules 28, no. 5: 2353. https://doi.org/10.3390/molecules28052353
APA StyleKusov, P. A., Kotelevtsev, Y. V., & Drachev, V. P. (2023). Cortisol Monitoring Devices toward Implementation for Clinically Relevant Biosensing In Vivo. Molecules, 28(5), 2353. https://doi.org/10.3390/molecules28052353






