The Effects of Acid and Water in the Formation of Anodic Alumina: DFT and Experiment Study
Abstract
1. Introduction
2. Results
2.1. The Adsorption of Molecules
2.2. The Acid Radicals Replacing the Adsorbed H2O
2.3. The Acid Radicals Bonding with the H of Adsorbed H2O
2.4. The Acid Radicals Stripping off H from Adsorbed H2O and OH
2.5. The Reasons for Acid Radicals Stripping off H Atoms of H2O
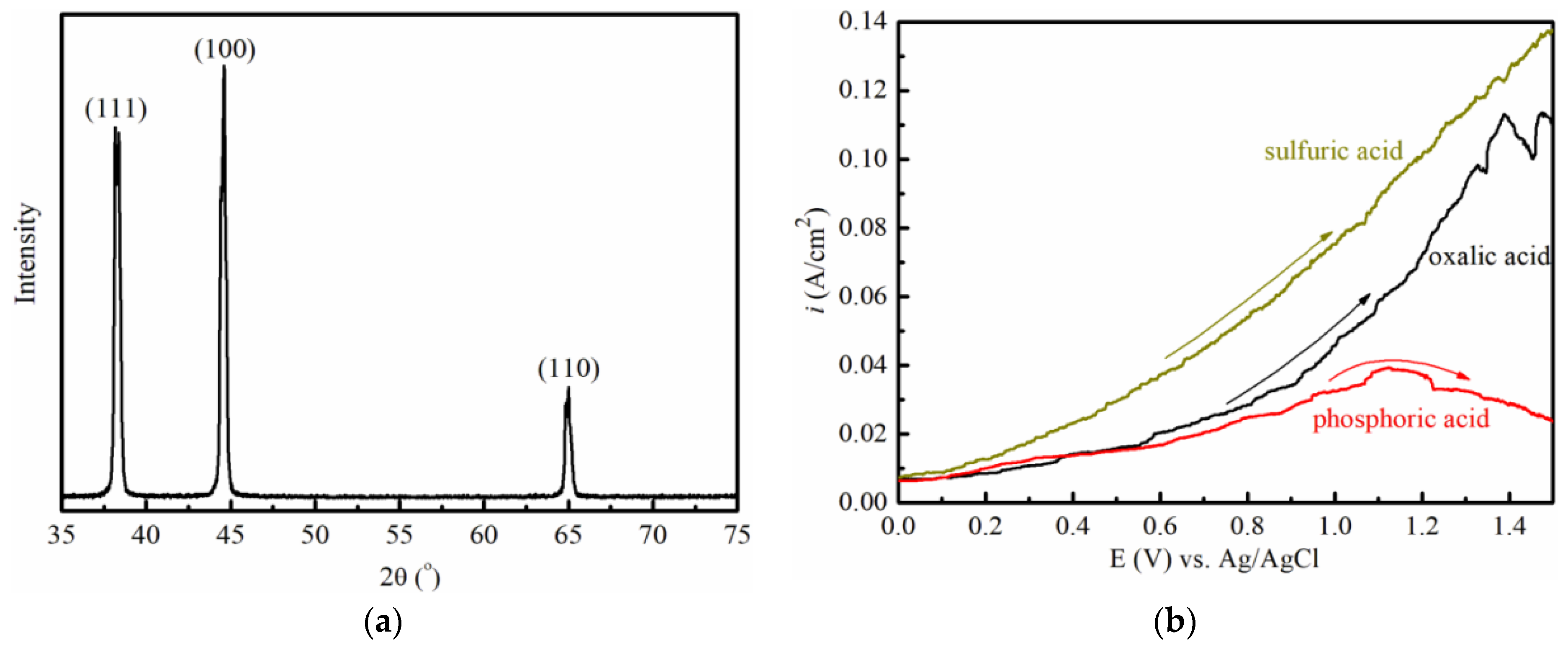
2.6. The Experimental Verification
3. Materials and Methods
3.1. Computation
3.2. Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Senbahavalli, R.; Mohanapriya, S.; Raj, V. Enhanced corrosion resistance of anodic non-porous alumina (ANPA) coatings on aluminium fabricated from mixed organic-inorganic electrolytes. Mater. Discov. 2016, 3, 29–37. [Google Scholar] [CrossRef]
- Hourdakis, E.; Nassiopoulou, A.G. High-Density MIM Capacitors with Porous Anodic Alumina Dielectric. IEEE Trans. Electron Devices 2010, 57, 2679–2683. [Google Scholar] [CrossRef]
- Thorat, S.B.; Diaspro, A.; Salerno, M. In vitro investigation of coupling-agent-free dental restorative composite based on nano-porous alumina fillers. J. Dent. 2014, 42, 279–286. [Google Scholar] [CrossRef]
- Macias, G.; Hernández-Eguía, L.P.; Ferré-Borrull, J.; Pallares, J.; Marsal, L.F. Gold-Coated Ordered Nanoporous Anodic Alumina Bilayers for Future Label-Free Interferometric Biosensors. ACS Appl. Mater. Interfaces 2013, 5, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Stępniowski, W.J.; Zasada, D.; Bojar, Z. First step of anodization influences the final nanopore arrangement in anodized alumina. Surf. Coat. Technol. 2011, 206, 1416–1422. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-Stępniowska, A.; Bojar, Z. Quantitative arrangement analysis of anodic alumina formed by short anodizations in oxalic acid. Mater. Charact. 2013, 78, 79–86. [Google Scholar] [CrossRef]
- Shingubara, S.; Morimoto, K.; Sakaue, H.; Takahagi, T. Self-Organization of a Porous Alumina Nanohole Array Using a Sulfuric/Oxalic Acid Mixture as Electrolyte. Electrochem. Solid-State Lett. 2004, 7, E15–E17. [Google Scholar] [CrossRef]
- Kashi, M.A.; Ramazani, A.; Rahmandoust, M.; Noormohammadi, M. The effect of pH and composition of sulfuric–oxalic acid mixture on the self-ordering configuration of high porosity alumina nanohole arrays. J. Phys. D Appl. Phys. 2007, 40, 4625–4630. [Google Scholar] [CrossRef]
- Zaraska, L.; Stępniowski, W.J.; Ciepiela, E.; Sulka, G.D. The effect of anodizing temperature on structural features and hexagonal arrangement of nanopores in alumina synthesized by two-step anodizing in oxalic acid. Thin Solid Films 2013, 534, 155–161. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Michalska-Domańska, M.; Norek, M.; Czujko, T. Fast Fourier transform based arrangement analysis of poorly organized alumina nanopores formed via self-organized anodization in chromic acid. Mater. Lett. 2014, 117, 69–73. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-Stępniowska, A.; Presz, A.; Czujko, T.; Varin, R.A. The effects of time and temperature on the arrangement of anodic aluminum oxide nanopores. Mater. Charact. 2014, 91, 1–9. [Google Scholar] [CrossRef]
- Chi, C.-S.; Lee, J.-H.; Kim, I.; Oh, H.-J. Effects of Microstructure of Aluminum Substrate on Ordered Nanopore Arrays in Anodic Alumina. J. Mater. Sci. Technol. 2015, 31, 751–758. [Google Scholar] [CrossRef]
- Sevgi, A.; Evrim, B.; Birgül, Y. The nanoporous anodic alumina oxide formed by two-step anodization. Thin Solid Films 2018, 648, 94–102. [Google Scholar]
- Roslyakov, I.V.; Gordeeva, E.O.; Napolskii, K.S. Role of Electrode Reaction Kinetics in Self-Ordering of Porous Anodic Alumina. Electrochim. Acta 2017, 241, 362–369. [Google Scholar] [CrossRef]
- Kondo, R.; Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Superhydrophilic and superhydrophobic aluminum alloys fabricated via pyrophosphoric acid anodizing and fluorinated SAM modification. J. Alloys Compd. 2017, 725, 379–387. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shigehara, Y.; Ono, S.; Asoh, H. Heat-induced structural transformations of anodic porous alumina formed in phosphoric acid. Microporous Mesoporous Mater. 2018, 265, 77–83. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, W.; Zhang, S. Morphology evolution of porous anodic alumina in mixed H3PO4/NH4F electrolytes. Surf. Coat. Technol. 2018, 334, 500–508. [Google Scholar] [CrossRef]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Sakaguchi, N.; Suzuki, R.O. Self-ordered Porous Alumina Fabricated via Phosphonic Acid Anodizing. Electrochim. Acta 2016, 190, 471–479. [Google Scholar] [CrossRef]
- Kikuchi, T.; Takenaga, A.; Natsui, S.; Suzuki, R.O. Advanced hard anodic alumina coatings via etidronic acid anodizing. Surf. Coat. Technol. 2017, 326, 72–78. [Google Scholar] [CrossRef]
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Exploration for the Self-ordering of Porous Alumina Fabricated via Anodizing in Etidronic Acid. Electrochim. Acta 2016, 211, 515–523. [Google Scholar] [CrossRef]
- Cojocaru, C.S.; Padovani, J.M.; Wade, T.; Mandoli, C.; Jaskierowicz, G.; Wegrowe, J.E.; Fontcuberta i Morral, A.; Pribat, D. Conformal Anodic Oxidation of Aluminum Thin Films. Nano Lett. 2005, 5, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Pashchanka, M.; Schneider, J.J. Self-Ordering Regimes of Porous Anodic Alumina Layers Formed in Highly Diluted Sulfuric Acid Electrolytes. J. Phys. Chem. C 2016, 120, 14590–14596. [Google Scholar] [CrossRef]
- Kondo, R.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Fabrication of self-ordered porous alumina via anodizing in sulfate solutions. Mater. Lett. 2016, 183, 285–289. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yamamoto, T.; Natsui, S.; Suzuki, R.O. Fabrication of Anodic Porous Alumina by Squaric Acid Anodizing. Electrochim. Acta 2014, 123, 14–22. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nakajima, D.; Kawashima, J. Fabrication of anodic porous alumina via anodizing in cyclicoxocarbon acids. Appl. Surf. Sci. 2014, 313, 276–285. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Self-Ordering Behavior of Anodic Porous Alumina via Selenic Acid Anodizing. Electrochim. Acta 2014, 137, 728–735. [Google Scholar] [CrossRef]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Nanostructural characterization of large-scale porous alumina fabricated via anodizing in arsenic acid solution. Appl. Surf. Sci. 2017, 403, 652–661. [Google Scholar] [CrossRef]
- Su, Z.; Bühl, M.; Zhou, W. Dissociation of Water During Formation of Anodic Aluminum Oxide. J. Am. Chem. Soc. 2009, 131, 8697–8702. [Google Scholar] [CrossRef]
- Thompson, G.E.; Xu, Y.; Skeldon, P.; Shimizu, K.; Han, S.H.; Wood, G.C. Anodic oxidation of aluminium. Philos. Mag. B 1987, 55, 651–667. [Google Scholar] [CrossRef]
- Garcia-Vergara, S.J.; Molchan, I.S.; Zhou, F.; Habazaki, H.; Kowalski, D.; Skeldon, P.; Thompson, G.E. Incorporation and migration of phosphorus species within anodic films on an Al-W alloy. Surf. Interface Anal. 2011, 43, 893–902. [Google Scholar] [CrossRef]
- Xu, Y.; Thompson, G.; Wood, G. Direct observation of the cell material comprising porous anodic films formed on aluminium. Electrochim. Acta 1982, 27, 1623–1625. [Google Scholar] [CrossRef]
- Fleming, I. Molecular Orbitals and Organic Chemical Reactions; Reference Edition; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 138–139. [Google Scholar]
- Blanck, S.; Loehlé, S.; Steinmann, S.N.; Michel, C. Adhesion of lubricant on aluminium through adsorption of additive head-groups on alumina: A DFT study. Tribol. Int. 2020, 145, 106140. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
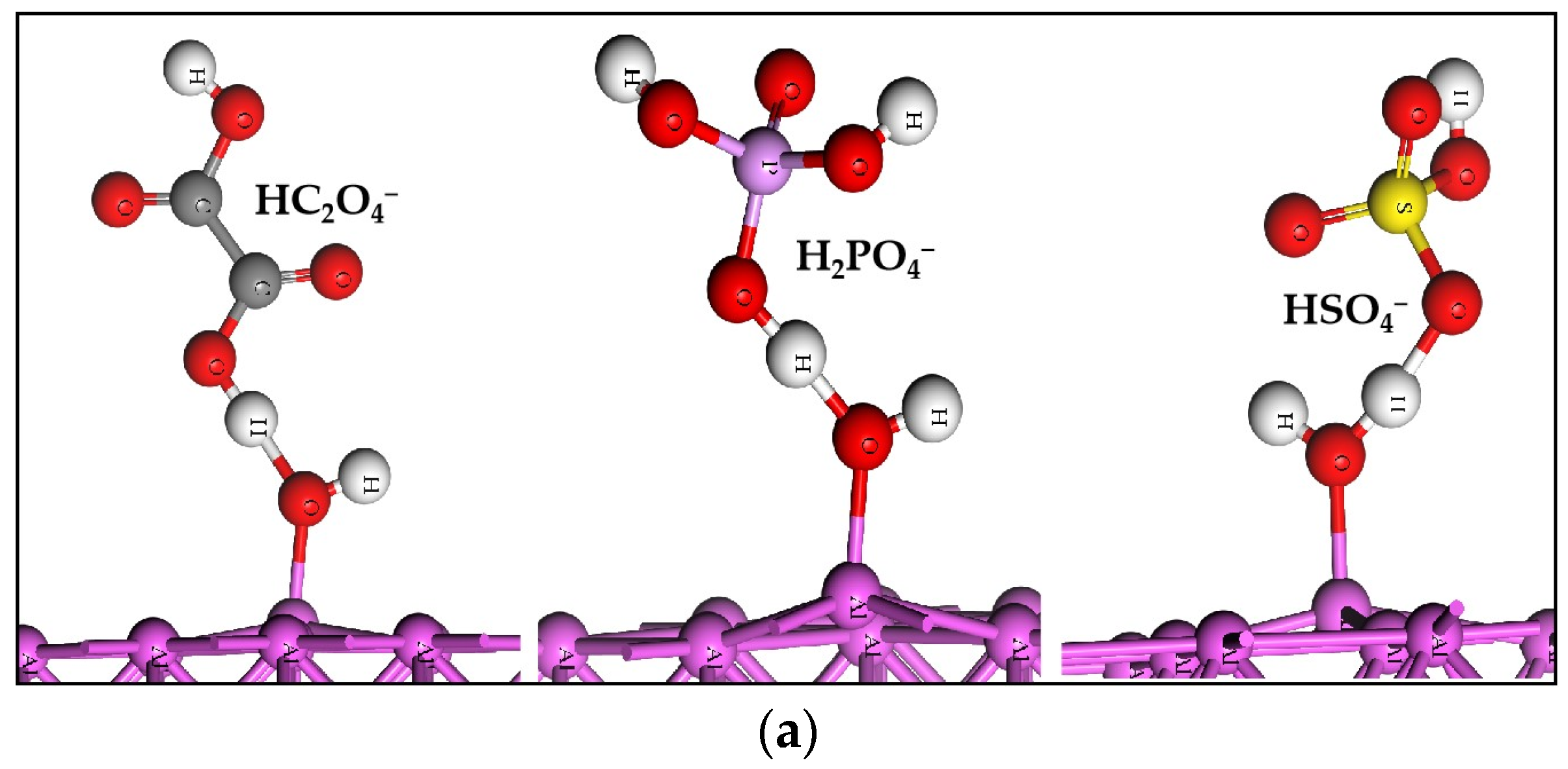
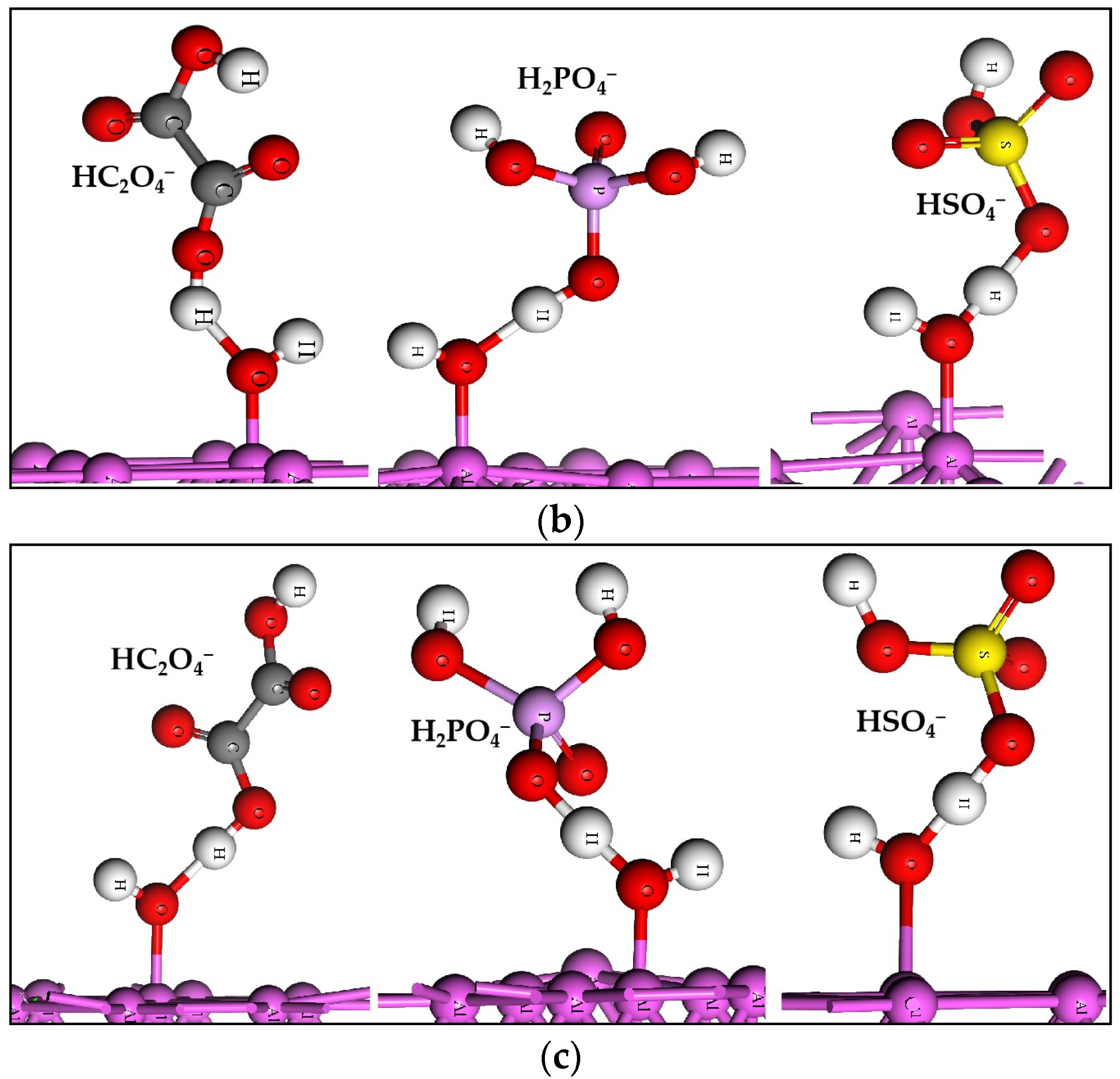
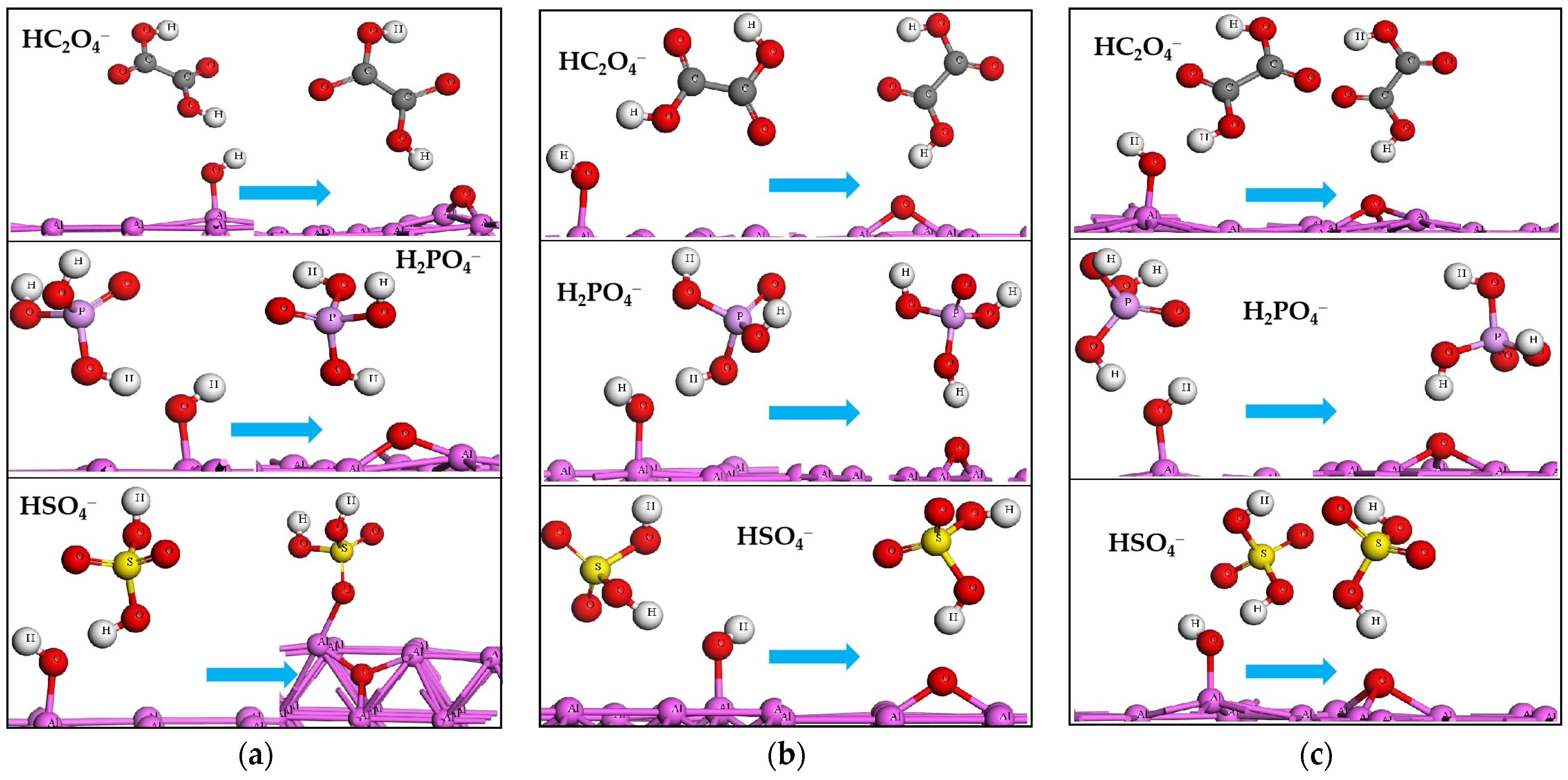


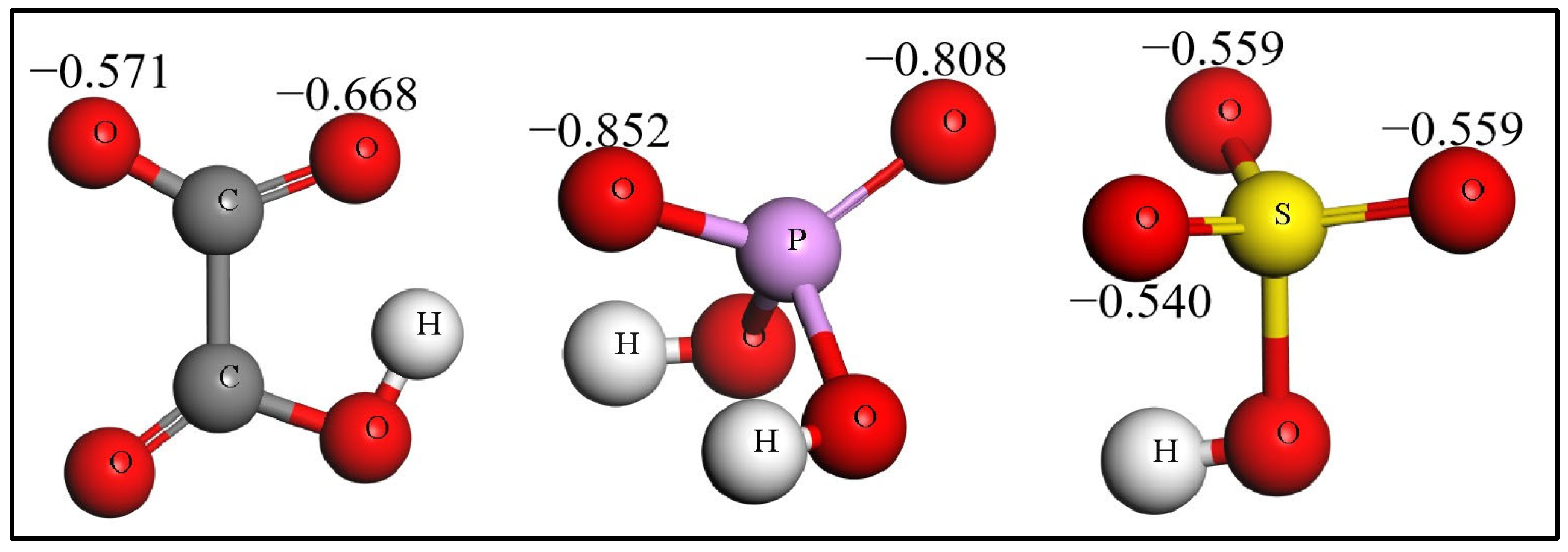
| Slabs | H2O | HC2O4− | H2PO4− | HSO4− |
|---|---|---|---|---|
| (100)0 | −0.34 | −3.65 | −4.06 | −2.93 |
| (110)0 | −0.47 | −3.39 | −3.28 | −3.08 |
| (111)0 | −0.36 | −2.78 | −3.70 | −3.18 |
| (100)+1 | −0.50 | −4.62 | −5.16 | −3.69 |
| (110)+1 | −0.53 | −3.90 | −3.67 | −3.75 |
| (111)+1 | −0.56 | −3.99 | −5.22 | −3.80 |
| Slabs | HC2O4− | H2PO4− | HSO4− |
|---|---|---|---|
| (100)0 | −3.31 | −3.72 | −2.59 |
| (110)0 | −2.92 | −2.81 | −2.61 |
| (111)0 | −2.42 | −3.33 | −2.82 |
| (100)+1 | −4.11 | −4.65 | −3.18 |
| (110)+1 | −3.37 | −3.14 | −3.22 |
| (111)+1 | −3.42 | −4.65 | −3.23 |
| Slabs | HC2O4− | H2PO4− | HSO4− |
|---|---|---|---|
| (100)0 | −2.23 | −1.41 | −0.52 |
| (110)0 | −1.70 | −0.83 | −0.04 |
| (111)0 | −2.49 | −1.73 | −0.80 |
| Slabs | a1 | b1 | a1 + b1 | c1 | d1 | c1 + d1 | e1 | f1 | e1 + f1 |
|---|---|---|---|---|---|---|---|---|---|
| (100)+1 | −3.21 | −2.54 | −5.75 | −3.24 | −2.57 | −5.81 | −2.57 | −1.91 | −4.48 |
| (110)+1 | −2.33 | −2.15 | −4.48 | −2.42 | −2.24 | −4.66 | −1.76 | −1.58 | −3.34 |
| (111)+1 | −2.97 | −3.37 | −6.34 | −3.08 | −3.48 | −6.56 | −2.49 | −2.89 | −5.38 |
| Slabs | H | H | Slabs | H |
|---|---|---|---|---|
| (100)0-H2O | 0.274 | 0.274 | (100)0-OH | 0.261 |
| (110)0-H2O | 0.267 | 0.267 | (110)0-OH | 0.260 |
| (111)0-H2O | 0.282 | 0.282 | (111)0-OH | 0.298 |
| (100)+1-H2O | 0.322 | 0.322 | (100)+1-OH | 0.323 |
| (110)+1-H2O | 0.311 | 0.311 | (110)+1-OH | 0.312 |
| (111)+1-H2O | 0.328 | 0.328 | (111)+1-OH | 0.343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kang, J.; Li, X.; Li, P.; Du, Y.; Qin, Y.; Li, N.; Li, J. The Effects of Acid and Water in the Formation of Anodic Alumina: DFT and Experiment Study. Molecules 2023, 28, 2427. https://doi.org/10.3390/molecules28062427
Zhang Z, Kang J, Li X, Li P, Du Y, Qin Y, Li N, Li J. The Effects of Acid and Water in the Formation of Anodic Alumina: DFT and Experiment Study. Molecules. 2023; 28(6):2427. https://doi.org/10.3390/molecules28062427
Chicago/Turabian StyleZhang, Zhengwei, Jin Kang, Xiaodong Li, Ping Li, Yali Du, Yufan Qin, Ningyi Li, and Jiebin Li. 2023. "The Effects of Acid and Water in the Formation of Anodic Alumina: DFT and Experiment Study" Molecules 28, no. 6: 2427. https://doi.org/10.3390/molecules28062427
APA StyleZhang, Z., Kang, J., Li, X., Li, P., Du, Y., Qin, Y., Li, N., & Li, J. (2023). The Effects of Acid and Water in the Formation of Anodic Alumina: DFT and Experiment Study. Molecules, 28(6), 2427. https://doi.org/10.3390/molecules28062427






