The Investigation of the Chemical Composition and Applicability of Gold Nanoparticles Synthesized with Amygdalus communis (Almond) Leaf Aqueous Extract as Antimicrobial and Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition
2.2. Characterization of Biogenic AuNPs
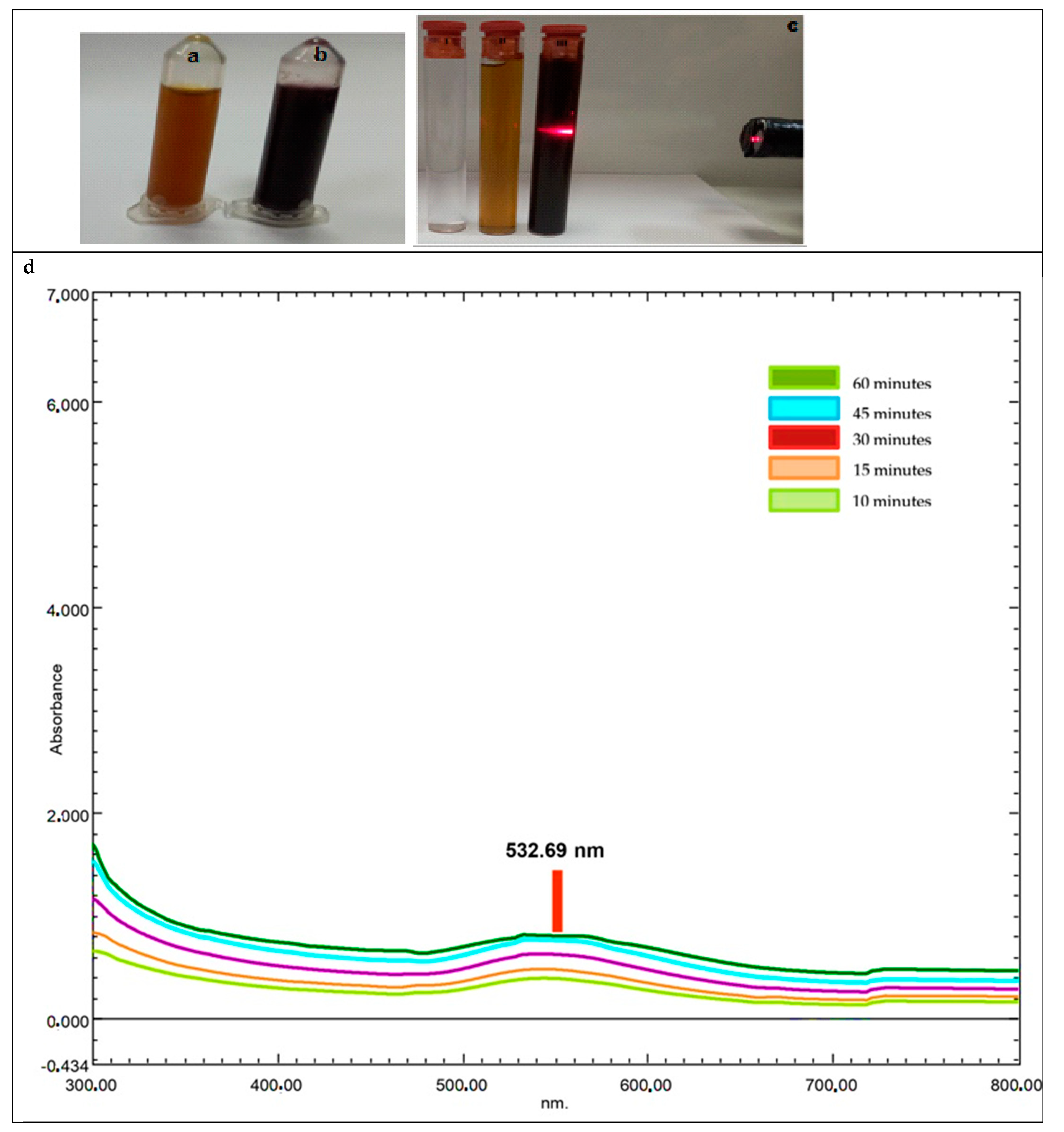
2.2.1. UV-Vis Spectrum Data of AC-AuNPs
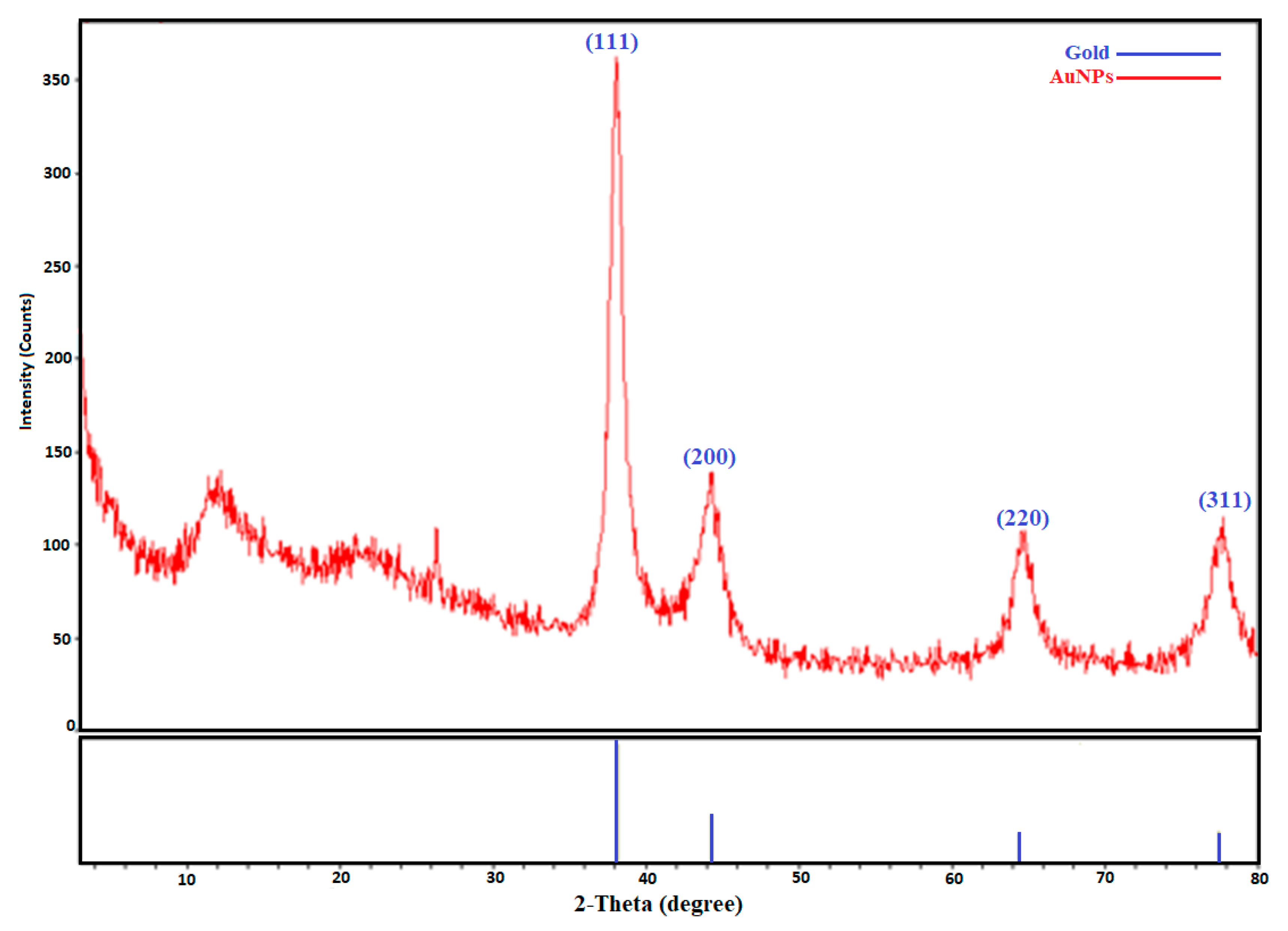
2.2.2. X-ray Diffraction Analysis Data
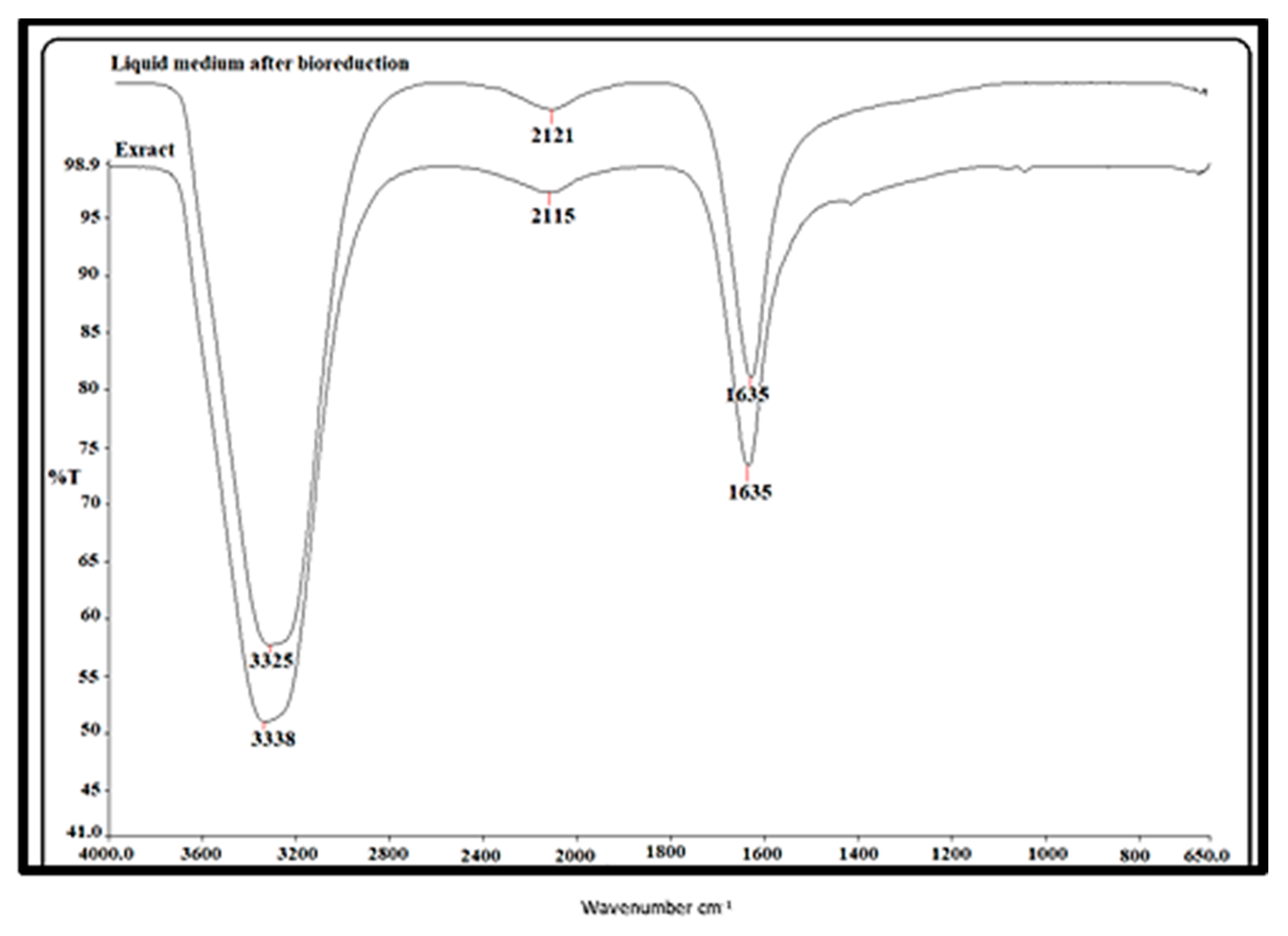
2.2.3. FTIR Spectroscopy Data
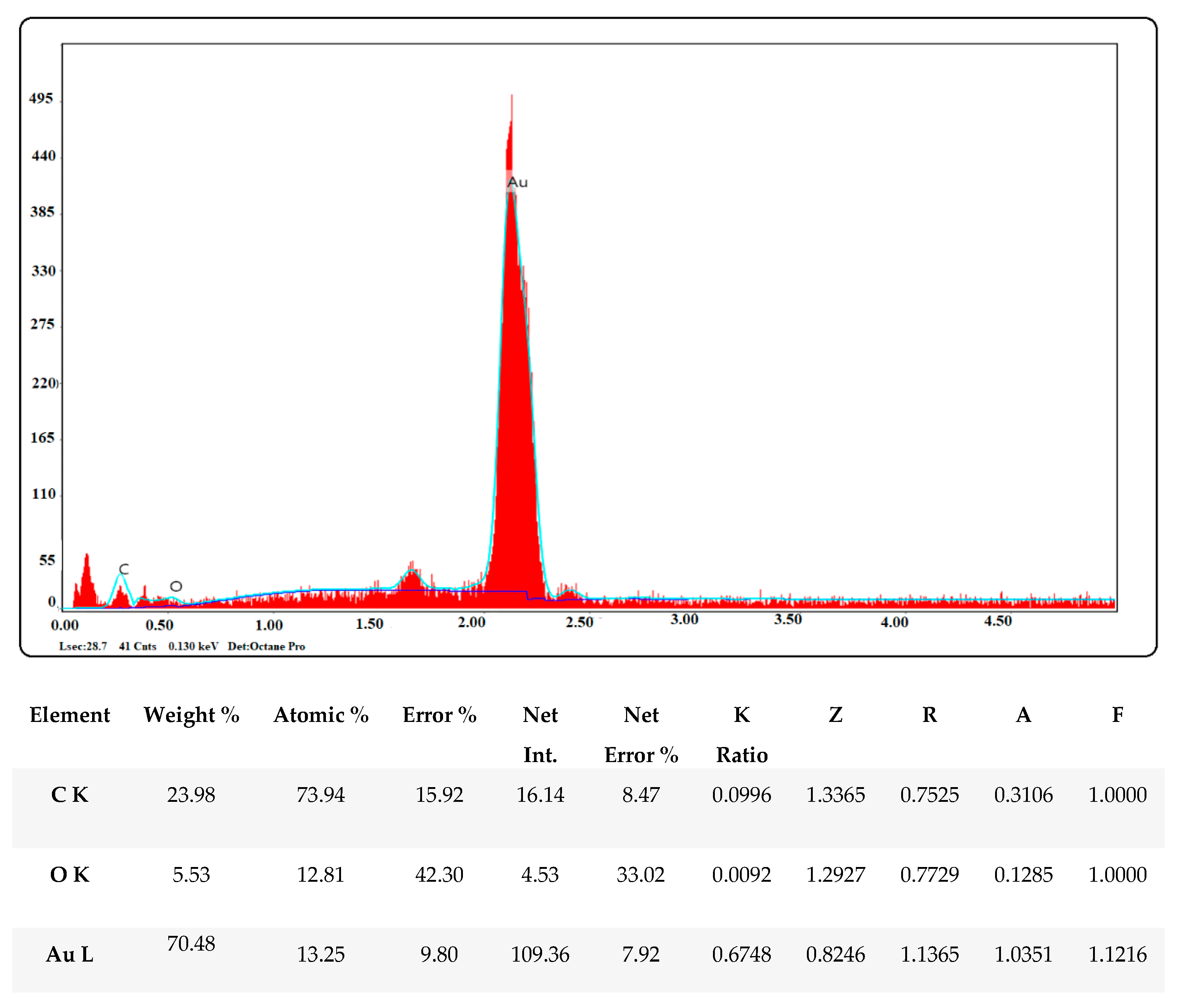
2.2.4. EDX Pattern of Biogenic Gold Nanoparticles
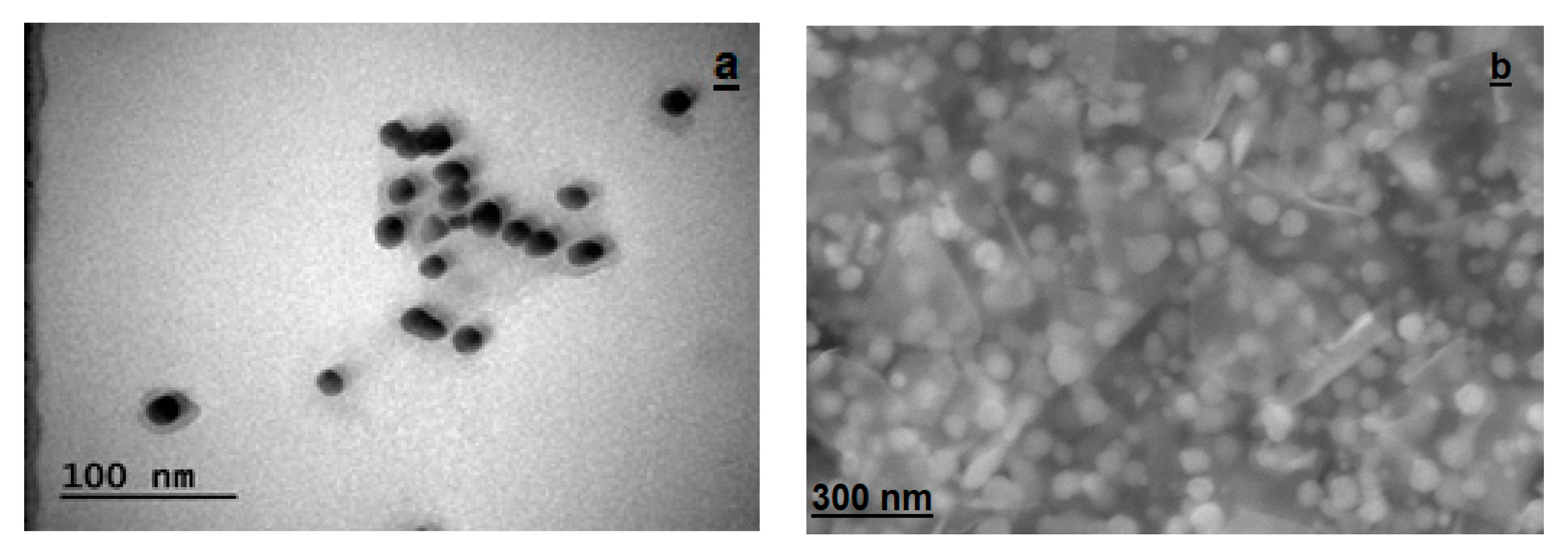
2.2.5. Morphological Structures of Synthesized AC-AuNPs
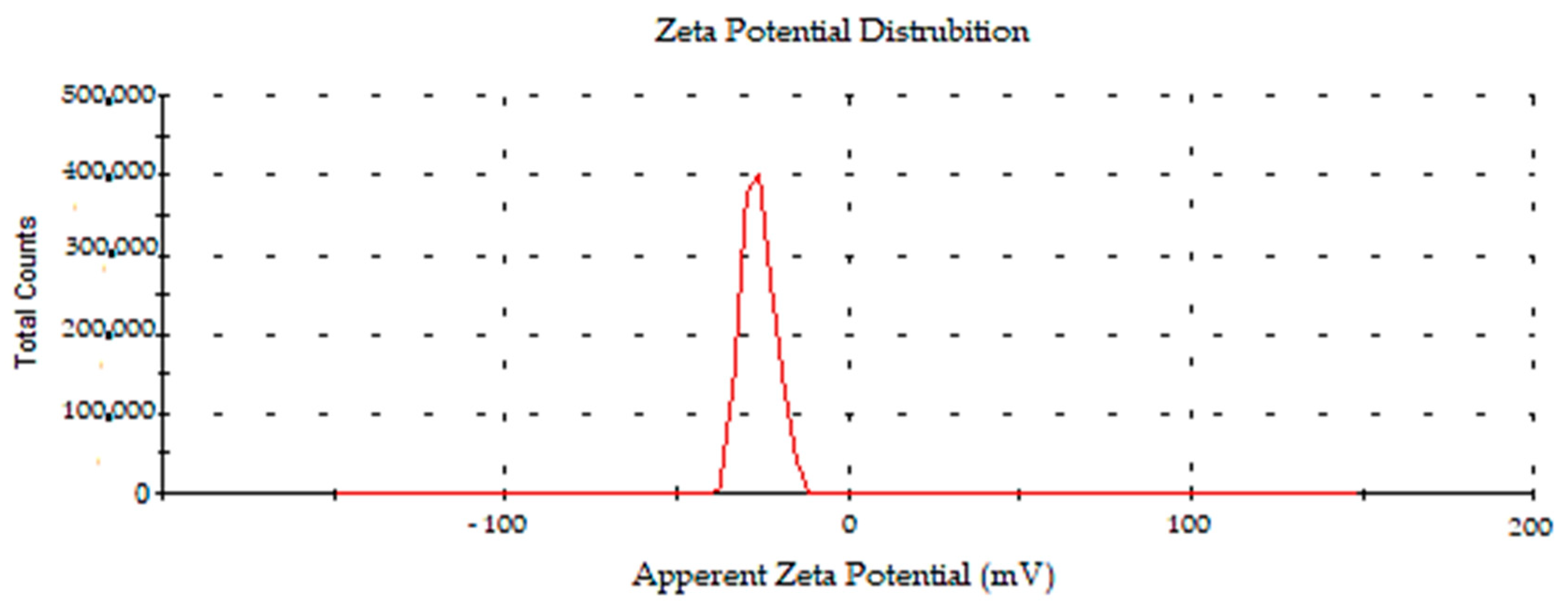
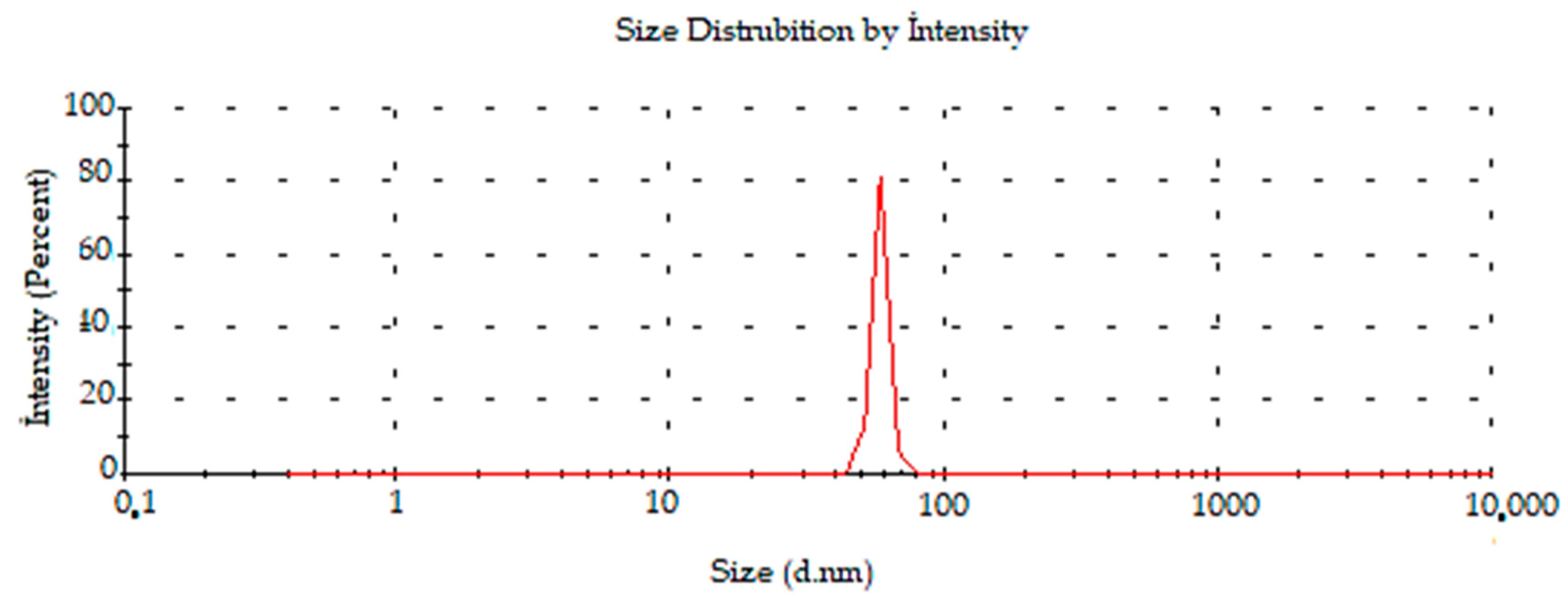
2.2.6. Surface Charge and Size Distributions of Synthesized AC-AuNPs
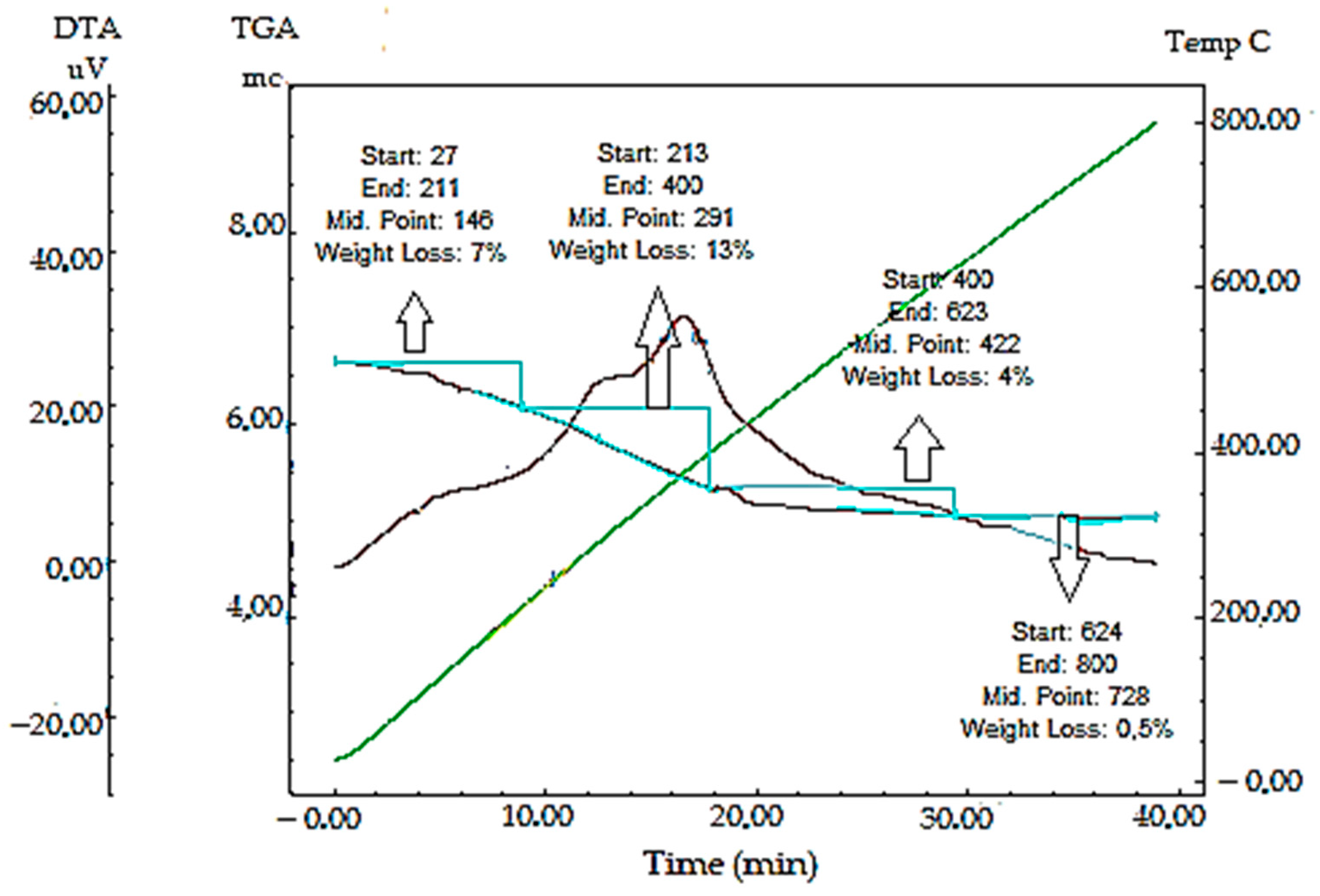
2.2.7. TGA-DTA Analysis Results of Synthesized AC-AuNPs
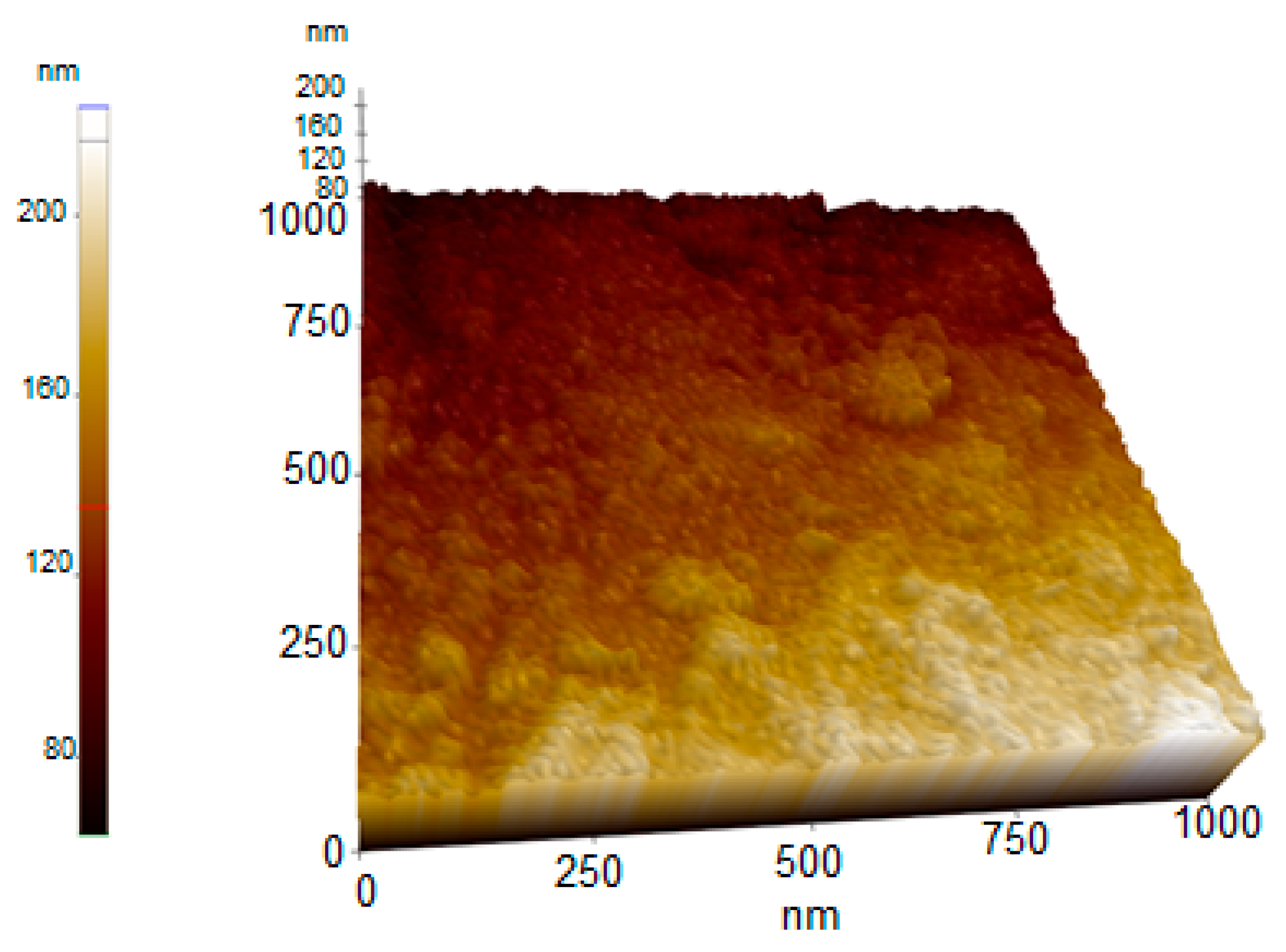
2.2.8. AFM Analysis Results of Synthesized AC-AuNPs
2.3. Biomedical Application of AC-AuNPs
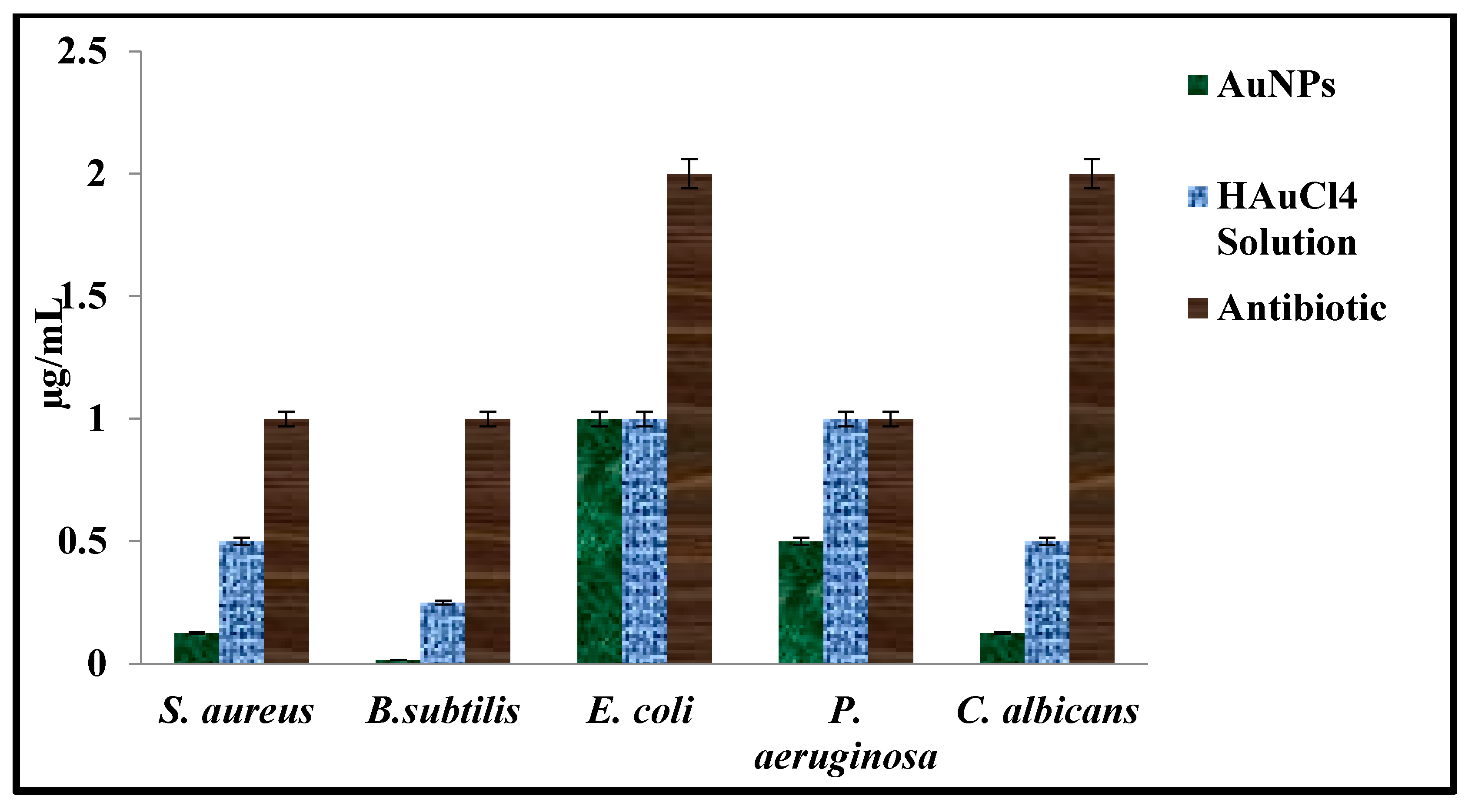
2.3.1. Antimicrobial Assay
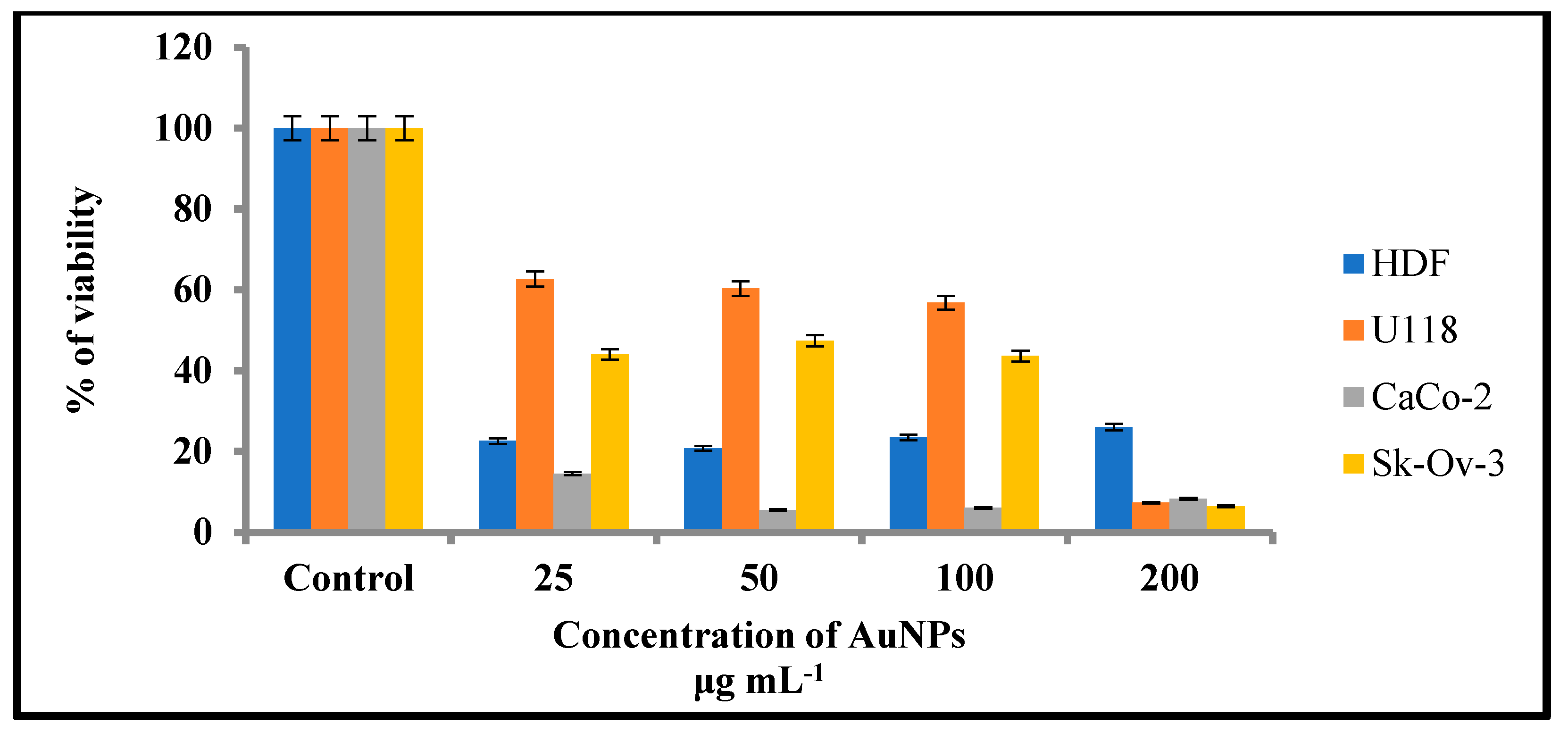
2.3.2. Assessment of Cell Viability—MTT Assay
3. Materials and Methods
3.1. Materials
3.1.1. Plant Materials
3.1.2. Standard and Reagents
3.2. Methods
3.2.1. Determination of Phenolic Compounds by LC-ESI-MS/MS
3.2.2. Synthesis and Characterization of AuNPs
3.2.3. Determination of Growth Suppression Effects of AC-AuNPs against Pathogenic Microorganisms
3.2.4. Determination of Anticancer Effects of AC-AuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Kwon, H.J.; Shin, K.; Soh, M.; Chang, H.; Kim, J.; Lee, J.; Ko, G.; Kim, B.H.; Kim, D.; Hyeon, T. Large-scale synthesis and medical applications of uniform-sized metal oxide nanoparticles. Adv. Mater. 2018, 30, 1704290. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Yao, B.; Folorunso, A.S. Green synthesis, characterization, and antibacterial investigation of synthesized gold nanoparticles (AuNPs) from Garcinia kola pulp extract. Plasmonics 2021, 16, 157–165. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine; Spherical Nucleic Acids: Stanford, CA, USA, 2020; pp. 55–90. [Google Scholar]
- Dou, X.; Wang, X.; Qian, S.; Liu, N.; Yuan, X. From understanding the roles of tetraoctylammonium bromide in the two-phase Brust–Schiffrin method to tuning the size of gold nanoclusters. Nanoscale 2020, 12, 19855–19860. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2020, 14, 53. [Google Scholar] [CrossRef]
- Rehman, A.; John, P.; Bhatti, A. Biogenic selenium nanoparticles: Potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. J. Nanomater. 2021, 11, 2005. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev. Mol. Diagn. 2021, 21, 627–630. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V.V. Photothermal and photodynamic therapy of tumors with plasmonic nanoparticles: Challenges and prospects. Materials 2022, 15, 1606. [Google Scholar] [CrossRef]
- Ferreira, D.; Fontinha, D.; Martins, C.; Pires, D.; Fernandes, A.R.; Baptista, P.V. Gold nanoparticles for vectorization of nucleic acids for cancer therapeutics. Molecules 2020, 25, 3489. [Google Scholar] [CrossRef]
- Ye, J.; Wen, Q.; Wu, Y.; Fu, Q.; Zhang, X.; Wang, J.; Gao, S.; Song, J. Plasmonic anisotropic gold nanorods: Preparation and biomedical applications. Nano Res. 2022, 15, 6372–6398. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef]
- Nawade, B.; Yahyaa, M.; Reuveny, H.; Shaltiel-Harpaz, L.; Eisenbach, O.; Faigenboim, A.; Holland, D.; Ibdah, M. Profiling of volatile terpenes from almond (Prunus dulcis) young fruits and characterization of seven terpene synthase genes. Plant Sci. 2019, 287, 110187. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Özcan, M.M. Characterization of physico-chemical and bioactive properties of oils of some important almond cultivars by cold press and soxhlet extraction. J. Food Sci. Technol. 2020, 57, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Tlili, N.; Kirkan, B.; Sarikurkcu, C. LC–ESI–MS/MS characterization, antioxidant power and inhibitory effects on α-amylase and tyrosinase of bioactive compounds from hulls of Amygdalus communis: The influence of the extracting solvents. Ind. Crops Prod. 2019, 128, 147–152. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M.; Safavi, M. Sour-Cherry Seed Polyphenol Contents, Antioxidant Activity and Nutritional Components as a Potential Bioactive Source. Nutr. Food Sci. Res. 2022, 9, 19–29. [Google Scholar] [CrossRef]
- Sahiba, N.; Sethiya, A.K.; Agarwal, D.; Agarwal, S. An Overview on Immunity Booster Foods in Coronavirus Disease (COVID-19). Comb. Chem. High Throughput Screen. 2022, 26, 1251–1284. [Google Scholar] [CrossRef]
- Fraihat, A.; Hamdan, F.R.; Abu-Irmaileh, B.; Abbasi, R.; Abu-Irmaileh, B.; Bustanji, Y. Evaluation of the antiproliferative Activities of Anthemis bornmuelleri L. and Amygdalis communis L. Extracts Against six Human Cancer cell lines. Res. J. Pharm. Technol. 2018, 11, 2512–2516. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of polyphenolic compounds extracted from different varieties of almond hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond by-products: Valorization for sustainability and competitiveness of the industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Sheng, Q.; Yi, L.; Zhong, B.; Wu, X.; Liu, L.; Zhang, B. Shikimic acid biosynthesis in microorganisms: Current status and future direction. Biotechnol. Adv. 2023, 62, 108073. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Chen, S.; Wang, L.; Zhao, X.H.; Piao, C.L. Pharmacological effects of polydatin in the treatment of metabolic diseases: A review. Phytomedicine 2022, 102, 154161. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evid.-Based Complement. Alternat. Med. 2021, 2021, 9913179. [Google Scholar] [CrossRef] [PubMed]
- Younis, F.A.; Ahmed, H.A.; Ahmed, F.M.; Awad, R.M.; Gibril, M. Green Synthesis and Characterization of Gold Nanoparticles (AuNPs) Using Fenugreek Seeds Extract (Trigonella foenum-graecum). Eur. J. Pharm. Sci. 2018, 5, 100–107. [Google Scholar]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Hussain, M.D. Process optimization for green synthesis of gold nanoparticles mediated by extract of Hygrophila spinosa T. Anders and their biological applications. Phys. E Low-Dimens. Syst. Nanostructures 2020, 121, 113830. [Google Scholar] [CrossRef]
- Vahidi, H.; Kobarfard, F.; Kosar, Z.; Mahjoub, M.A.; Saravanan, M.; Barabadi, H. Mycosynthesis and characterization of selenium nanoparticles using standard penicillium chrysogenum PTCC 5031 and their antibacterial activity: A novel approach in microbial nanotechnology. Nanomed. J. 2020, 7, 315–323. [Google Scholar] [CrossRef]
- Kumar, P.S.; Jeyalatha, M.V.; Malathi, J.; Ignacimuthu, S. Anticancer effects of one-pot synthesized biogenic gold nanoparticles (Mc-AuNPs) against laryngeal carcinoma. J. Drug Deliv. Sci. Technol. 2018, 44, 118–128. [Google Scholar] [CrossRef]
- Keskin, C.; Atalar, M.N.; Firat Baran, M.; Baran, A. Environmentally friendly rapid synthesis of gold nanoparticles from Artemisia absinthium plant extract and application of antimicrobial activities. J. Inst. Sci. Technol. 2021, 11, 365–375. [Google Scholar] [CrossRef]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, antioxidant and anticancer activities of gold nanoparticles green synthesized using Mangifera indica seed aqueous extract. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Eisa, N.E.; Virk, P.; Hendi, A.A.; Ortashi, K.M.; Mahgoub, A.S.; Eissa, F.Z. Green synthesis of gold nanoparticles: Preparation, characterization, cytotoxicity, and anti-bacterial activities. Mater. Lett. 2019, 256, 126608. [Google Scholar] [CrossRef]
- Baran, A.; Baran, M.F.; Keskin, C.; Kandemir, S.I.; Valiyeva, M.; Mehraliyeva, S.; Khalilov, R.; Eftekhari, A. Ecofriendly/rapid synthesis of silver nanoparticles using extract of waste parts of artichoke (Cynara scolymus L.) and evaluation of their cytotoxic and antibacterial activities. J. Nanomater. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Nadhe, S.B.; Wadhwani, S.A.; Singh, R.; Chopade, B.A. Green synthesis of AuNPs by Acinetobacter sp. GWRVA25: Optimization, characterization, and its antioxidant activity. Front. Chem. 2020, 8, 474. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Malik, N.; Cho, M.H.; Khan, M.M. Recent progress of algae and blue–green algae-assisted synthesis of gold nanoparticles for various applications. Bioprocess Biosyst. Eng. 2019, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Tripathy, A.; Behera, M.; Rout, A.S.; Biswal, S.K.; Phule, A.D. Optical, structural, and antimicrobial study of gold nanoparticles synthesized using an aqueous extract of mimusops elengi raw fruits. Biointerface Res. Appl. Chem. 2020, 10, 7085–7096. [Google Scholar] [CrossRef]
- Chinnaiyan, S.K.; Soloman, A.M.; Perumal, R.K.; Gopinath, A.; Balaraman, M. 5 Fluorouracil-loaded biosynthesised gold nanoparticles for the in vitro treatment of human pancreatic cancer cell. IET Nanobiotechnol. 2019, 13, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Chellapandian, C.; Ramkumar, B.; Puja, P.; Shanmuganathan, R.; Pugazhendhi, A.; Kumar, P. Gold nanoparticles using red seaweed Gracilaria verrucosa: Green synthesis, characterization and biocompatibility studies. Process Biochem. 2019, 80, 58–63. [Google Scholar] [CrossRef]
- Baran, M.F. Synthesis, characterization and investigation of antimicrobial activity of silver nanoparticles from Cydonia oblonga leaf. Appl. Ecol. Environ. Res. 2019, 17, 2583–2592. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Rapid green synthesis of gold nanoparticles: Synthesis, characterization and antimicrobial activities. Prog. Nutr. 2021, 23, e2021242. [Google Scholar] [CrossRef]
- Nirala, N.R.; Prakash, R. One step synthesis of AuNPs@ MoS2-QDs composite as a robust peroxidase-mimetic for instant unaided eye detection of glucose in serum, saliva and tear. Sens. Actuators B Chem. 2018, 263, 109–119. [Google Scholar] [CrossRef]
- Rauf, A.; Ahmad, T.; Khan, A.; Maryam; Uddin, G.; Ahmad, B.; Mabkhot, Y.N.; Bawazeer, S.; Riaz, N.; Malikovna, B.K.; et al. Green synthesis and biomedicinal applications of silver and gold nanoparticles functionalized with methanolic extract of Mentha longifolia. Artif. Cells Nanomed. Biotechnol. 2021, 49, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ. Sci. Pollut. Res. 2017, 24, 17347–17357. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.A.; Raman, T.; Veerappan, A. Future prospects of antibacterial metal nanoparticles as enzyme inhibitor. Mater. Sci. Eng. C 2016, 68, 939–947. [Google Scholar] [CrossRef]
- Baran, A.; Hatipoğlu, A.; Firat Baran, M.; Aktepe, N. Evaluation of Synthesis and Antimicrobial Activities of Gold Nanoparticles from Hawthorn (Crataegus monogyna) Fruit Extract. Eur. J. Sci. Technol. 2021, 32, 974–978. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Green synthesis of gold nanoparticles from Prunus cerasifera pissardii nigra leaf and their antimicrobial activities on some food pathogens. Prog. Nutr. 2021, 23, e2021241. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud. Univ. Sci. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Oh, B. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Sivakumar, A.S.; Prasannakumar, P.; Kumar, V.A.; Shim, K.S.; Yun, S.I. Anticancer activity of biologically synthesized silver and gold nanoparticles on mouse myoblast cancer cells and their toxicity against embryonic zebrafish. Mater. Sci. Eng. C 2017, 73, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Keskin, C.; Baran, A.; Baran, M.F.; Hatipoğlu, A.; Adican, M.T.; Atalar, M.N.; Huseynova, I.; Khalilov, R.; Ahmadian, E.; Yavuz, Ö.; et al. Green Synthesis, Characterization of Gold Nanomaterials using Gundelia tournefortii Leaf Extract, and Determination of Their Nanomedicinal (Antibacterial, Antifungal, and Cytotoxic) Potential. J. Nanomater. 2022, 2022, 7211066. [Google Scholar] [CrossRef]
- Mishra, P.; Ray, S.; Sinha, S.; Das, B.; Khan, M.I.; Behera, S.K.; Mishra, A. Facile bio-synthesis of gold nanoparticles by using extract of Hibiscus sabdariffa and evaluation of its cytotoxicity against U87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016, 105, 264–272. [Google Scholar] [CrossRef]
- Hutchinson, N.; Wu, Y.; Wang, Y.; Kanungo, M.; DeBruine, A.; Kroll, E.; Gilmore, D.; Eckrose, Z.; Gaston, S.; Matel, P.; et al. Green Synthesis of Gold Nanoparticles Using Upland Cress and Their Biochemical Characterization and Assessment. J. Nanomater. 2021, 12, 28. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2019, 12, 4719–4730. [Google Scholar] [CrossRef]
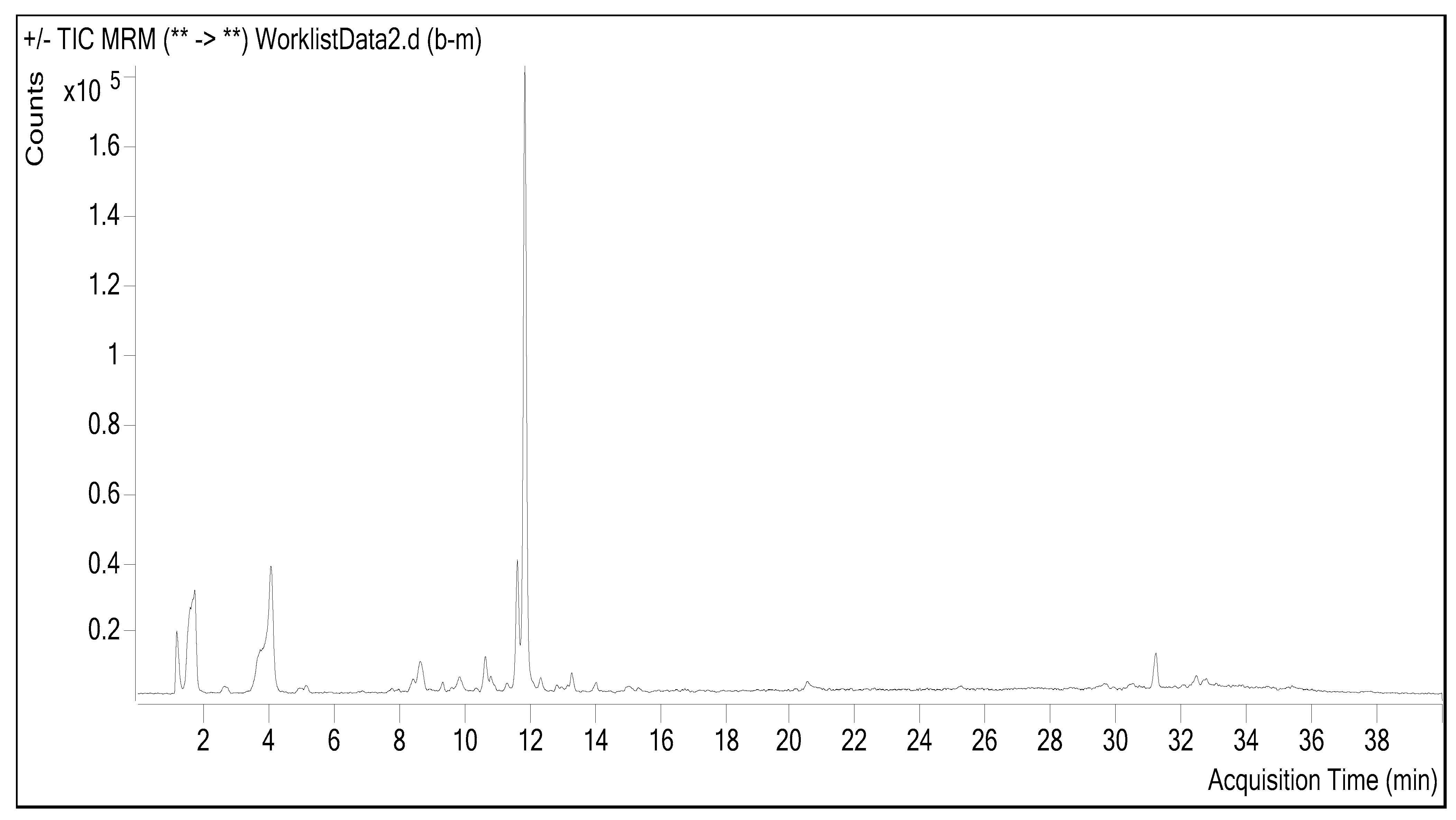
| Number | Standard | (RT) | R2 | RSD | MI (m/z) | Linearity Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) | Recovery (%) | Amygdalus communis Final Conc (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Shikimic acid | 1.20 | 0.99 | 1.92 | 159.2 | 0.1–5 | 12.1 | 16.2 | 99.80 | 18,034.30 |
| 2 | Gallic acid | 1.70 | 0.99 | 1.58 | 168.9 | 0.1–5 | 13.2 | 17.0 | 100.10 | 1213.10 |
| 3 | Protocatechuic acid | 2.70 | 0.96 | 1.39 | 152.8 | 0.1–5 | 21.9 | 38.6 | 99.72 | 425.70 |
| 4 | Gentisic acid | 2.30 | 0.99 | 1.84 | 152.7 | 0.1–5 | 18.5 | 28.2 | 99.60 | ND |
| 5 | Catechin | 4.10 | 0.99 | 2.10 | 288.6 | 0.2–10 | 55.0 | 78.0 | 100.20 | 178.10 |
| 6 | 4-Hydroxybenzoic acid | 8.70 | 0.99 | 1.20 | 137.3 | 0.2–10 | 68.4 | 88.1 | 100.30 | ND |
| 7 | Chlorogenic acid | 7.50 | 0.99 | 2.10 | 353.1 | 0.1–5 | 13.1 | 17.6 | 100.00 | ND |
| 8 | 4-Hydroxybenzaldehyde | 5.80 | 0.99 | 2.20 | 121.9 | 0.1–5 | 20.1 | 36.1 | 100.00 | ND |
| 9 | Vanillic acid | 7.80 | 0.99 | 1.90 | 107.9 | 1–50 | 141.9 | 164.9 | 100.20 | ND |
| 10 | Caffeic Acid | 6.10 | 0.99 | 1.10 | 178.8 | 0.05–2.5 | 7.7 | 9.5 | 100.20 | ND |
| 11 | Epicatechin | 4.10 | 0.99 | 1.5 | 288.8 | 1–50 | 139.6 | 161.6 | 100.10 | ND |
| 12 | Syringic acid | 8.40 | 0.99 | 1.20 | 197.1 | 1–50 | 82.3 | 104.5 | 100.10 | ND |
| 13 | P-coumaric acid | 8.50 | 0.99 | 1.90 | 162.9 | 0.1–5 | 25.9 | 34.9 | 100.50 | 162.40 |
| 14 | Salicylic Acid | 8.70 | 0.99 | 1.40 | 136.9 | 0.1–5 | 6.0 | 8.3 | 99.90 | 694.80 |
| 15 | Taxifolin | 9.10 | 0.99 | 1.50 | 199.2 | 0.1–5 | 9.2 | 12.1 | 99.90 | ND |
| 16 | Polydatine | 9.30 | 0.99 | 1.40 | 182.8 | 0.1–5 | 12.1 | 19.2 | 100.00 | 315.60 |
| 17 | Trans-ferulic acid | 9.50 | 0.99 | 1.40 | 196.3 | 1–50 | 11.8 | 15.6 | 99.50 | ND |
| 18 | Sinapic acid | 11.40 | 0.99 | 1.45 | 222.9 | 0.2–10 | 65.2 | 82.3 | 99.90 | ND |
| 19 | Quercimeritrin | 10.7 | 0.99 | 1.90 | 137.9 | 0.1–5 | 68.5 | 88.2 | 99.60 | 2015.4 |
| 20 | Coumarin | 10.6 | 0.99 | 2.20 | 152.3 | 0.1–5 | 214.2 | 247.3 | 99.50 | ND |
| 21 | Scutellarin | 12.9 | 0.99 | 1.30 | 198.3 | 0.1–5 | 16.2 | 21.2 | 99.50 | ND |
| 22 | O-coumaric acid | 8.5 | 0.99 | 2.10 | 163.0 | 0.1–5 | 31.8 | 40.4 | 100.00 | ND |
| 23 | Cynarin | 11.4 | 0.99 | 1.55 | 289.3 | 0.1–5 | 19.5 | 28.5 | 100.00 | ND |
| 24 | Protocatechuic ethyl ester | 11.2 | 0.99 | 1.45 | 137.2 | 0.1–5 | 15.4 | 22.2 | 100.00 | ND |
| 25 | Hyperoside | 11.6 | 0.99 | 1.80 | 289.1 | 0.1–5 | 139.5 | 161.5 | 99.90 | 1773.1 |
| 26 | Quercetin-3-glucoside | 11.9 | 0.99 | 1.75 | 304.1 | 0.1–5 | 4.9 | 6.5 | 100.00 | 2448.0 |
| 27 | Rutin | 11.9 | 0.99 | 2.10 | 610.2 | 0.1–5 | 15.9 | 22.9 | 99.80 | 16,564.6 |
| 28 | Resveratrol | 11.7 | 0.99 | 1.10 | 301.1 | 0.1–5 | 7.1 | 9.1 | 100.00 | ND |
| 29 | Naringin | 11.1. | 0.99 | 1.25 | 269.8 | 0.1–5 | 2.6 | 3.9 | 100.60 | ND |
| 30 | Rosmarinic acid | 12.5 | 0.99 | 1.55 | 360.1 | 0.1–5 | 16.2 | 21.2 | 100.60 | ND |
| 31 | Quercetin-3-D-xyloside | 12.5 | 0.99 | 1.65 | 305.2 | 0.1–5 | N.A | N.A | 100.10 | ND |
| 32 | Hesperidin | 12.5 | 0.99 | 1.60 | 450.1 | 0.1–5 | 19.0 | 26.0 | 99.70 | ND |
| 33 | Kaemerol-3-glucoside | 13.3 | 0.99 | 1.35 | 285.1 | 0.1–5 | 10.4 | 15.6 | 99.90 | 53.2 |
| 34 | Fisetin | 13.4 | 0.99 | 1.25 | 285.0 | 0.1–5 | 10.1 | 12.7 | 99.80 | ND |
| 35 | Oleuropein | 13.9 | 0.99 | 1.20 | 540.2 | 0.1–5 | 24.6 | 30.6 | 99.90 | ND |
| 36 | Baicalin | 13.8 | 0.99 | 1.25 | 154.2 | 0.1–5 | 24.3 | 30.2 | 99.90 | ND |
| 37 | Trans-cinnamic acid | 14.3 | 0.99 | 1.30 | 149.8 | 0.1–5 | 215.1 | 240.2 | 99.90 | ND |
| 38 | Ellagic acid | 15.0 | 0.99 | 1.50 | 302.1 | 0.1–5 | 56.9 | 71.1 | 100.10 | ND |
| 39 | Quercetin | 15.0 | 0.99 | 1.65 | 301.1 | 0.1–5 | 15.5 | 19.0 | 99.70 | 298.7 |
| 40 | Naringenin | 15.1 | 0.99 | 2.35 | 270.8 | 0.1–5 | 2.6 | 3.9 | 100.60 | 66.3 |
| 41 | Silibinin | 15.8 | 0.99 | 2.20 | 440.1 | 0.1–5 | 19.3 | 28.3 | 99.90 | ND |
| 42 | Hesperetin | 15.4 | 0.99 | 2.50 | 301.2 | 0.1–5 | 7.1 | 9.1 | 100.00 | ND |
| 43 | Morin | 13.2 | 0.99 | 2.10 | 152.9 | 0.1–5 | 22.3 | 28.4 | 100.00 | ND |
| 44 | Kaempferol | 19.5 | 0.99 | 1.85 | 286.1 | 0.1–5 | 10.2 | 15.4 | 99.90 | ND |
| 45 | Tamarixetin | 17.5 | 0.99 | 1.90 | 163.1 | 0.1–5 | 25.8 | 34.8 | 99.90 | ND |
| 46 | Baicalein | 17.6 | 0.99 | 1.75 | 158.2 | 0.1–5 | 23.9 | 32.7 | 99.90 | ND |
| 47 | 7-Hydroxyflavone | 18.6 | 0.99 | 1.65 | 222.2 | 0.1–5 | 64.9 | 82.1 | 99.90 | ND |
| 48 | 6-Hydroxyflavone | 19.8 | 0.99 | 1.55 | 138.1 | 0.1–5 | 5.9 | 8.2 | 100.00 | ND |
| 49 | Biochanin A | 20.5 | 0.99 | 1.20 | 145.2 | 0.1–5 | 212.4 | 244.2 | 99.80 | 89.5 |
| 50 | Chrysin | 20.8 | 0.99 | 1.55 | 147.1 | 0.1–5 | 0.012 | 0.012 | 99.90 | ND |
| 51 | 5-Hydroxyflavone | 23.9 | 0.99 | 1.65 | 252.7 | 0.1–5 | 9.7 | 11.5 | 100.00 | ND |
| 52 | 6,2,4-Trimetoxyflavone | 24.9 | 0.99 | 1.70 | 585.2 | 0.1–5 | 11.1 | 15.5 | 99.90 | ND |
| 53 | Diosgenin | 30.5 | 0.99 | 1.10 | 285.5 | 0.1–5 | 11.8 | 16.6 | 100.00 | 36.9 |
| Mass Loss Point | Temperature (°C) | Mass Loss (%) |
|---|---|---|
| First | 27–211 | 7 |
| Second | 213–400 | 13 |
| Third | 400–623 | 4 |
| Fourth | 624–800 | 0.5 |
| Tested Microorganism | AuNPs µg mL−1 | HAuCI4 Solution µg mL−1 | * Antibiotics µg mL−1 |
|---|---|---|---|
| E. coli | 1.00 | 1.00 | 2.00 |
| P. aeruginosa | 0.50 | 1.00 | 1.00 |
| S. aureus | 0.12 | 0.50 | 1.00 |
| B. subtilis | 0.02 | 0.25 | 1.00 |
| C. albicans | 0.12 | 0.50 | 2.00 |
| MIC Values of AuNPs µg mL−1 | ||||
|---|---|---|---|---|
| Green Synthesis Source | Gram Positive S.aureus/B. subtilis | Gram Negative E.coli/P. aeruginosa | C. albicans | Ref. |
| Cydonia oblonga | 0.15 | 0.05 | 0.13 | [41] |
| Crataegus monogyna | 0.05/0.02 | 0.50/0.25 | 0.11 | [48] |
| Prunus cerasifera | 0.25/0.12 | 1.00/0.50 | 0.50 | [49] |
| Jatropha integerrima Jacq. | 10.0/5.00 | 2.5 | - | [50] |
| Zingiber officinale | 30 | - | - | [51] |
| Cell Lines | Concentrations (µg mL−1) | |||
|---|---|---|---|---|
| 25 | 50 | 100 | 200 | |
| HDF | 22.6 * | 20.8 | 23.5 | 26.1 |
| U118 | 62.6 | 60.3 | 56.8 | 7.3 |
| Caco-2 | 14.5 | 5.5 | 6.0 | 8.3 |
| Sk-ov-3 | 44.0 | 47.4 | 43.6 | 6.4 |
| Green Synthesis Source | Cell Line | Dimension (nm) | Shape | Effective Concentration (µg mL−1) | Ref. |
|---|---|---|---|---|---|
| H. spinosa | Sk-ov-3 | 68.4 | Spherical | 47.5 | [28] |
| M. indica | MCF-7 | 19.5 | Spherical | 400 | [32] |
| G. tournefortii | U118 | 5–10 | Spherical | 100 | [53] |
| H. sabdariffa | U87 | 30 | Spherical | 2.5 | [54] |
| B. verna | HeLa | 11 | Spherical | 2 | [55] |
| C. baccata | Caco-2 | 8.4 | Spherical | 400 | [56] |
| A. communis | Caco-2, Sk-ov-3, U118 | 58 | Spherical | 25 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, M.F.; Keskin, C.; Baran, A.; Eftekhari, A.; Omarova, S.; Khalilov, R.; Adican, M.T.; Rosić, G.; Selakovic, D.; Yıldıztekin, M.; et al. The Investigation of the Chemical Composition and Applicability of Gold Nanoparticles Synthesized with Amygdalus communis (Almond) Leaf Aqueous Extract as Antimicrobial and Anticancer Agents. Molecules 2023, 28, 2428. https://doi.org/10.3390/molecules28062428
Baran MF, Keskin C, Baran A, Eftekhari A, Omarova S, Khalilov R, Adican MT, Rosić G, Selakovic D, Yıldıztekin M, et al. The Investigation of the Chemical Composition and Applicability of Gold Nanoparticles Synthesized with Amygdalus communis (Almond) Leaf Aqueous Extract as Antimicrobial and Anticancer Agents. Molecules. 2023; 28(6):2428. https://doi.org/10.3390/molecules28062428
Chicago/Turabian StyleBaran, Mehmet Fırat, Cumali Keskin, Ayşe Baran, Aziz Eftekhari, Sabina Omarova, Rovshan Khalilov, Mehmet Tevfik Adican, Gvozden Rosić, Dragica Selakovic, Mahmut Yıldıztekin, and et al. 2023. "The Investigation of the Chemical Composition and Applicability of Gold Nanoparticles Synthesized with Amygdalus communis (Almond) Leaf Aqueous Extract as Antimicrobial and Anticancer Agents" Molecules 28, no. 6: 2428. https://doi.org/10.3390/molecules28062428
APA StyleBaran, M. F., Keskin, C., Baran, A., Eftekhari, A., Omarova, S., Khalilov, R., Adican, M. T., Rosić, G., Selakovic, D., Yıldıztekin, M., Kurt, K., Aytuğ Ava, C., & Atalar, M. N. (2023). The Investigation of the Chemical Composition and Applicability of Gold Nanoparticles Synthesized with Amygdalus communis (Almond) Leaf Aqueous Extract as Antimicrobial and Anticancer Agents. Molecules, 28(6), 2428. https://doi.org/10.3390/molecules28062428






