Biofilm Formation and Control of Foodborne Pathogenic Bacteria
Abstract
1. Introduction
2. Overview of Biofilms
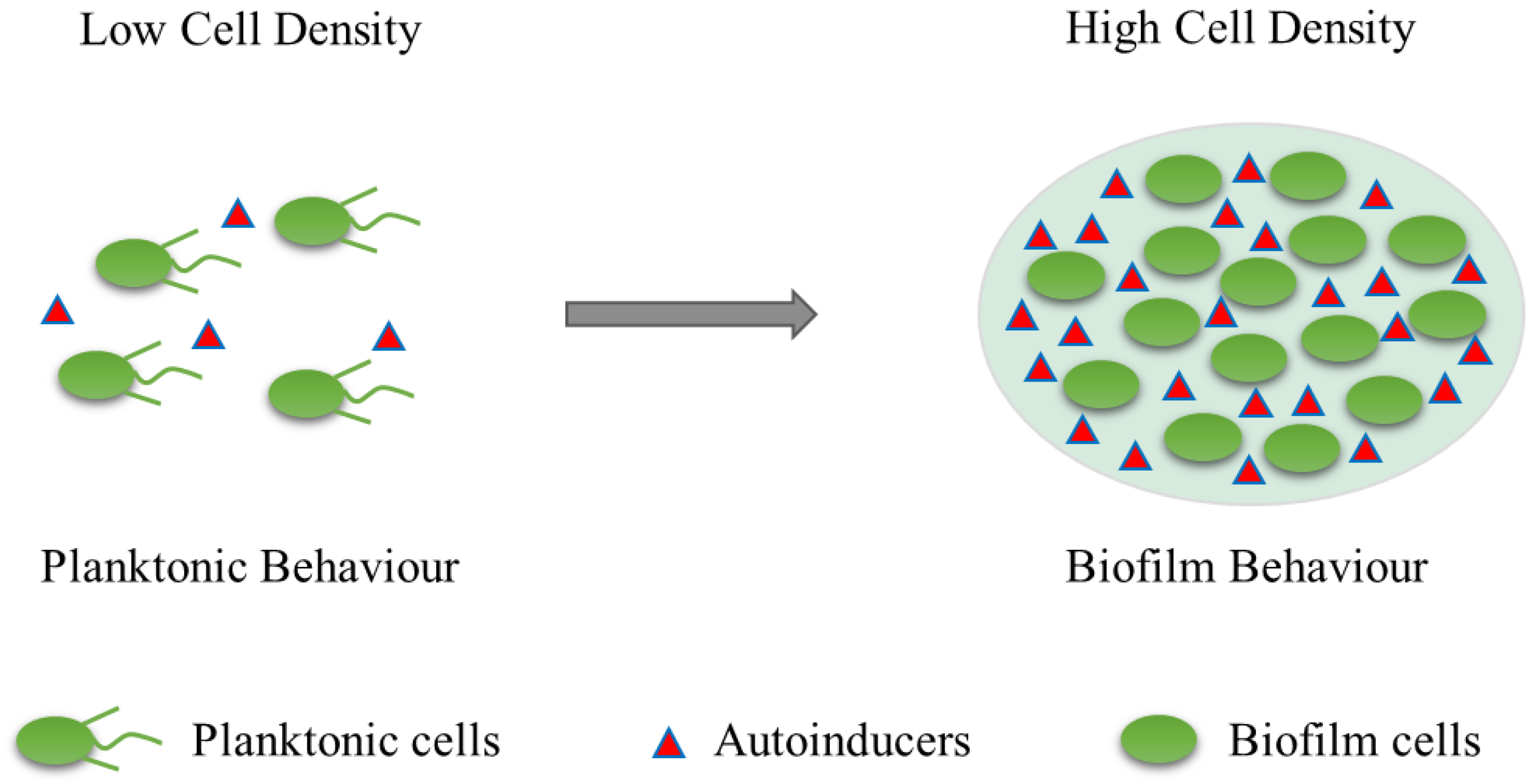
3. Biofilm Formation
4. Biofilm and Food Safety
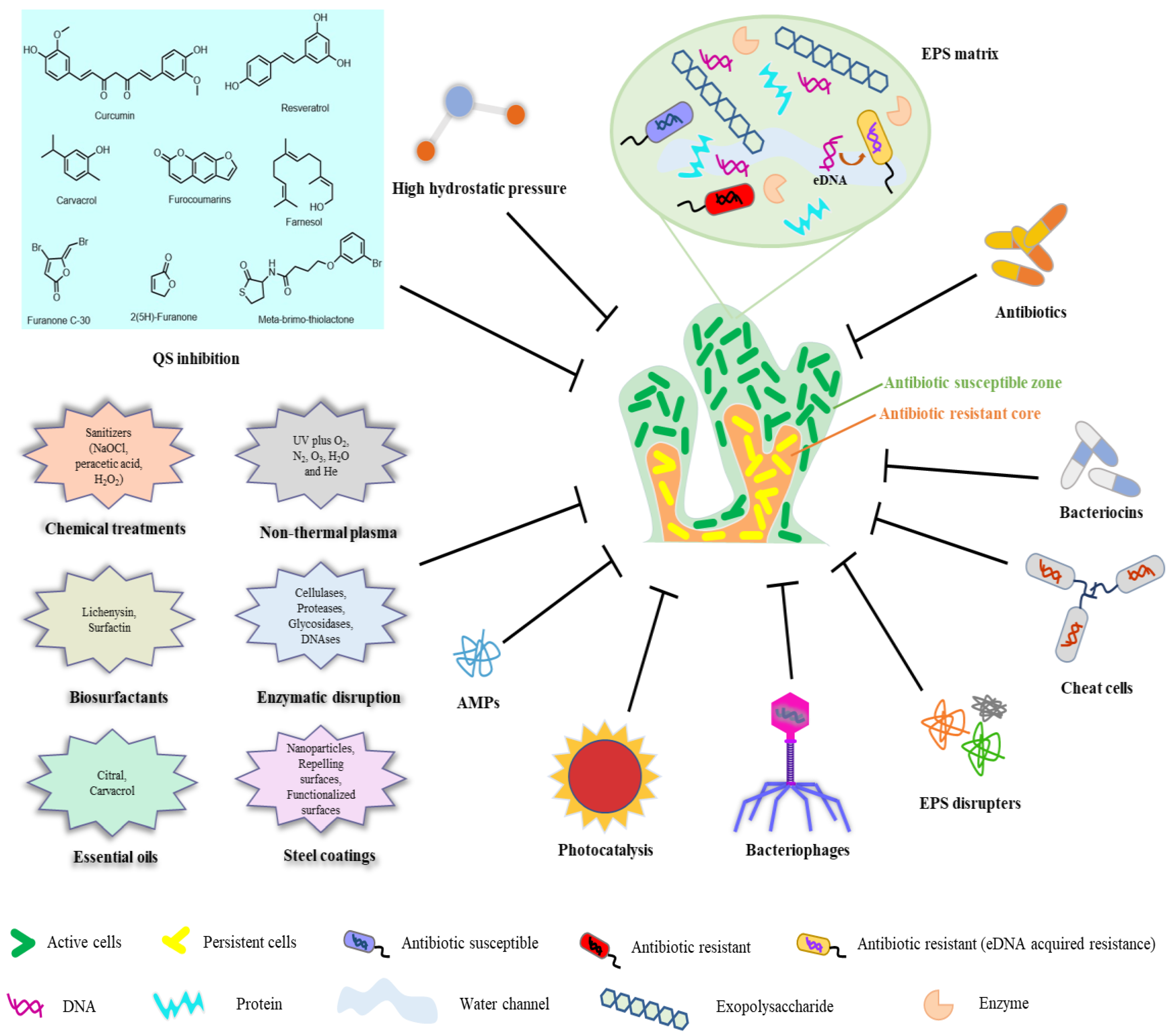
5. Biofilm Removal and Control in the Food Industry
6. Summary of Common Foodborne Pathogens and Their Implication for Food Safety
6.1. Listeria monocytogenes
6.2. Salmonella enterica
6.3. Staphylococcus aureus
6.4. Pseudomonas aeruginosa
6.5. Escherichia coli
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.-H. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Winkelstroter, L.; Teixeira, F.B.D.R.; Silva, E.P.; Alves, V.F.; De Martinis, E.C.P. Unraveling microbial biofilms of importance for food microbiology. Microb. Ecol. 2014, 68, 35–46. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control. 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Delgado, S.; Vázquez-Sánchez, D.; Martínez, B.; Cabo, M.L.; Rodríguez, A.; Herrera, J.J.; García, P. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef]
- Herrera, F.; Santos, J.; Otero, A.; Garcia-Lopez, M.-L. Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in spain. J. Appl. Microbiol. 2006, 100, 527–536. [Google Scholar] [CrossRef]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.-M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. npj Biofilms. Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef]
- Tremblay, Y.D.; Lévesque, C.; Segers, R.P.; Jacques, M. Method to grow Actinobacillus pleuropneumoniae biofilm on a biotic surface. BMC Vet. Res. 2013, 9, 213. [Google Scholar] [CrossRef]
- Accepts, A.E.M.; Society, A.; Reserved, A.R. Biofilm formation on biotic and abiotic surfaces in the presence of antimicrobials by Escherichia coli isolates from cases of bovine mastitis. Appl. Environ. Microbiol. 2014, 80, 6136–6145. [Google Scholar] [CrossRef]
- Bogino, P.C.; Oliva, M.D.L.M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef]
- Raghupathi, P.K.; Liu, W.; Sabbe, K.; Houf, K.; Burmølle, M.; Sørensen, S.J.; Raghupathi, P.K.; Liu, W.; Sabbe, K.; Houf, K.; et al. Synergistic interactions within a multispecies biofilm enhance individual species protection against grazing by a pelagic protozoan. Front. Microbiol. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Koczan, J.M.; Lenneman, B.R.; McGrath, M.J.; Sundin, G.W. Cell surface attachment structures contribute to biofilm formation and xylem colonization by erwinia amylovora. Appl. Environ. Microbiol. 2011, 77, 7031–7039. [Google Scholar] [CrossRef]
- Worthington, R.J.; Richards, J.J.; Melander, C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 2012, 10, 7457–7474. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Røder, H.L.; Herschend, J.; Russel, J.; Andersen, M.F.; Madsen, J.S.; Sørensen, S.J.; Burmølle, M. Enhanced bacterial mutualism through an evolved biofilm phenotype. ISME J. 2018, 12, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.; Ha, S. Current and recent advanced strategies for combating biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull 2011, 36, 347–355. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (Bio)control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold. Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Sanchez-Vizuete, P.; Orgaz, B.; Aymerich, S.; Le Coq, D.; Briandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Han, Q.; Song, X.; Zhang, Z.; Fu, J.; Wang, X.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. Removal of foodborne pathogen biofilms by acidic electrolyzed water. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Meesilp, N.; Mesil, N. Effect of microbial sanitizers for reducing biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa on stainless steel by cultivation with UHT milk. Food Sci. Biotechnol. 2019, 28, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial biofilms in the food industry—A comprehensive review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.S.; Flint, S.; Schmid, J.; Brooks, J.D. The role of surface charge and hydrophobicity in the attachment of Anoxybacillus flavithermus isolated from milk powder. J. Ind. Microbiol. Biotechnol. 2010, 37, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Vandriesche, G.; Coorevits, A.; Coudijzer, K.; De Jonghe, V.; Dewettinck, K.; De Vos, P.; Devreese, B.; Heyndrickx, M.; De Block, J. Heterogeneity of heat-resistant proteases from milk Pseudomonas species. Int. J. Food Microbiol. 2009, 133, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Korfhagen, T.R.; Irvin, R.T.; Schurr, M.J.; Sauer, K.; Lau, G.W.; Sutton, M.D.; Yu, H.; Hoiby, N. Pseudomonas aeruginosa biofilm infections in cystic fibrosis: Insights into pathogenic processes and treatment strategies. Expert. Opin. Ther. Targets. 2010, 14, 117–130. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Desvaux, M.; Hébraud, M.; Møretrø, T.; Langsrud, S.; Doulgeraki, A.; Nychas, G.-J.; Kačániová, M.; Czaczyk, K.; et al. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol. 2015, 6, 1–26. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Jahid, I.K.; Do Ha, S. The paradox of mixed-species biofilms in the context of food safety. Compr. Rev. Food Sci. Food Saf. 2014, 13, 990–1011. [Google Scholar] [CrossRef]
- Yin, W.; Xu, S.; Wang, Y.; Zhang, Y.; Chou, S.-H.; Galperin, M.Y.; He, J. Ways to control harmful biofilms: Prevention, inhibition, and eradication. Crit. Rev. Microbiol. 2021, 47, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Toté, K.; Horemans, T.; Vanden Berghe, D.; Maes, L.; Cos, P. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2010, 76, 3135–3142. [Google Scholar] [CrossRef]
- Cabeça, T.K.; Pizzolitto, A.C.; Pizzolitto, E.L. Activity of disinfectants against foodborne pathogens in suspension and adhered to stainless steel surfaces. Braz. J. Microbiol. 2012, 43, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Shikongo-Nambabi, M.N.N.N.; Kachigunda, B.; Venter, S.N. Evaluation of oxidising disinfectants to control vibrio biofilms in treated seawater used for fish processing. Water SA 2010, 36, 215–220. [Google Scholar]
- Park, H.W.; Yoon, W.B. A quantitative microbiological exposure assessment model for Bacillus cereus in pasteurized rice cakes using computational fluid dynamics and monte carlo simulation. Food Res. Int. 2019, 125, 108562. [Google Scholar] [CrossRef]
- Park, H.W.; Yoon, W.B. Computational fluid dynamics (CFD) modelling and application for sterilization of foods: A review. Processes 2018, 6, 62. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, S.H.; Kang, D.H. Thermal and non-thermal treatment effects on Staphylococcus aureus biofilms formed at different temperatures and maturation periods. Food Res. Int. 2020, 137, 1–8. [Google Scholar] [CrossRef]
- Chang, S.-S.; Han, A.; Reyes-De-Corcuera, J.; Powers, J.; Kang, D.-H. Evaluation of steam pasteurization in controlling Salmonella serotype enteritidis on raw almond surfaces. Lett. Appl. Microbiol. 2010, 50, 393–398. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, S.-H.; Kim, S.-S.; Kang, D.-H. Inactivation of Staphylococcus aureus biofilms on food contact surfaces by superheated steam treatment. J. Food Prot. 2019, 82, 1496–1500. [Google Scholar] [CrossRef]
- Ban, G.H.; Yoon, H.; Kang, D.H. A comparison of saturated steam and superheated steam for inactivation of Escherichia coli O157, H7, Salmonella typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Food Control. 2014, 40, 344–350. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Borges, A.; Borges, F.; Simões, M. Repurposing ibuprofen to control Staphylococcus aureus biofilms. Eur. J. Med. Chem. 2019, 166, 197–205. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in food processing environments: Challenges and opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Coronel-León, J.; Marqués, A.; Bastida, J.; Manresa, A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016, 120, 99–111. [Google Scholar] [CrossRef]
- Disson, O.; Moura, A.; Lecuit, M. Making sense of the biodiversity and virulence of Listeria monocytogenes. Trends. Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Rodríguez-Herrera, J.J.; Vázquez-Sánchez, D.; Cabo, M.L. Current knowledge on Listeria monocytogenes biofilms in food-related environments: Incidence, resistance to biocides, ecology and biocontrol. Foods 2018, 7, 85. [Google Scholar] [CrossRef]
- Rothrock, M.J.J.; Davis, M.L.; Locatelli, A.; Bodie, A.; McIntosh, T.G.; Donaldson, J.R.; Ricke, S.C. Listeria occurrence in poultry flocks: Detection and potential implications. Front. Vet. Sci. 2017, 4, 125. [Google Scholar] [CrossRef]
- Ćwiek, K.; Korzekwa, K.; Tabiś, A.; Bania, J.; Bugla-Płoskońska, G.; Wieliczko, A. Antimicrobial resistance and biofilm formation capacity of Salmonella enterica serovar enteritidis strains isolated from poultry and humans in poland. Pathogens 2020, 9, 643. [Google Scholar] [CrossRef]
- Abeysundara, P.D.A.; Dhowlaghar, N.; Nannapaneni, R.; Schilling, M.W.; Mahmoud, B.; Sharma, C.S.; Ma, D.P. Salmonella enterica growth and biofilm formation in flesh and peel cantaloupe extracts on four food-contact surfaces. Int. J. Food Microbiol. 2018, 280, 17–26. [Google Scholar] [CrossRef]
- Farahani, R.K.; Ehsani, P.; Ebrahimi-Rad, M.; Khaledi, A. Molecular detection, virulence genes, biofilm formation, and antibiotic resistance of Salmonella enterica serotype enteritidis isolated from poultry and clinical samples. Jundishapur. J. Microbiol. 2018, 11, e69504. [Google Scholar] [CrossRef]
- Nguyen, H.D.N.; Yang, Y.S.; Yuk, H.G. Biofilm formation of Salmonella typhimurium on stainless steel and acrylic surfaces as affected by temperature and pH level. LWT 2014, 55, 383–388. [Google Scholar] [CrossRef]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal food-borne disease: An ongoing challenge in public health. Biomed. Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Parastan, R.; Kargar, M.; Solhjoo, K.; Kafilzadeh, F. Staphylococcus aureus biofilms: Structures, antibiotic resistance, inhibition, and vaccines. Gene Rep. 2020, 20, 100739. [Google Scholar] [CrossRef]
- Crone, S.; Vives-Flórez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martínez-García, E.; Rojas-Acosta, C.; Gomez-Puerto, M.C.; Calum, H.; et al. The environmental occurrence of Pseudomonas aeruginosa. Apmis 2020, 128, 220–231. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Ban, G.H.; Kang, D.H. Inactivation of Escherichia coli O157, H7, Salmonella typhimurium, and Listeria monocytogenes on cherry tomatoes and oranges by superheated steam. Food Res. Int. 2018, 112, 38–47. [Google Scholar] [CrossRef]
- Rohde, L.E.; Clausell, N.; Ribeiro, J.P.; Goldraich, L.; Netto, R.; Dec, G.W.; DiSalvo, T.G.; Polanczyk, C.A. Inactivation of Escherichia coli O157, H7 in biofilm on food-contact surfaces by sequential treatments of aqueous chlorine dioxide and drying. Int. J. Food Microbiol. 2014, 191, 129–134. [Google Scholar] [CrossRef]
- Kim, N.H.; Rhee, M.S. Synergistic bactericidal action of phytic acid and sodium chloride against Escherichia coli O157, H7 cells protected by a biofilm. Int. J. Food Microbiol. 2016, 227, 17–21. [Google Scholar] [CrossRef]
- Weerarathne, P.; Payne, J.; Saha, J.; Kountoupis, T.; Jadeja, R.; Jaroni, D. Evaluating the efficacy of sodium acid sulfate to reduce Escherichia coli O157, H7 and its biofilms on food-contact surfaces. LWT 2021, 139, 110501. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Bucur, F.I.; Borda, D.; Alexa, E.-A.; Neagu, C.; Nicolau, A.I. Biofilms formed by pathogens in food and food processing environments. Bact. Biofilms. 2020, 1, 1–32. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hu, Q.; Xu, F.; Ding, S.-Y.; Zhu, K. Characterization of Bacillus cereus in dairy products in china. Toxins 2020, 12, 454. [Google Scholar] [CrossRef]
- Tewari, A.; Abdullah, S. Bacillus cereus food poisoning: International and indian perspective. J. Food Sci. Technol. 2015, 52, 2500–2511. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef]
- Klančnik, A.; Šimunović, K.; Sterniša, M.; Ramić, D.; Možina, S.S.; Bucar, F. Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 2021, 20, 55–84. [Google Scholar] [CrossRef]
- Shamloo, E.; Hosseini, H.; Moghadam, Z.A.; Larsen, M.H.; Haslberger, A.; Alebouyeh, M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iran J. Vet. Res. 2019, 20, 241–254. [Google Scholar]
- Orsi, R.H.; Wiedmann, M. Characteristics and distribution of Listeria spp., including Listeria species newly described since 2009. Appl. Microbiol. Biotechnol. 2016, 100, 5273–5287. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Salazar, J.K.; Wu, Z.; Yang, W.; Freitag, N.E.; Tortorello, M.L.; Wang, H.; Zhang, W. Roles of a novel Crp/Fnr family transcription factor Lmo0753 in soil survival, biofilm production and surface attachment to fresh produce of Listeria monocytogenes. PLoS ONE 2013, 8, e75736. [Google Scholar] [CrossRef]
- Pérez-Trallero, E.; Zigorraga, C.; Artieda, J.; Alkorta, M.; Marimón, J.M. Two outbreaks of Listeria monocytogenes infection, northern spain. Emerg. Infect. Dis. 2014, 20, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Okike, I.O.; Lamont, R.F.; Heath, P.T. Do we really need to worry about Listeria in newborn infants? Pediatr. Infect. Dis. J. 2013, 32, 405–406. [Google Scholar] [CrossRef]
- Camacho-Gonzalez, A.; Spearman, P.W.; Stoll, B.J. Neonatal infectious diseases: Evaluation of neonatal sepsis. Pediatr. Clin. North Am. 2013, 60, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in fresh produce: Outbreaks, prevalence and contamination levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef]
- Duze, S.T.; Marimani, M.; Patel, M. Tolerance of Listeria monocytogenes to biocides used in food processing environments. Food Microbiol. 2021, 97, 103758. [Google Scholar] [CrossRef]
- Schlech, W.F. Epidemiology and clinical manifestations of Listeria monocytogenes infection. Gram-Positive Pathog. 2019, 50, 793–802. [Google Scholar] [CrossRef]
- Wu, S.; Wu, Q.; Zhang, J.; Chen, M.; Yan, Z.; Hu, H. Listeria monocytogenes prevalence and characteristics in retail raw foods in china. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Ayele, Y.; Kundu, P.; Jadhav, V.J. Growing importance of listeriosis as food borne disease. J. Exp. Food Chem. 2017, 3, 4. [Google Scholar] [CrossRef]
- Aryal, M.; Muriana, P.M. Efficacy of commercial sanitizers used in food processing facilities for inactivation of Listeria monocytogenes, E. coli O157, H7, and Salmonella biofilms. Foods 2019, 8, 639. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic bacilli inhibit Salmonella biofilm formation without killing planktonic cells. Front. Microbiol. 2021, 12, 615328. [Google Scholar] [CrossRef]
- Dekker, J.P.; Frank, K.M. Salmonella, Shigella, and Yersinia. Clin. Lab. Med. 2015, 35, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Zhu, S.; Zhao, J.-H.; Bao, H.-X.; Liu, H.; Ding, T.-M.; Liu, G.-R.; Li, Y.-G.; Johnston, R.N.; Cao, F.-L.; et al. Genetic boundaries delineate the potential human pathogen Salmonella bongori into discrete lineages: Divergence and speciation. BMC Genom. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ruby, T.; McLaughlin, L.; Gopinath, S.; Monack, D. Salmonella’s long-term relationship with its host. FEMS Microbiol. Rev. 2012, 36, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Chen, Q.; Guo, A.; Liu, W.; Ruan, Y.; Zhang, X.; Nou, X. Differential effects of growth medium salinity on biofilm formation of two Salmonella enterica strains. J. Food Prot. 2020, 83, 196–203. [Google Scholar] [CrossRef]
- Sarowska, J.; Frej-Mądrzak, M.; Jama-Kmiecik, A.; Kilian, A.; Teryks-Wołyniec, D.; Choroszy-Krol, I. Detection of Salmonella in foods using a reference PN-ISO method and an alternative method based on loop-mediated isothermal amplification coupled with bioluminescence. Adv. Clin. Exp. Med. 2016, 25, 945–950. [Google Scholar] [CrossRef]
- Akil, L.; Ahmad, H.A. Quantitative risk assessment model of human salmonellosis resulting from consumption of broiler chicken. Diseases 2019, 7, 19. [Google Scholar] [CrossRef]
- Corcoran, M.; Morris, D.; De Lappe, N.; O’Connor, J.; Lalor, P.; Dockery, P.; Cormican, M. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl. Environ. Microbiol. 2014, 80, 1507–1514. [Google Scholar] [CrossRef]
- Rodrigues, D.; Teixeira, P.; Oliveira, R.; Azeredo, J. Salmonella enterica enteritidis biofilm formation and viability on regular and triclosan-impregnated bench cover materials. J. Food Prot. 2011, 74, 32–37. [Google Scholar] [CrossRef]
- Lira, M.C.; Givisiez, P.E.N.; De Sousa, F.G.C.; Magnani, M.; De Souza, E.L.; Spricigo, D.A.; Gebreyes, W.A.; De Oliveira, C.J.B. Biofilm-forming and antimicrobial resistance traits of Staphylococci isolated from goat dairy plants. J. Infect. Dev. Ctries. 2016, 10, 932–938. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (mrsa). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. The combination of ultrasound and chlorogenic acid to inactivate Staphylococcus aureus under planktonic, biofilm, and food systems. Ultrason. Sonochem. 2021, 80, 105801. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.B.D.S.; de Carvalho, R.J.; de Souza, N.T.; Oliveira, K.D.S.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control. 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef]
- Singh, R.; Sahore, S.; Kaur, P.; Rani, A.; Ray, P. Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog. Dis. 2016, 74, 1–20. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert. Rev. Anti. Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front Cell Infect Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef]
- Huber, P.; Basso, P.; Reboud, E.; Attrée, I. Pseudomonas aeruginosa renews its virulence factors. Environ. Microbiol. Rep. 2016, 8, 564–571. [Google Scholar] [CrossRef]
- Gellatly, S.L.; Hancock, R.E.W. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef]
- Behzadi, P.; Baráth, Z.; Gajdács, M. It’s not easy being green: A narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, B.I.; Schniederberend, M.; Jain, R. Cross-regulation of Pseudomonas motility systems: The intimate relationship between flagella, pili and virulence. Curr. Opin. Microbiol. 2015, 28, 78–82. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa biofilm, a programmed bacterial life for fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, L.Z. Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2013, 14, 20983–21005. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Karami, P.; Khaledi, A.; Mashoof, R.Y.; Yaghoobi, M.H.; Karami, M.; Dastan, D.; Alikhani, M.Y. The correlation between biofilm formation capability and antibiotic resistance pattern in Pseudomonas aeruginosa. Gene Rep. 2020, 18, 100561. [Google Scholar] [CrossRef]
- Ma, A.; Neumann, N.; Chui, L. Phenotypic and genetic determination of biofilm formation in heat resistant Escherichia coli possessing the locus of heat resistance. Microorganisms 2021, 9, 403. [Google Scholar] [CrossRef]
- Ryu, J.H.; Beuchat, L.R. Biofilm formation by Escherichia coli O157, H7 on stainless steel: Effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 2005, 71, 247–254. [Google Scholar] [CrossRef]
- Barilli, E.; Vismarra, A.; Frascolla, V.; Rega, M.; Bacci, C. Escherichia coli strains isolated from retail meat products: Evaluation of biofilm formation ability, antibiotic resistance, and phylogenetic group analysis. J. Food Prot. 2020, 83, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, V.O.; Adedeji, A.O.; Kwaga, J. Assessment of the contamination potentials of some foodborne bacteria in biofilms for food products. Asian Pac. J. Trop. Med. 2014, 7, S232–S237. [Google Scholar] [CrossRef] [PubMed]

| Disinfectants | Characteristics | Function | Types of Microorganisms Acting | References |
|---|---|---|---|---|
| Sodium hypochlorite (NaClO) | strong oxidising agents |
| Staphylococcus aureus, Prevotella intermedia, Peptostreptococcus miros, Streptococcus intermedius, Fusobacterium nucleatum, Enterococcus faecalis, Listeria monocytogenes, Pseudomonas fragi, Staphylococcus xylosus, Bacillus cereus | [20,43] |
| Quaternary ammonium (QACs) | surface-active agents, membrane-active agents, hydrophobic activity |
| Listeria monocytogenes, Bacillus cereus, Staphylococcus spp., Pseudomonas spp. | [20,32] |
| Peracetic acid (PAA) | strong oxidising agents |
| Listeria monocytogenes, Staphylococcus aureus, Pseudomonas aeruginosa | [32,44] |
| Hydrogen peroxide (H2O2) | highly oxidising capacity |
| Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio spp., | [5,43,45] |
| Methodology | Mechanism of Action | Description | Reference |
|---|---|---|---|
| Electrolyzed water | Promote biofilm dispersion | acidic and slightly acidified electrolyzed water can efficiently remove L. innocua, L. monocytogenes, Vibrio parahaemolyticus, E. coli, and B. cereus biofilms. | [54] |
| Bacteriophages | Cell lysis | can not only directly kill bacteria, but also induce host bacteria to express EPS degradation enzymes, thus accelerating the clearance of mature biofilms. | [53] |
| Nonthermal atmospheric plasmas | Bactericidal | demonstrated high disinfectant capacity, contact-free and waterless, over conventional chemical-based disinfection. | [54] |
| Bacteriocins | Cell membrane alteration | Such as the bacteriocins nisin, subtilomycin, lichenicidin, enterocin B3A-B3B, enterocin AS-48, and sonorensin. | [54] |
| Biosurfactants | Inhibition of bacterial adhesion | Avoid biofilm formation and even inhibit QS molecules | [55] |
| Enzymatic disruption | Extracellular matrix disruption | Such as cellulases, proteases, glycosidases, and DNAses. | [29] |
| QS inhibition | Downregulation of adhesion and virulence mechanisms | Binding of inhibitors to QS receptors (lactic acid), enzymatic degradation of QS signals (paroxonases), sRNA post-transcriptional control, inhibition of QS signals biosynthesis. | [29] |
| High hydrostatic pressure | Bactericidal and endospores removal | high hydrostatic pressure (up to 900 MPa) combined with thermal treatments (50–100 °C) | [29] |
| Novel physical microbial inactivation technologies | Inactivation of microorganisms within biofilms | Such as photodynamic inactivation using pulsed ultraviolet light, electron beam irradiation, steam heating, light at 405 nm, and treatment of the surfaces using ozone, ultrasounds, and gaseous chlorine dioxide. | [54] |
| Foodborne Pathogens | Characteristics | Contaminated Food | The Main Symptoms of FOOD Poisoning | Examples of Harmful Spoilage Effects | References |
|---|---|---|---|---|---|
| Listeria monocytogenes | Gram-positive, rod-shaped, facultative anaerobic, non-spore forming | meat (especially beef), eggs, poultry, seafood, vegetable, salad, juice, milk, cheese, dairy, ice-cream | diarrhea and fever | meningitis, encephalitis, endocarditis, sepsis, pneumonia, and other central nervous system infections | [56,57,58] |
| Salmonella enterica | Gram-negative, rod-shaped, facultative anaerobic, flagellate, non-spore forming | eggs, egg products, poultry meat | fever, diarrhea, and abdominal cramps | gastroenteritis, sepsis | [59,60,61,62] |
| Staphylococcus aureus | Gram-positive, spherical, facultative anaerobic, flagellate, non-spore forming, non-motile | meat products, dairy products, egg products, poultry, salads, bakery products (especially cream-filled pastries and cakes, and sandwich fillings) | nausea, vomiting, spasmodic pain in the middle and upper abdomen, diarrhea | osteomyelitis, endocarditis, chronic wound infection, eye infection, multimicrobial biofilm infection, renal abscess | [63,64] |
| Pseudomonas aeruginosa | Gram-negative, rod-shaped, obligate aerobic, flagellate, motile | fruits, vegetables, meat, low-acid dairy products | fever, ulceration, diarrhea, expectoration | postoperative wound infection, urinary tract infection, bedsore, abscess, external otitis, otitis media, keratitis, folliculitis, sepsis, cystic fibrosis | [65,66,67] |
| Escherichia coli | Gram-negative, rod-shaped, non-spore forming, metabolically active | fresh meat, fruits, vegetables, raw milk, dairy products | nausea, vomiting, abdominal cramps, bloody diarrhea, fever | gastrointestinal infections, urinary tract infections, septic infections, hemorrhagic colitis, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, kidney failure | [68,69,70,71] |
| Bacillus cereus | Gram-positive, rod-shaped, facultative aerobic, spore-forming, motile | dairy products, vegetables, meat, rice | diarrhoea and vomiting symptoms | meningitis, brain abscess, cellulitis, endophthalmitis, pneumonia, endocarditis, and osteomyelitis | [72,73,74] |
| Campylobacter jejuni | Gram-negative, rod-shaped, microaerophilic, flagellate, non-spore forming, motile | Animals, poultry, vegetables, fruits, all kinds of cooked food, milk, dairy products | bloody diarrhoea, fever, stomach cramps, nausea, and vomiting | gastrointestinal infection, acute enteritis, septicemia, meningitis, arthritis, pyelonephritis | [75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. https://doi.org/10.3390/molecules28062432
Liu X, Yao H, Zhao X, Ge C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules. 2023; 28(6):2432. https://doi.org/10.3390/molecules28062432
Chicago/Turabian StyleLiu, Xiaoli, Huaiying Yao, Xihong Zhao, and Chaorong Ge. 2023. "Biofilm Formation and Control of Foodborne Pathogenic Bacteria" Molecules 28, no. 6: 2432. https://doi.org/10.3390/molecules28062432
APA StyleLiu, X., Yao, H., Zhao, X., & Ge, C. (2023). Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules, 28(6), 2432. https://doi.org/10.3390/molecules28062432






