Research Progress and Future Trends of Low Temperature Plasma Application in Food Industry: A Review
Abstract
1. Introduction
2. Sterilization Mechanism of Low Temperature Plasma
2.1. Ultraviolet Light
2.2. Charged Particles
2.3. Active Ingredients
3. Factors Affecting Sterilization Effect
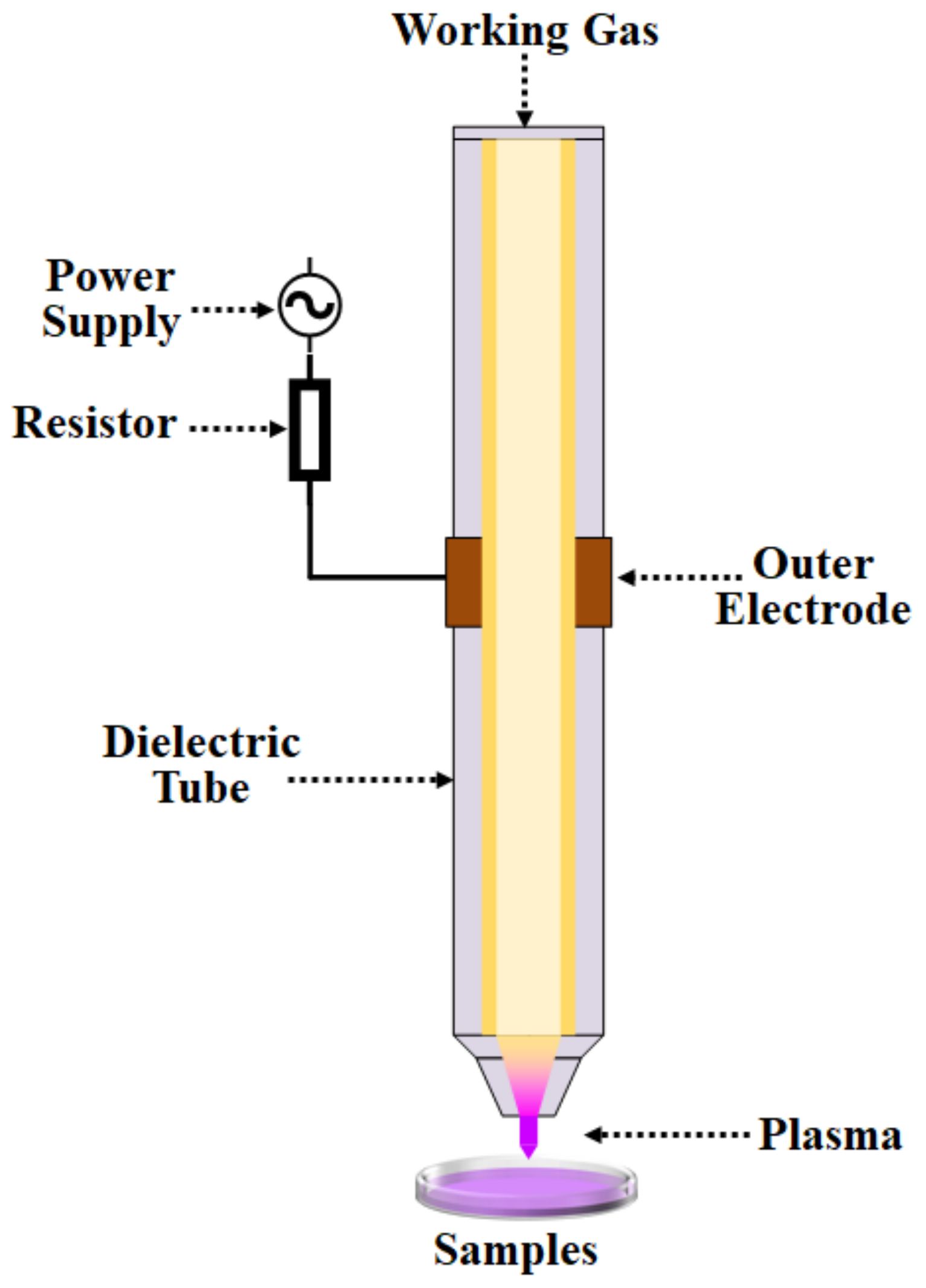
3.1. Plasma Equipment Factors
3.2. Reactant and Microorganism Factors
4. Application Status of Plasma in Food Industry
4.1. Application of Plasma in Different Foods
4.1.1. Application of Plasma in Vegetables and Fruits
4.1.2. Application of Plasma in Meat Food
4.1.3. Application of Plasma in Grain Industry
4.1.4. Application of Plasma in Dairy Products
4.2. Application of Plasma in Other Ways
4.2.1. Application of Plasma in Food Packaging
4.2.2. Application of Plasma-Activated Water
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chau, T.T.; Kao, K.C.; Blank, G.; Madrid, F. Microwave plasma for low temperature dry sterilization. Biomaterials 1996, 17, 1273–1277. [Google Scholar] [CrossRef]
- Choi, M.S.; Cheigh, C.-I.; Jeong, E.-A.; Shin, J.-K.; Chung, M.-S. Nonthermal sterilization of Listeria monocytogenes in infant foods by intense pulsed-light treatment. J. Food Eng. 2010, 97, 504–509. [Google Scholar] [CrossRef]
- Fitriyanti, M.; Narsimhan, G. Synergistic effect of low power ultrasonication on antimicrobial activity of cecropin P1 against E. coli in food systems. LWT 2018, 96, 175–181. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Prevosto, L.; Grijalba, P.E.; Zilli, C.G.; Cejas, E.; Mancinelli, B.; Balestrasse, K.B. Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status. Heliyon 2019, 5, e01495. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.W.; Han, R.W.; Yuan, M.D.; Xi, Q.; Du, Q.J.; Li, P.; Yang, Y.X.; Applegate, B.; Wang, J. Ultrasound combined with slightly acidic electrolyzed water thawing of mutton: Effects on physicochemical properties, oxidation and structure of myofibrillar protein. Ultrason. Sonochem. 2023, 93, 106309. [Google Scholar] [CrossRef]
- Park, J.N.; Song, B.-S.; Kim, J.-H.; Choi, J.-I.; Sung, N.-Y.; Han, I.-J.; Lee, J.-W. Sterilization of ready-to-cook Bibimbap by combined treatment with gamma irradiation for space food. Radiat. Phys. Chem. 2012, 81, 1125–1127. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Ibañez, V.N.; Varela, A.; Cejas, E.; Ferreyra, M.; Chamorro-Garcés, J.C.; Zilli, C.; Vallecorsa, P.; Fina, B.; Prevosto, L.; et al. Non-Thermal Plasmas Affect Plant Growth and DNA Methylation Patterns in Glycine max. J. Plant Growth Regul. 2022, 41, 2732–2742. [Google Scholar] [CrossRef]
- Stoops, J.; Jansen, M.; Claes, J.; Van Campenhout, L. Decontamination of powdery and granular foods using Continuous Wave UV radiation in a dynamic process. J. Food Eng. 2013, 119, 254–259. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.; Ding, T. Understanding the Impact of Nonthermal Plasma on Food Constituents and Microstructure—A Review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Liao, X.Y.; Cullen, P.J.; Liu, D.; Xiang, Q.; Wang, J.; Chen, S.; Ye, X.; Ding, T. Effects of Nonthermal Plasma Technology on Functional Food Components. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1379–1394. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; Álvarez, C.; Cullen, P.J.; Tiwari, B.K. Effect of cold plasma on the techno-functional properties of animal protein food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 58, 102205. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga toxin-producing Escherichia coli, and Listeria monocytogenes by a novel surface discharge cold plasma design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Ziuzina, D.; Misra, N.N.; Han, L.; Cullen, P.J.; Moiseev, T.; Mosnier, J.P.; Keener, K.; Gaston, E.; Vilaró, I.; Bourke, P. Investigation of a large gap cold plasma reactor for continuous in-package decontamination of fresh strawberries and spinach. Innov. Food Sci. Emerg. Technol. 2020, 59, 102229. [Google Scholar] [CrossRef]
- Wang, J.; Han, R.W.; Liao, X.Y.; Ding, T. Application of Plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. LWT 2021, 137, 110465. [Google Scholar] [CrossRef]
- Wang, H.X.; Li, Y.X.; Xi, Q.; Han, R.; Cullen, P.J.; Du, Q.; Yang, Y.; Forghani, F.; Zhang, J.; Wang, J. Application of plasma activated water for Escherichia coli decontamination and shelf-life extension of kale. Food Qual. Saf. 2022, 6, fyac041. [Google Scholar] [CrossRef]
- Liao, X.Y.; Cullen, P.J.; Liu, D.H.; Muhammad, A.I.; Chen, S.; Ye, X.; Wang, J.; Ding, T. Combating Staphylococcus aureus and its methicillin resistance gene (mecA) with cold plasma. Sci. Total Environ. 2018, 645, 1287–1295. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Li, Y.; Liao, X.Y.; Liu, D.; Ye, X.; Chen, S.; Hu, Y.; Wang, J.; Ding, T. Effect of dielectric barrier discharge plasma on background microflora and physicochemical properties of tiger nut milk. Food Control 2019, 96, 119–127. [Google Scholar] [CrossRef]
- Wei, G.F.; Gao, W.Y.; Qin, Y.J.; Zhang, Y.J. Review and prospect of low-temperature plasma sterilization. Chin. Med. Equip. J. 2005, 26, 27–29. [Google Scholar]
- Chizoba Ekezie, F.-G.; Sun, D.-W.; Han, Z.; Cheng, J.-H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold Plasma Decontamination of Foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Feichtinger, J.; Hertel, C. Response of Deinococcus radiodurans to low-pressure low-temperature plasma sterilization processes. J. Appl. Microbiol. 2010, 109, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Trompeter, F.J.; Neff, W.J.; Franken, O.; Heise, M.; Saveljew, A.B. Reduction of Bacillus subtilis and Aspergillus niger spores using nonthermal atmospheric gas discharges. IEEE Trans. Plasma Sci. 2002, 30, 1416–1423. [Google Scholar] [CrossRef]
- Vleugels, M.; Shama, G.; Deng, X.T.; Greenacre, E.; Brocklehurst, T.; Kong, M.G. Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Trans. Plasma Sci. 2005, 33, 824–828. [Google Scholar] [CrossRef]
- Mendis, D.A.; Rosenberg, M.; Azam, F. A note on the possible electrostatic disruption of bacteria. Plasma Sci. IEEE Trans. 2002, 28, 1304–1306. [Google Scholar] [CrossRef]
- Pothakamury, U.R.; Monsalve-Gonzalez, A.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Escherichia coli and Staphylococcus aureus in model foods by pulsed electric field technology. Food Res. Int. 1995, 28, 167–171. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of ozone in the food industry. LWT—Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Dolezalova, E.; Lukes, P. Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 2015, 103, 7–14. [Google Scholar] [CrossRef]
- Weng, C.C.; Wu, Y.-T.; Liao, J.-D.; Kao, C.-Y.; Chao, C.-C.; Chang, J.-E.; Hsu, B.-W. Inactivation of bacteria by a mixed argon and oxygen micro-plasma as a function of exposure time. Int. J. Radiat. Biol. 2009, 85, 362–368. [Google Scholar] [CrossRef]
- Hury, S.; Vidal, D.R.; Desor, F.; Pelletier, J.; Lagarde, T. A parametric study of the destruction efficiency of Bacillus spores in low pressure oxygen-based plasmas. Lett. Appl. Microbiol. 1998, 26, 417–421. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.W.; Wang, S.Q. Effect of atmospheric pressure low temperature plasma on sterilization rate of Escherichia coli on sliced cucumber surface and quality attributes. Sci. Technol. Cereals Oils Foods 2018, 1, 67–69. [Google Scholar]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jäger, A.; Polívka, L.; Syková, E.; Dejneka, A.; Kubinová, Š. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef]
- Gaunt, L.F.; Beggs, C.B.; Georghiou, G.E. Bactericidal Action of the Reactive Species Produced by Gas-Discharge Nonthermal Plasma at Atmospheric Pressure: A Review. IEEE Trans. Plasma Sci. 2006, 34, 1257–1269. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Cheng, J.-H.; Sun, D.-W. Inactivation of Listeria monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT 2020, 118, 108635. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Free, L.; Hinds, L.M.; Vijayaraghavan, R.K.; Daniels, S.; O’Donnell, C.P.; Tiwari, B.K. Effect of non-thermal plasma technology on microbial inactivation and total phenolic content of a model liquid food system and black pepper grains. LWT 2020, 118, 108716. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhu, X.; Yuan, Y.; Gao, Z.; Yue, T. Application of electrical discharge plasma on the inactivation of Zygosaccharomyces rouxii in apple juice. LWT 2020, 121, 108974. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Wemlinger, E.; Pedrow, P.; Barbosa-Cánovas, G.; Garcia-Perez, M. Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food Control 2013, 34, 149–157. [Google Scholar] [CrossRef]
- Misra, N.N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.P.; Keener, K.M.; Cullen, P.J. In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 2014, 125, 131–138. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, D.D.; Peng, J.; Tu, K.; Ma, P.P. Efficacy of cold plasma on microbial decontamination and storage quality of blueberries. Food Sci. 2018, 39, 101–107. [Google Scholar]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Zhao, N.; Ge, L.; Huang, Y.; Wang, Y.; Wang, Y.; Lai, H.; Wang, Y.; Zhu, Y.; Zhang, J. Impact of cold plasma processing on quality parameters of packaged fermented vegetable (radish paocai) in comparison with pasteurization processing: Insight into safety and storage stability of products. Innov. Food Sci. Emerg. Technol. 2020, 60, 102300. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Misra, N.N.; Cullen, P.J. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innov. Food Sci. Emerg. Technol. 2013, 19, 153–157. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhong, C.-S.; Mujumdar, A.S.; Yang, X.-H.; Deng, L.-Z.; Wang, J.; Xiao, H.-W. Cold plasma pretreatment enhances drying kinetics and quality attributes of chili pepper (Capsicum annuum L.). J. Food Eng. 2019, 241, 51–57. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Jarmoluk, A.; Marycz, K. Antimicrobial activity of low-pressure plasma treatment against selected foodborne bacteria and meat microbiota. Ann. Microbiol. 2015, 65, 1537–1546. [Google Scholar] [CrossRef]
- Choi, S.; Puligundla, P.; Mok, C. Effect of corona discharge plasma on microbial decontamination of dried squid shreds including physico-chemical and sensory evaluation. LWT 2017, 75, 323–328. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; de Alba, M.; Harrison, S.M.; Brunton, N.P.; Cullen, P.J.; Tiwari, B.K. Effects of cold atmospheric plasma on mackerel lipid and protein oxidation during storage. LWT 2020, 118, 108697. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Park, S.; Choe, W.; Jo, C. Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Curr. Appl. Phys. 2013, 13, 1420–1425. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; In Yong, H.; Choe, J.H.; Jeon, H.-J.; Choe, W.; Jo, C. The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Sci. 2015, 108, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Spinelli, A.C.; Govindan, B.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Cold plasma treatment of ready-to-eat ham: Influence of process conditions and storage on inactivation of Listeria innocua. Food Res. Int. 2019, 123, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Gök, V.; Aktop, S.; Özkan, M.; Tomar, O. The effects of atmospheric cold plasma on inactivation of Listeria monocytogenes and Staphylococcus aureus and some quality characteristics of pastırma—A dry-cured beef product. Innov. Food Sci. Emerg. Technol. 2019, 56, 102188. [Google Scholar] [CrossRef]
- Yong, H.I.; Lee, H.; Park, S.; Park, J.; Choe, W.; Jung, S.; Jo, C. Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat’s physicochemical properties. Meat Sci. 2017, 123, 151–156. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Lim, Y.; Choe, W.; Yong, H.I.; Jo, C. Direct infusion of nitrite into meat batter by atmospheric pressure plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 113–118. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Leipold, F.; Kusano, Y.; Hansen, F.; Jacobsen, T. Decontamination of a rotating cutting tool during operation by means of atmospheric pressure plasmas. Food Control 2010, 21, 1194–1198. [Google Scholar] [CrossRef]
- Misra, N.N.; Kaur, S.; Tiwari, B.K.; Kaur, A.; Singh, N.; Cullen, P.J. Atmospheric pressure cold plasma (ACP) treatment of wheat flour. Food Hydrocoll. 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Thirumdas, R.; Trimukhe, A.; Deshmukh, R.R.; Annapure, U.S. Functional and rheological properties of cold plasma treated rice starch. Carbohydr. Polym. 2017, 157, 1723–1731. [Google Scholar] [CrossRef]
- Sarangapani, C.; Thirumdas, R.; Devi, Y.; Trimukhe, A.; Deshmukh, R.R.; Annapure, U.S. Effect of low-pressure plasma on physico–chemical and functional properties of parboiled rice flour. LWT Food Sci. Technol. 2016, 69, 482–489. [Google Scholar] [CrossRef]
- Potluri, S.; Sangeetha, K.; Santhosh, R.; Nivas, G.; Mahendran, R. Effect of low-pressure plasma on bamboo rice and its flour. J. Food Process. Preserv. 2018, 42, e13846. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, H.C.; Chen, Y.K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna radiate). LWT 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.-J.; Woo, K.S.; Jo, C.; Kim, J.-K.; Kim, S.H.; Park, H.Y.; Oh, S.-K.; Kim, W.H. Evaluation of cold plasma treatments for improved microbial and physicochemical qualities of brown rice. LWT 2016, 73, 442–447. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. Technol. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Datta, N.; Deeth, H.C. Diagnosing the cause of proteolysis in UHT milk. LWT Food Sci. Technol. 2003, 36, 173–182. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Manzoor, M.F.; Tan, Y.-C.; Inam-ur-Raheem, M.; Aadil, R.M. Effect of dielectric barrier discharge (DBD) plasma on the structure and antioxidant activity of bovine serum albumin (BSA). Int. J. Food Sci. Technol. 2020, 55, 2824–2831. [Google Scholar] [CrossRef]
- Gurol, C.; Ekinci, F.Y.; Aslan, N.; Korachi, M. Low Temperature Plasma for decontamination of E. coli in milk. Int. J. Food Microbiol. 2012, 157, 1–5. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Microbial safety and quality attributes of milk following treatment with atmospheric pressure encapsulated dielectric barrier discharge plasma. Food Control 2015, 47, 451–456. [Google Scholar] [CrossRef]
- Korachi, M.; Ozen, F.; Aslan, N.; Vannini, L.; Guerzoni, M.E.; Gottardi, D.; Ekinci, F.Y. Biochemical changes to milk following treatment by a novel, cold atmospheric plasma system. Int. Dairy J. 2015, 42, 64–69. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Fernandes, L.M.; Moraes, J.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; Borges, F.O.; et al. Processing chocolate milk drink by low-pressure cold plasma technology. Food Chem. 2019, 278, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Peng, P.; Zhou, N.; Cheng, Y.; Min, M.; Ma, Y.; Mao, Q.; Chen, P.; Chen, C.; Ruan, R. Evaluation of Cronobacter sakazakii inactivation and physicochemical property changes of non-fat dry milk powder by cold atmospheric plasma. Food Chem. 2019, 290, 270–276. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, S.; Jung, H.S.; Park, S.H.; Jo, C. Evaluation of a Dielectric Barrier Discharge Plasma System for Inactivating Pathogens on Cheese Slices. J. Anim. Sci. Technol. 2012, 54, 191–198. [Google Scholar] [CrossRef]
- Wan, Z.; Pankaj, S.K.; Mosher, C.; Keener, K.M. Effect of high voltage atmospheric cold plasma on inactivation of Listeria innocua on Queso Fresco cheese, cheese model and tryptic soy agar. LWT 2019, 102, 268–275. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Turtoi, M.; Nicolau, A. Intense light pulse treatment as alternative method for mould spores destruction on paper–polyethylene packaging material. J. Food Eng. 2007, 83, 47–53. [Google Scholar] [CrossRef]
- Lei, J.; Yang, L.; Zhan, Y.; Wang, Y.; Ye, T.; Li, Y.; Deng, H.; Li, B. Plasma treated polyethylene terephthalate/polypropylene films assembled with chitosan and various preservatives for antimicrobial food packaging. Colloids Surf. B Biointerfaces 2014, 114, 60–66. [Google Scholar] [CrossRef]
- Lee, T.; Puligundla, P.; Mok, C. Inactivation of foodborne pathogens on the surfaces of different packaging materials using low-pressure air plasma. Food Control 2015, 51, 149–155. [Google Scholar] [CrossRef]
- Oh, Y.A.; Roh, S.H.; Min, S.C. Cold plasma treatments for improvement of the applicability of defatted soybean meal-based edible film in food packaging. Food Hydrocoll. 2016, 58, 150–159. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Jiménez, A.; Bourke, P.; Cullen, P.J. Characterization of polylactic acid films for food packaging as affected by dielectric barrier discharge atmospheric plasma. Innov. Food Sci. Emerg. Technol. 2014, 21, 107–113. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Luo, Y.; Murad, M.S.; Zhu, L.; Mu, G. Improved packing performance and structure-stability of casein edible films by dielectric barrier discharges (DBD) cold plasma. Food Packag. Shelf Life 2020, 24, 100471. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Liao, X.; Su, Y.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Wang, J.; Ding, T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2020, 59, 102256. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Q.; Cullen, P.J.; Su, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT 2020, 117, 108649. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, W.; Zhao, D.; Niu, L.; Li, K.; Bai, Y. Synergistic inactivation of Escherichia coli O157:H7 by plasma-activated water and mild heat. Food Control 2019, 106, 106741. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Kim, S.B.; Ryu, S.; Lim, J.; Hong, E.J.; Byeon, Y.S.; Chun, H.H. Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.). Food Control 2019, 98, 501–509. [Google Scholar] [CrossRef]
- Lo Porto, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
| Food Matrix | Conditions | Results | References |
|---|---|---|---|
| black pepper | voltages of 15 and 30 kV for 3–20 min | Up to 2 log and 1 log reduction in Bacillus subtilis vegetative cells and spores achieved, respectively | [36] |
| apple juice | voltages of 21 kV for 30 min | 5.6 log reduction in yeast (Zygosaccharomyces rouxii) reached | [37] |
| lettuce, carrots and tomatoes | 3.95 kV up to 12.83 kV (60 Hz) in argon, from 30 s to 10 min | the highest voltage and longest treatment time could reach 1.6 log reduction in pathogenic Escherichia coli | [38] |
| strawberries | 60 kV for 5 min | 2 log reduction in the background microflora (aerobic mesophilic bacteria, yeast, and mold) achieved | [39] |
| blueberries | 45 kV for 50 s | Total aerobic mesophilic bacteria and yeast/mold counts were decreased by 1.75 and 1.77 log reduction | [40] |
| fresh-cut pitaya | 60 kV for 5 min | significantly inhibited the growth of total aerobic bacterial counts, increased the cutting-induced phenolic accumulation, and enhanced antioxidant activity in fresh-cut pitaya fruit | [41] |
| fermented vegetable (radish paocai) | 60 kV for 60 s | efficiently eliminated yeasts, especially gas-producing yeast | [42] |
| pomegranate juice | 5 cm3 sample volume, and 0.75 dm3/min gas flow at 6 W for 3 min | Reached the greatest anthocyanin stability | [43] |
| blueberry juice | 11 kV for 4 min | Significantly increased the content of phenolics and better kept the original color | [44] |
| tomato | 30, 40 and 50 kV for different time | the activity of tomato peroxidase decreased with the increase in treatment time and volt-age | [45] |
| blueberries | 80 kV for 5 min | The degradation efficacy of pesticides of 80.18% for boscalid and 75.62% for Imidacloprid reached, respectively, | [46] |
| chili pepper | 750 W for 15, 30, 45, and 60 s | improve the drying speed and anti-oxidation ability, and can effectively retain the red pigment content | [47] |
| Food Matrix | Conditions | Results | References |
|---|---|---|---|
| Pork and beef | 21 kV for 10 min | the total number of microorganisms, yeasts, and molds, and psychrotrophic microorganisms was reduced in the range of 1.14–1.48 log cycles for pork and 0.98–2.09 log cycles for beef | [48] |
| dried squid shreds | 20 kV for 0–3 min | aerobic bacteria, marine bacteria, and Staphylococcus aureus were inactivated by 2.0, 1.6, and 0.9 log units, respectively. | [49] |
| mackerel | 80 kV for 5 min | no significant changes were found in lipid oxidation, as well as the fatty acid composition or nutritional quality indices after treatment | [50] |
| pork loin | 3 kV for 5 and 10 min | E. coli was reduced by 0.26 and 0.55 log cycles, while Listeria monocytogenes was reduced from 0.17 to 0.59 log cycles | [51] |
| sausage | 10 W/cm2 | there were no noticeable effects on the total aerobic bacterial counts, color, and peroxide values of sausages | [52] |
| ready-to-eat ham | 300 W for 3 min | a significant reduction in L. innocua of 1.51 to 1.75 log CFU/cm2 at 4 °C, while 1.43 to 1.78 log CFU/cm2 at 23 °C | [53] |
| dry-cured beef product | 25 kV for 5 min | Maximum reduction of 0.85 log CFU/cm2 for S. aureus and 0.83 log CFU/cm2 for L. monocytogenes, while 1.41 log CFU/cm2 for aerobic bacteria and 1.66 log CFU/cm2 for yeast–mold counts, respectively | [54] |
| beef jerky | flexible thin-layer plasma 10 min treatment | E. coli O157:H7, L. monocytogenes, Salmonella Typhimurium, and Aspergillus flavus were reduced by approximately 2 to 3 log CFU/g | [55] |
| meat batter | 550 W 30 min | Total aerobic bacterial count of meat batter was not influenced and the nitrite level increased to 65.96 ppm | [56] |
| Food Matrix | Conditions | Results | References |
|---|---|---|---|
| wheat flour | voltages of 60 and 70 kV for 5 and 10 min. | an improvement in the dough strength and optimum mixing time for both strong and weak wheat flours. | [59] |
| rice starch | two different power levels 40 W and 60 W for 5 and 10 min | change the structure, function, and rheology of natural rice starch | [60] |
| rice flour | at varying power of 30 W, 40 W and 50 W for duration of 5, 10 and 15 min | the water absorption rate of steamed rice after heating would increase and the cooking time could by shortening 8 min, the texture characteristics will also be improved | [61] |
| bamboo rice | 15, 20, and 25 W/cm2 for 5 and 10 min | The soaking rate of bamboo rice was increased by 15% and the cooking time was shortened by about 12 min | [62] |
| brown rice | ranging from 1 to 3 kV for 10 min | the germination rate, seedling length, and water absorption of brown rice would increase | [63] |
| Thai germinated brown rice | 100–200 W for 75 s | the germination percentage, root length, and seedling height measurements of the most sensitive rice cultivar increased by 84%, 57%, and 69%, respectively | [64] |
| Wheat | 60 W, 80 W and 100 W for 15 s | improve seed germination potential (6.0%) and germination rate (6.7%) | [65] |
| mung beans | two different power levels 40 W and 60 W for 10, 15 and 20 min | increased the germination rate by 36.2%, radical root length by 20% and conductivity of seeds by 102% | [66] |
| brown rice | 250 W for periods of 5, 10 and 20 min | a 20 min plasma treatment resulted in a reduction in bacterial counts by approximately 2.30 log CFU/g | [67] |
| wheat germs | voltages of 20 and 24 kV for 5–35 min | 25 min ACP treatment resulted in reduction in lipase and lipoxygenase activity of WG to 25.03% and 49.98% of initial extent, respectively. | [68] |
| Food Matrix | Conditions | Results | References |
|---|---|---|---|
| milk | 9 kV for 3, 6, 9, 12, 15 and 20 min | 4.15 log CFU/mL E. coli in whole milk decreased; did not cause any significant change to the pH and color values | [71] |
| milk | 250 W for 5 and 10 min | Total aerobic bacterial count (0.98 log CFU/mL) was eliminated. Approximately 2.40 log CFU/mL decrease in Escherichia coli, Listeria monocytogenes, and Salmonella Typhimurium achieved | [72] |
| milk | 9 kV for 20 min | Significantly increased the total aldehyde content. No significant difference was observed in the total ketone or alcohol levels | [73] |
| chocolate milk | 400 W at gas flow rates of 10, 20, and 30 mL/min for 5, 10, and 15 min | Different treatment condition showed different effect on physio-chemical characteristics, bioactive compounds, fatty acid composition, and volatile compounds profile of chocolate milk drink | [74] |
| milk powder | 4.4 kV for 20–120 s | Led to 1.17–3.27 log10 reductions in Cronobacter sakazakii | [75] |
| Cheese Slices | 3.5 kV for 1, 5, 10 and 15 min | 0.09–1.47 log CFU/g and 0.05–1.98 log CFU/g decrease in E. coli with helium and He/O2, while 0.05 to 0.45 log CFU/g and 0.08 to 0.91 log CFU/g decrease in S. aureus | [76] |
| cheese | 100 kV for 5 min | 1.6 log CFU/g decrease in Listeria innocua achieved | [77] |
| Whey protein isolate | 70 kV for 1, 5, 10, 15, 30 and 60 min | an increase in carbonyl groups and the surface hydrophobicity, while the reduction in free SH groups indicated mild oxidation occurred in the proteins | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Du, Q.; Yang, Y.; Zhang, J.; Han, R.; Wang, J. Research Progress and Future Trends of Low Temperature Plasma Application in Food Industry: A Review. Molecules 2023, 28, 4714. https://doi.org/10.3390/molecules28124714
Zhang J, Du Q, Yang Y, Zhang J, Han R, Wang J. Research Progress and Future Trends of Low Temperature Plasma Application in Food Industry: A Review. Molecules. 2023; 28(12):4714. https://doi.org/10.3390/molecules28124714
Chicago/Turabian StyleZhang, Jiacheng, Qijing Du, Yongxin Yang, Jing Zhang, Rongwei Han, and Jun Wang. 2023. "Research Progress and Future Trends of Low Temperature Plasma Application in Food Industry: A Review" Molecules 28, no. 12: 4714. https://doi.org/10.3390/molecules28124714
APA StyleZhang, J., Du, Q., Yang, Y., Zhang, J., Han, R., & Wang, J. (2023). Research Progress and Future Trends of Low Temperature Plasma Application in Food Industry: A Review. Molecules, 28(12), 4714. https://doi.org/10.3390/molecules28124714





