Insights into the Structures of Bilirubin and Biliverdin from Vibrational and Electronic Circular Dichroism: History and Perspectives
Abstract
:1. Introduction and Historical Note
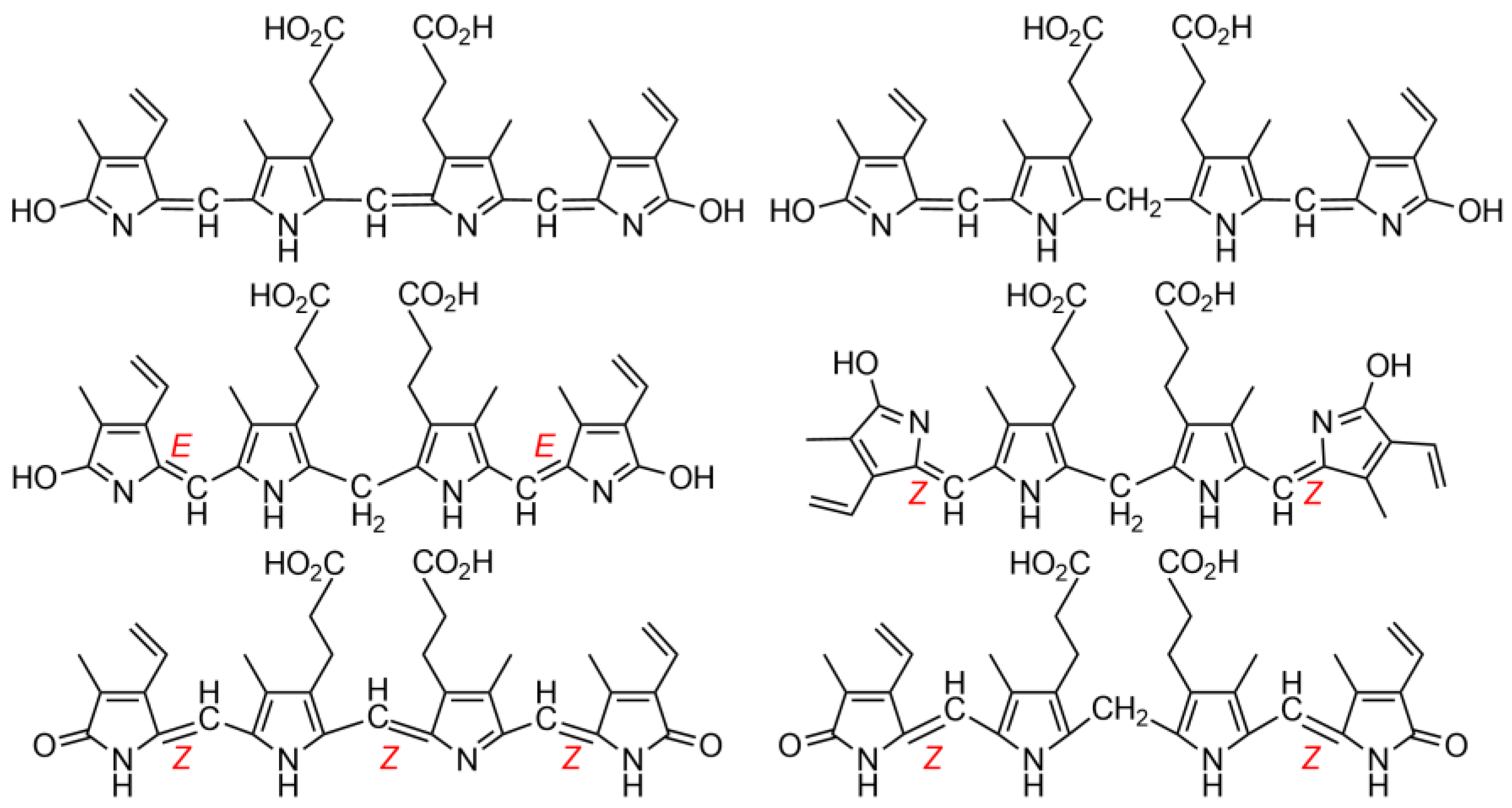
1.1. Fundamentals and Basic Chemistry: Foundations
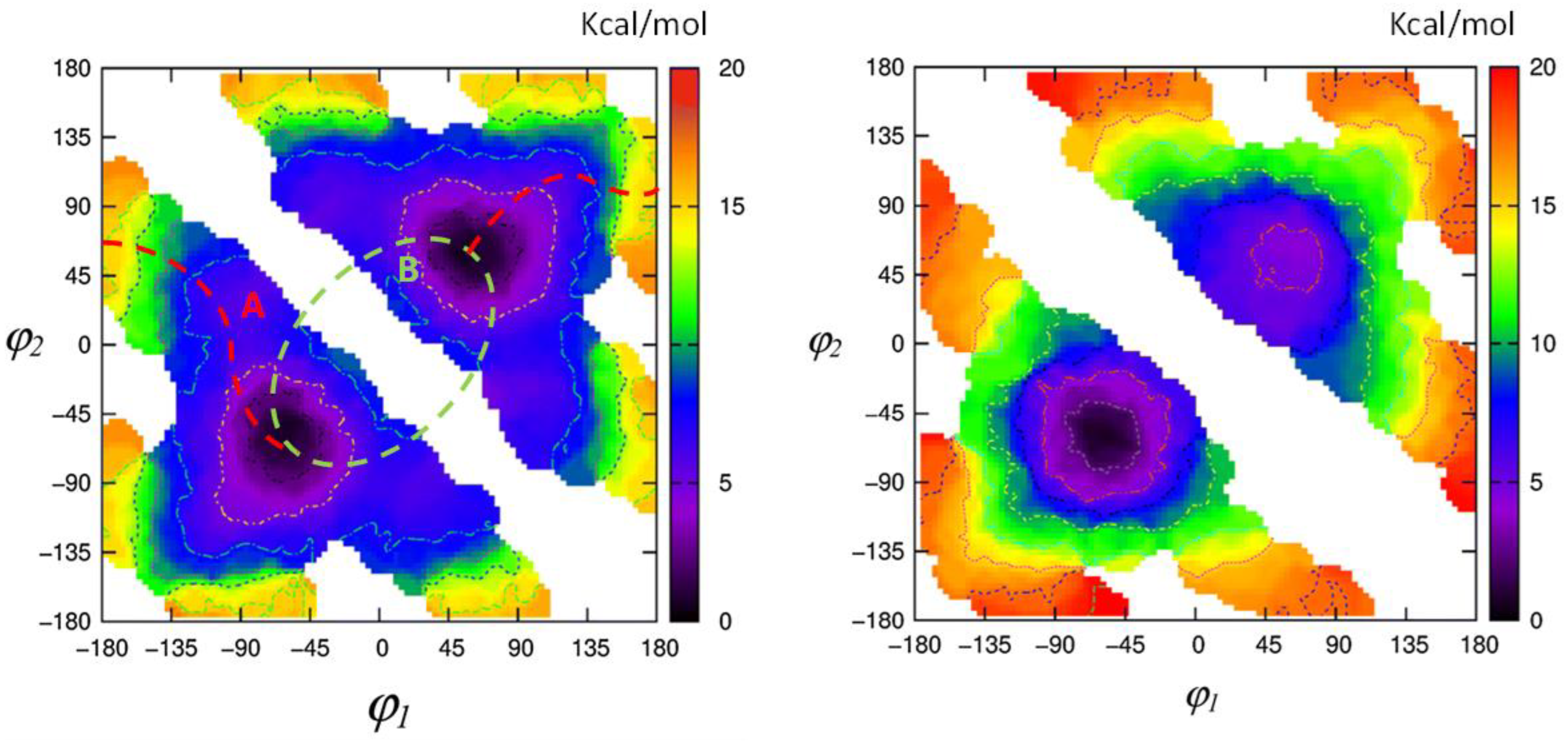
1.2. Experimental and Computational Structure Determinations
1.3. Spectroscopic Studies
2. Chiroptical Spectroscopies
2.1. The Advent of Bilirubinoid Chiroptical Spectroscopies
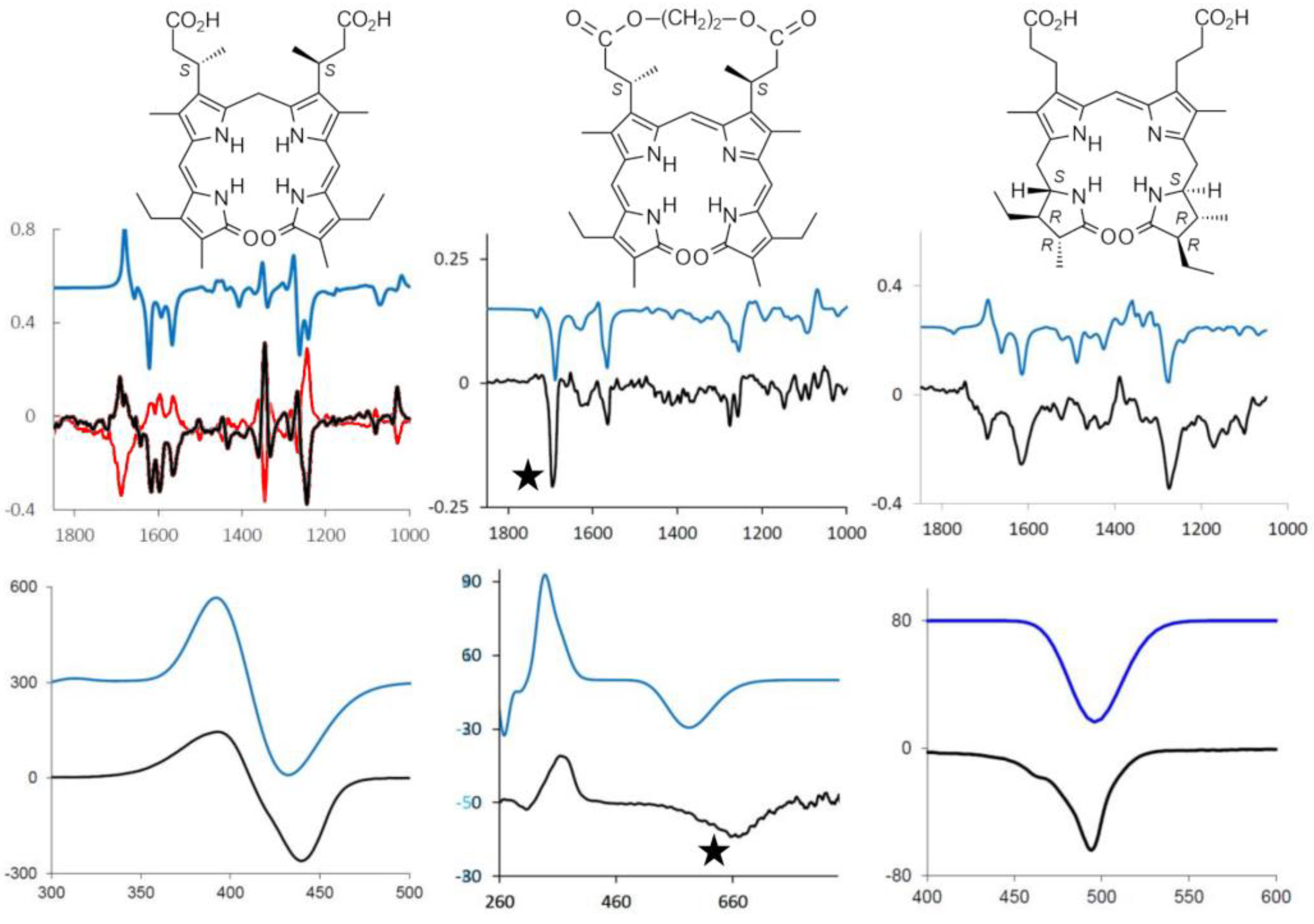
2.2. Chiral Derivatives (ECD and VCD Experiments and Calculations)
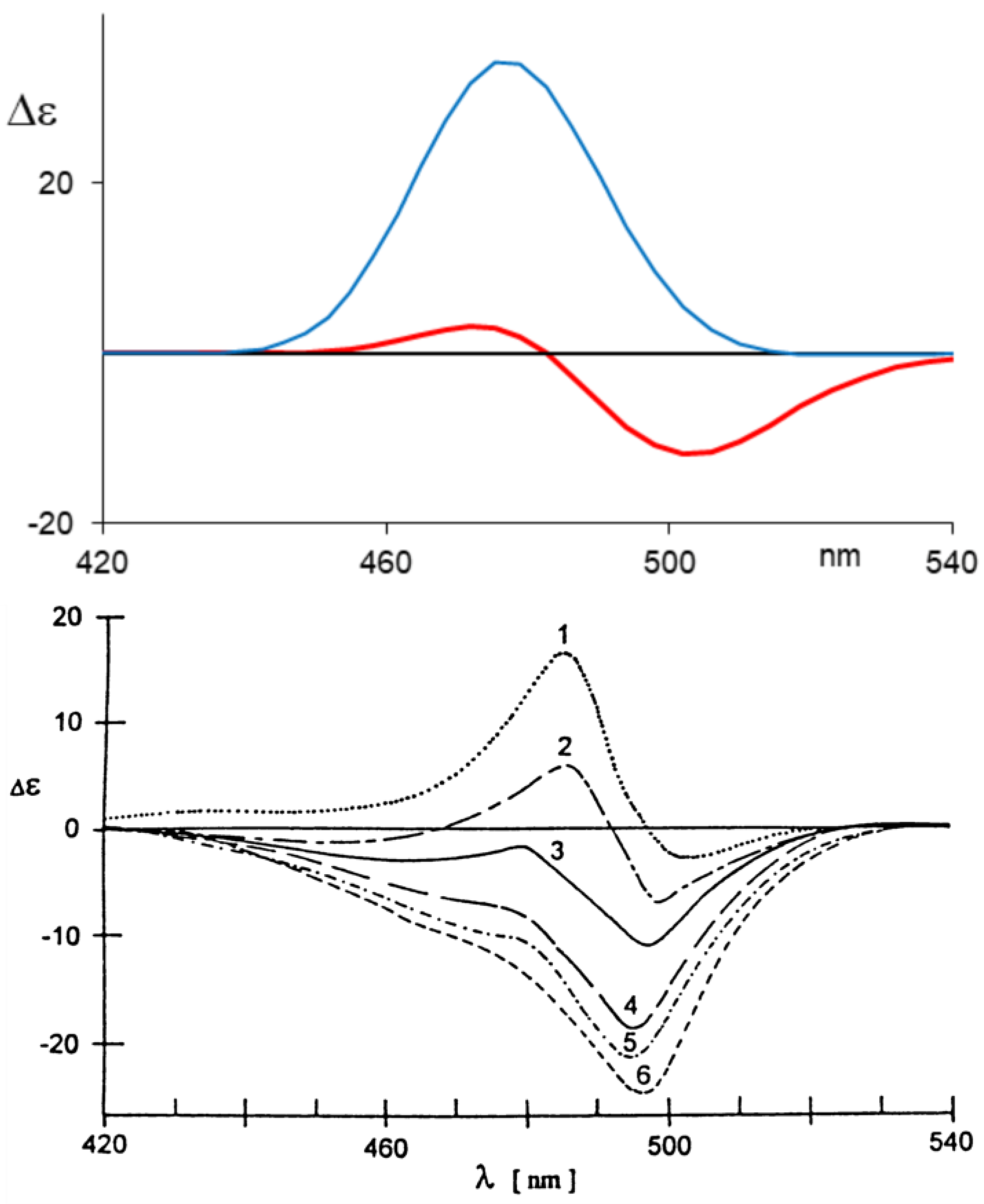
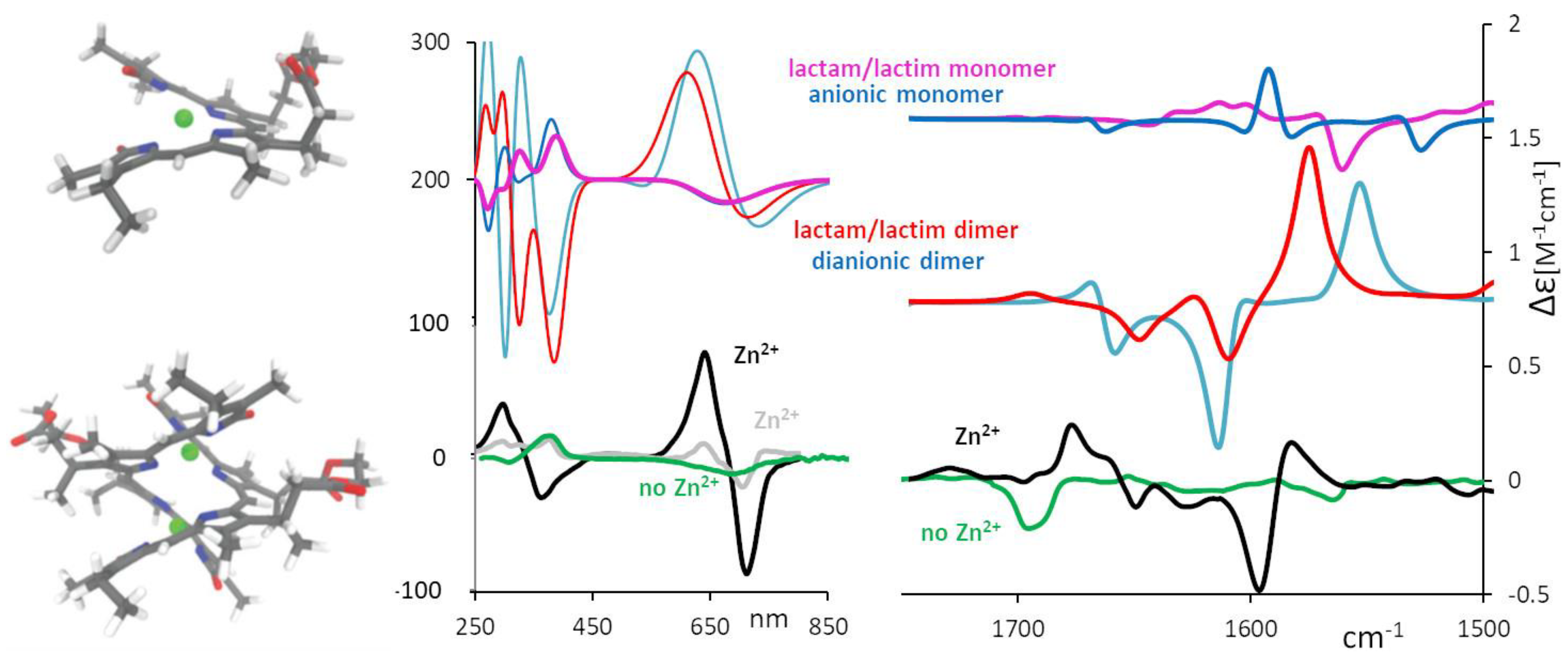
2.3. Aggregation and Sensing (of Bilirubin and Biliverdin Chiral Derivatives)
3. Deracemization with Chiral Agents
3.1. Small Molecules
3.2. Cyclodextrin and Surfactants
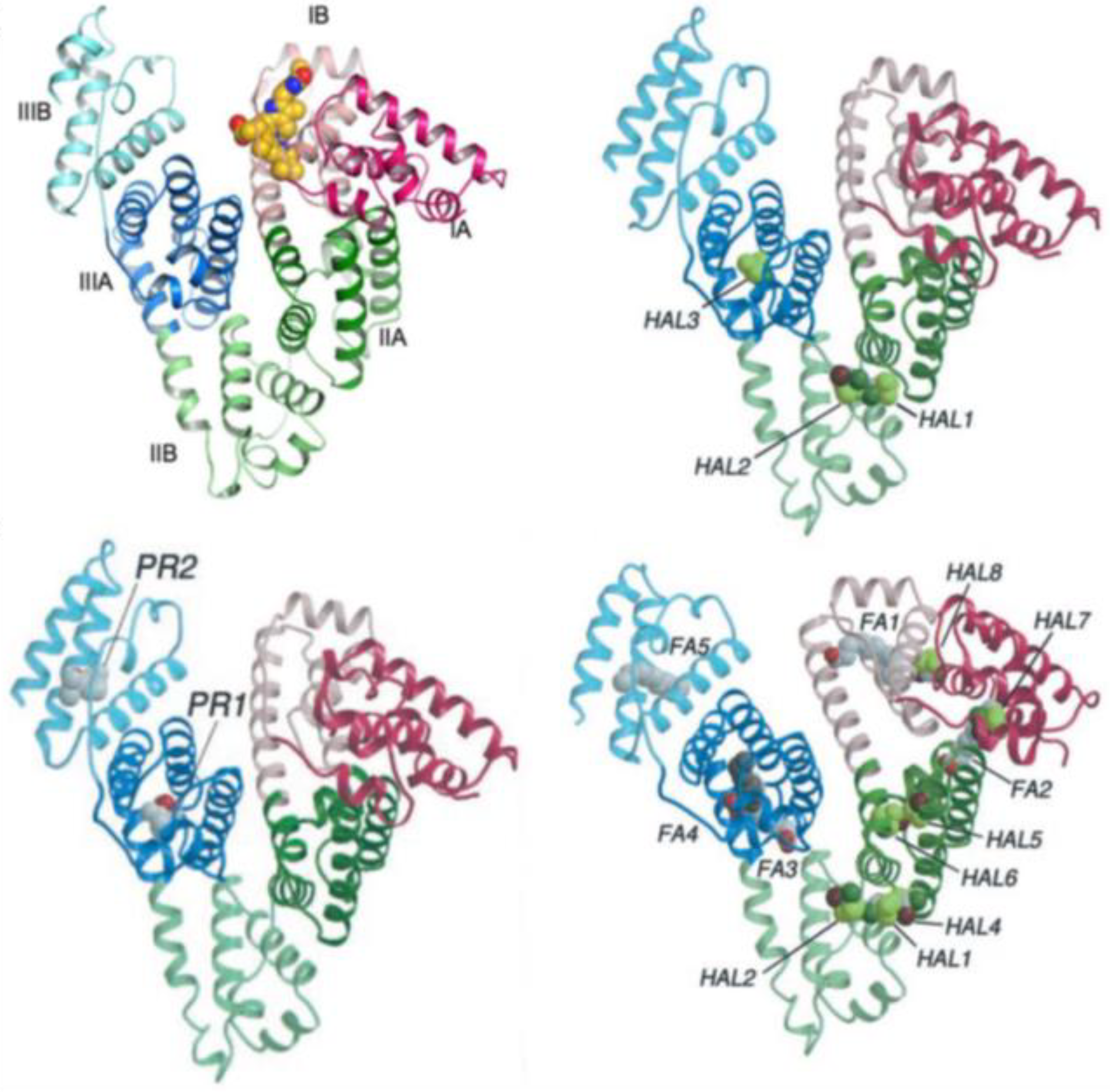
3.3. Serum Albumin: Discussion of an Example Dealing with Pharmaceutically Active Molecules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falk, H. The Chemistry of Linear Oligopyrroles and Bile Pigments; Springer: Vienna, Austria, 1989; ISBN 978-3-7091-6938-4. [Google Scholar]
- Lightner, D.A. Bilirubin: Jekyll and Hyde Pigment of Life Pursuit of Its Structure Through Two World Wars to the New Millenium; Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 2013; ISBN 978-3-7091-1637-1. [Google Scholar]
- Fischer, H.; Orth, H. Die Chemie Des Pyrrols; Akademische Verlagsgesellschaft: Leipzig, German, 1934. [Google Scholar]
- Fischer, H.; Orth, H. Die Chemie Des Pyrrols, Bd II. Pyrrolfarbstoffe. 1. Hälfte; Akademische Verlagsgesellschaft: Leipzig, German, 1937. [Google Scholar]
- Fischer, H.; Stern, A. Die Chemie Des Pyrrols, Bd II. Pyrrolfarbstoffe. 2. Hälfte; Akademische Verlagsgesellschaft: Leipzig, German, 1940. [Google Scholar]
- Fischer, H.; Zeile, K. Synthese des Hämatoporphyrins, Protoporphyrins und Hämins. Justus Liebigs Ann. Chem. 1929, 468, 98–116. [Google Scholar] [CrossRef]
- Fischer, H.; Plieninger, H. Synthese Des Biliverdins (Uteroverdins) Und Bilirubins, Der Biliverdine XIII α Und III α, Sowie Der Vinylneoxanthosäure. Hoppe-Seyler’S Z. Für Physiol. Chem. 1942, 274, 231–260. [Google Scholar] [CrossRef]
- Lemberg, R.; Legge, J.W. Hematin Compounds and Bile Pigments; Interscience Publishers: New York, NY, USA; London, UK, 1949. [Google Scholar]
- Wislicenus, J. Über Die Räumliche Anordnung Der Atome in Organischen Molekulen Und Ihre Bestimmung in Geometrisch-Isomeren Ungesättigten Verbindungen. In Abhandlungen der kőniglich sächsichen Geselschaft der Wissenschaften zu Leipzig; S. Hirzel: Leipzig, German, 1887; Volume 24, pp. 1–77. [Google Scholar]
- Anschütz, R. Zur Geschichte Der Isomerie Der Fumarsäure Und Der Maleïnsäure. Justus Liebigs Ann. Chem. 1889, 254, 168–182. [Google Scholar] [CrossRef]
- Fischer, H. Vorlesungen über Organische Chemie, Teil I. Aliphatische Chemie; Treibs, A., Ed.; Akademischer Verlag Dr. Peter Belej: München, German, 1950. [Google Scholar]
- Fischer, H. Vorlesungen über Organische Chemie, Teil II. Benzol Derivate; Treibs, A., Ed.; Akademischer Verlag Dr. Peter Belej: München, German, 1950. [Google Scholar]
- Manitto, P.; Severini-Ricca, G.S.; Monti, D. The Conformation of Bilirubin and Its Esters. Gazz Chim. Ital. 1974, 104, 633–637. [Google Scholar]
- Holm, F.; Städeler, G. Untersuchung Über Das Hämatoidin. J. Prakt. Chem. 1867, 100, 142. [Google Scholar] [CrossRef] [Green Version]
- Salkowski, E.L. Hoppe-Seyler’s Medicinisch-Chemische Untersuchungen. Von Hoppe-Seyler Berl. 1868, 436. [Google Scholar]
- Lightner, D.A.; McDonagh, A.F. Molecular Mechanisms of Phototherapy for Neonatal Jaundice. Acc. Chem. Res. 1984, 17, 417–424. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Lightner, D.A. ‘Like a Shrivelled Blood Orange’—Bilirubin, Jaundice, and Phototherapy. Pediatrics 1985, 75, 443–455. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Lightner, D.A. Attention to Stereochemistry. Chem. Eng. News 2003, 81, 2–3. [Google Scholar] [CrossRef] [Green Version]
- Ritter, S. Mind Your E’s and Z’s. Chem. Eng. News 2003, 81, 29–30. [Google Scholar]
- Čepa, A.; Dejmková, V.; Lešetický, L.; Jelínek, I.; Smrček, S.; Štícha, M.; Jašprová, J.; Urbanová, M.; Goncharova, I.; Dračínský, M.; et al. Physico-Chemical Characterization of Bilirubin-10-Sulfonate and Comparison of Its Acid–Base Behavior with Unconjugated Bilirubin. Sci. Rep. 2021, 11, 12896. [Google Scholar] [CrossRef]
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Lehninger Principles of Biochemistry; W. H. Freeman: New York, NY, USA, 2021; ISBN 978-1-319-38149-3. [Google Scholar]
- Lieberman, M.; Peet, A. Marks’ Basic Medical Biochemistry; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2022; ISBN 978-1-975174-71-2. [Google Scholar]
- Devlin, T.M. Textbook of Biochemistry with Clinical Correlations; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-60152-5. [Google Scholar]
- Chowdhury, J.R.; Wolkoff, A.W.; Chowdhury, N.R.; Arias, I.M. Hereditary Jaundice and Disorders of Bilirubin Metabolism. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Ostrow, J.D. Bile Pigments and Jaundice: Molecular, Metabolic, and Medical Aspects; Liver Series; Marcel Dekker: New York, NY, USA, 1986; ISBN 978-0-8247-7428-8. [Google Scholar]
- Sheldrick, W.S. Crystal and Molecular Structure of Biliverdin Dimethyl Ester. J. Chem. Soc. Perkin Trans. 2 1976, 1457–1462. [Google Scholar] [CrossRef]
- Bonnett, R.; Davies, J.E.; Hursthouse, M.B.; Sheldrick, W.S. The Structure of Bilirubin. Proc. R. Soc. Lond. B Biol. Sci. 1978, 202, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Davies, J.E.; Hursthouse, M.B. Structure of Bilirubin. Nature 1976, 262, 326–328. [Google Scholar] [CrossRef]
- Becker, W.; Sheldrick, W.S. Bile Pigment Structures. II. The Crystal Structure of Mesobilirubin IXα–Bis(Chloroform). Acta Crystallogr. B 1978, 34, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Le Bas, G.; Allegret, A.; Mauguen, Y.; de Rango, C.; Bailly, M. The Structure of Triclinic Bilirubin Chloroform–Methanol Solvate. Acta Crystallogr. B 1980, 36, 3007–3011. [Google Scholar] [CrossRef]
- Mugnoli, A.; Manitto, P.; Monti, D. Structure of Di-Isopropylammonium Bilirubinate. Nature 1978, 273, 568–569. [Google Scholar] [CrossRef]
- Mugnoli, A.; Manitto, P.; Monti, D. Structure of Bilirubin IXα (Isopropylammonium Salt) Chloroform Solvate, C33H34N4O62−.2C3H10N+.2CHCl3. Acta Crystallogr. C 1983, 39, 1287–1291. [Google Scholar] [CrossRef]
- Falk, H.; Grubmayr, K.; Thirring, K.; Gurker, N. Beiträge zur Chemie der Pyrrolpigmente, 21. Mitt.: Röntgenphotoelektronenspektrometrische Untersuchungen des N1s-Niveaus von Gallenpigmenten. Mon. Für Chem. 1978, 109, 1183–1189. [Google Scholar] [CrossRef]
- Hansen, P.E.; Jakobsen, H.J. A Natural Abundance15N NMR Investigation of Bilirubin IX-α. Org. Magn. Reson. 1984, 22, 668–670. [Google Scholar] [CrossRef]
- Eschenmoser, A. Organische Naturstoffsynthese heute Vitamin B12 als Beispiel. Naturwissenschaften 1974, 61, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.; Grubmayr, K.; Haslinger, E.; Schlederer, T.; Thirring, K. Beiträge zur Chemie der Pyrrolpigmente, 25. Mitt. Die diastereomeren (geometrisch isomeren) Biliverdindimethylester—Struktur, Konfiguration und Konformation. Mon. Für Chem.Chem. Mon. 1978, 109, 1451–1473. [Google Scholar] [CrossRef]
- Falk, H.; Grubmayr, K.; Thirring, K. Beiträge Zur Chemie Der Pyrrolpigmente, XXIII [1] Die Struktur Eines Bilatrien-Abc-Derivates Im Gelösten Zustande/On the Chemistry of Pyrrole Pigments, XXIII [1] The Structure of a Bilatriene-Abc Derivative in the State of Solution. Z. Für Nat. B 1978, 33, 924–931. [Google Scholar] [CrossRef]
- Kaplan, D.; Panigel, R.; Navon, G. Carbon-13 NMR Spectrum of Bilirubin. Spectrosc. Lett. 1977, 10, 881–892. [Google Scholar] [CrossRef]
- Kaplan, D.; Navon, G. Nuclear Magnetic Resonance Studies of the Conformation of Bilirubin and Its Derivatives in Solution. J. Chem. Soc. Perkin Trans. 2 1981, 1374–1381. [Google Scholar] [CrossRef]
- Kaplan, D.; Navon, G. NMR Spectroscopy of Bilirubin and Its Derivatives. Isr. J. Chem. 1983, 23, 177–186. [Google Scholar] [CrossRef]
- Falk, H.; Thirring, K. Beiträge zur chemie der pyrrolpigmente-XXXVII: Überbrückte gallenpiqmente: N21-N24-methylen-aetiobiliverdin- IV-γ und N21-N24-methylen-aetiobilirubin-IV-γ. Tetrahedron 1981, 37, 761–766. [Google Scholar] [CrossRef]
- Manitto, P.; Monti, D. Free-Energy Barrier of Conformational Inversion in Bilirubin. J. Chem. Soc. Chem. Commun. 1976, 122–123. [Google Scholar] [CrossRef]
- Navon, G.; Frank, S.; Kaplan, D. A Nuclear Magnetic Resonance Study of the Conformation and the Interconversion between the Enantiomeric Conformers of Bilirubin and Mesobilirubin in Solution. J. Chem. Soc. Perkin Trans. 2 1984, 1145–1149. [Google Scholar] [CrossRef]
- Person, R.V.; Peterson, B.R.; Lightner, D.A. Bilirubin Conformational Analysis and Circular Dichroism. J. Am. Chem. Soc. 1994, 116, 42–59. [Google Scholar] [CrossRef]
- Ghidinelli, S.; Longhi, G.; Abbate, S.; Boiadjiev, S.E.; Lightner, D.A. Bilirubin and Its Congeners: Conformational Analysis and Chirality from Metadynamics and Related Computational Methods. Mon. Für Chem. Chem. Mon. 2019, 150, 801–812. [Google Scholar] [CrossRef]
- Goncharova, I.; Urbanová, M. Stereoselective Bile Pigment Binding to Polypeptides and Albumins: A Circular Dichroism Study. Anal. Bioanal. Chem. 2008, 392, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, I.; Urbanová, M. Vibrational and Electronic Circular Dichroism Study of Bile Pigments: Complexes of Bilirubin and Biliverdin with Metals. Anal. Biochem. 2009, 392, 28–36. [Google Scholar] [CrossRef]
- Abbate, S.; Lebon, F.; Longhi, G.; Boiadjiev, S.E.; Lightner, D.A. Vibrational and Electronic Circular Dichroism of Dimethyl Mesobilirubins-XIIIα. J. Phys. Chem. B 2012, 116, 5628–5636. [Google Scholar] [CrossRef] [PubMed]
- Novotná, P.; Goncharova, I.; Urbanová, M. Mutual Structural Effect of Bilirubin and Model Membranes by Vibrational Circular Dichroism. Biochim. Biophys. Acta BBA Biomembr. 2014, 1838, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Ghidinelli, S.; Longhi, G.; Mazzeo, G.; Abbate, S.; Boiadjiev, S.E.; Lightner, D.A. On the Aggregation of Bilirubinoids in Solution as Evidenced by VCD and ECD Spectroscopy and DFT Calculations. Chirality 2018, 30, 19–28. [Google Scholar] [CrossRef]
- Ghidinelli, S.; Abbate, S.; Mazzeo, G.; Boiadjiev, S.E.; Lightner, D.A.; Longhi, G. Biliverdin Chiral Derivatives as Chiroptical Switches for PH and Metal Cation Sensing. Phys. Chem. Chem. Phys. 2021, 23, 20138–20151. [Google Scholar] [CrossRef] [PubMed]
- Ghidinelli, S.; Abbate, S.; Boiadjiev, S.E.; Lightner, D.A.; Longhi, G. l -Stercobilin-HCl and d -Urobilin-HCl. Analysis of Their Chiroptical and Conformational Properties by VCD, ECD, and CPL Experiments and MD and DFT Calculations. J. Phys. Chem. B 2018, 122, 12351–12362. [Google Scholar] [CrossRef]
- Nagano, S.; Sadeghi, M.; Balke, J.; Fleck, M.; Heckmann, N.; Psakis, G.; Alexiev, U. Improved Fluorescent Phytochromes for in Situ Imaging. Sci. Rep. 2022, 12, 5587. [Google Scholar] [CrossRef] [PubMed]
- Keum, H.; Yoo, D.; Jon, S. Photomedicine Based on Heme-Derived Compounds. Adv. Drug Deliv. Rev. 2022, 182, 114134. [Google Scholar] [CrossRef]
- Zagorec-Marks, W.; Dodson, L.G.; Weis, P.; Schneider, E.K.; Kappes, M.M.; Weber, J.M. Intrinsic Structure and Electronic Spectrum of Deprotonated Biliverdin: Cryogenic Ion Spectroscopy and Ion Mobility. J. Am. Chem. Soc. 2021, 143, 17778–17785. [Google Scholar] [CrossRef] [PubMed]
- Modi, V.; Donnini, S.; Groenhof, G.; Morozov, D. Protonation of the Biliverdin IXα Chromophore in the Red and Far-Red Photoactive States of a Bacteriophytochrome. J. Phys. Chem. B 2019, 123, 2325–2334. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, H.; Knobloch, G.; Plieninger, H.; Ehl, K.; Ruppert, J.; Moscowitz, A.; Watson, C.J. The Absolute Configuration of Natural (-)-Stercobilin and Other Urobilinoid Compounds. Proc. Natl. Acad. Sci. USA 1971, 68, 2141–2144. [Google Scholar] [CrossRef] [Green Version]
- Moscowitz, A.; Krueger, W.C.; Kay, I.T.; Skewes, G.; Bruckenstein, S. On the Origin of the Optical Activity in the Urobilins. Proc. Natl. Acad. Sci. USA 1964, 52, 1190–1194. [Google Scholar] [CrossRef] [Green Version]
- Lightner, D.A.; Docks, E.L.; Horwitz, J.; Moscowitz, A. Circular Dichroism Studies at Variable Temperature: Urobilinoid Conformation. Proc. Natl. Acad. Sci. USA 1970, 67, 1361–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.; Watson, C.J., II. Isolation of a Dextrorotatory Urobilin from Human Fistula Bile. Exp. Biol. Med. 1942, 49, 641–643. [Google Scholar] [CrossRef]
- Watson, C.J. The Origin Of Natural Crystalline Urobilin (Stercobilin). J. Biol. Chem. 1936, 114, 47–57. [Google Scholar] [CrossRef]
- Weigang, O.E.; Turner, J.A.; Trouard, P.A. Emission Polarization and Circular Dichroism of Hexahelicene. J. Chem. Phys. 1966, 45, 1126–1134. [Google Scholar] [CrossRef]
- Lightner, D.A.; Hefelfinger, D.T.; Frank, G.W.; Powers, T.W.; Trueblood, K.N. Absolute Configuration of Hexahelicene. Nat. Phys. Sci. 1971, 232, 124–125. [Google Scholar] [CrossRef]
- Scholtan, W.; Gloxhuber, C. Protein Binding of Bilirubin and Bromsulfalein. Arzneimittelforschung 1966, 16, 520–528. [Google Scholar]
- Blauer, G.; Harmatz, D.; Snir, J. Optical Properties of Bilirubin-Serum Albumin Complexes in Aqueous Solution. Biochim. Biophys. Acta BBA Protein Struct. 1972, 278, 68–88. [Google Scholar] [CrossRef]
- Boiadjiev, S.E.; Lightner, D.A. Stereocontrol of Bilirubin Conformation. Tetrahedron Asymmetry 1996, 7, 1309–1322. [Google Scholar] [CrossRef]
- Boiadjiev, S.E.; Pfeiffer, W.P.; Lightner, D.A. Synthesis and Stereochemistry of Bilirubin Analogs Lacking Carboxylic Acids. Tetrahedron 1997, 53, 14547–14564. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Boiadjiev, S.E.; Lightner, D.A. On the Aggregation of Bilirubins Using Vapor Pressure Osmometry. J. Heterocycl. Chem. 2000, 37, 863–870. [Google Scholar] [CrossRef]
- Boiadjiev, S.E.; Watters, K.; Wolf, S.; Lai, B.N.; Welch, W.H.; McDonagh, A.F.; Lightner, D.A. P K a and Aggregation of Bilirubin: Titrimetric and Ultracentrifugation Studies on Water-Soluble Pegylated Conjugates of Bilirubin and Fatty Acids. Biochemistry 2004, 43, 15617–15632. [Google Scholar] [CrossRef]
- Boiadjiev, S.E.; Anstine, D.T.; Lightner, D.A. Synthesis and Stereochemistry of Optically Active Biliverdin Cyclic Esters. Tetrahedron Asymmetry 1995, 6, 901–912. [Google Scholar] [CrossRef]
- Struckmeier, G.; Thewalt, U.; Fuhrhop, J.H. Structures of Zinc Octaethyl Formylbiliverdinate Hydrate and Its Dehydrated Bis-Helical Dimer. J. Am. Chem. Soc. 1976, 98, 278–279. [Google Scholar] [CrossRef]
- Bonfiglio, J.V.; Bonnett, R.; Buckley, D.G.; Hamzetash, D.; Hursthouse, M.B.; Malik, K.M.A.; McDonagh, A.F.; Trotter, J. Linear Tetrapyrroles as Ligands: Syntheses and x-Ray Analyses of Boron and Nickel Complexes of Octaethyl-21H,24H-Bilin-1,19-Dione. Tetrahedron 1983, 39, 1865–1874. [Google Scholar] [CrossRef]
- Pu, Y.-M.; Lightner, D.A. Intramolecular Exciton Coupling and Induced Circular Dichroism From Bilirubin-Ephedrine Heteroassociation Complexes. Stereochemical Models for Protein Binding. Croat. Chem. Acta 1989, 62, 301–324. [Google Scholar]
- Lightner, D.A.; An, J.-Y. Circular Dichroism of Bilirubin-Amine Heteroassociation Complexes. Tetrahedron 1987, 43, 4287–4296. [Google Scholar] [CrossRef]
- Gawronski, J.K.; Polonski, T.; Lightner, D.A. Sulfoxides as Chiral Complexation Agents. Conformational Enantiomer Resolution and Induced Circular Dichroism of Bilirubins. Tetrahedron 1990, 46, 8053–8066. [Google Scholar] [CrossRef]
- Trull, F.R.; Shrout, D.P.; Lightner, D.A. Chiral Recognition by Sulfoxides. Induced Circular Dichroism from Symmetric Mesobilirubin Analogs. Tetrahedron 1992, 48, 8189–8198. [Google Scholar] [CrossRef]
- Lightner, D.A.; Gawronski, J.K.; Gawronska, K. Conformational Enantiomerism in Bilirubin. Selection by Cyclodextrins. J. Am. Chem. Soc. 1985, 107, 2456–2461. [Google Scholar] [CrossRef]
- Goncharova, I.; Urbanová, M. Bile Pigment Complexes with Cyclodextrins: Electronic and Vibrational Circular Dichroism Study. Tetrahedron Asymmetry 2007, 18, 2061–2068. [Google Scholar] [CrossRef]
- Reisinger, M.; Lightner, D.A. Bilirubin Conformational Enantiomer Selection in Sodium Deoxycholate Chiral Micelles. J. Incl. Phenom. 1985, 3, 479–485. [Google Scholar] [CrossRef]
- Novotná, P.; Králík, F.; Urbanová, M. Chiral Recognition of Bilirubin and Biliverdin in Liposomes and Micelles. Biophys. Chem. 2015, 205, 41–50. [Google Scholar] [CrossRef]
- Sorrenti, A.; Altieri, B.; Ceccacci, F.; Di Profio, P.; Germani, R.; Giansanti, L.; Savelli, G.; Mancini, G. Deracemization of Bilirubin as the Marker of the Chirality of Micellar Aggregates. Chirality 2012, 24, 78–85. [Google Scholar] [CrossRef]
- Bombelli, C.; Bernardini, C.; Elemento, G.; Mancini, G.; Sorrenti, A.; Villani, C. Concentration as the Switch for Chiral Recognition in Biomembrane Models. J. Am. Chem. Soc. 2008, 130, 2732–2733. [Google Scholar] [CrossRef]
- Ceccacci, F.; Giansanti, L.; Mortera, S.L.; Mancini, G.; Sorrenti, A.; Villani, C. Enantiodiscrimination of Bilirubin-IXα Enantiomers in Biomembrane Models: Has Chirality a Role in Bilirubin Toxicity? Bioorganic Chem. 2008, 36, 252–254. [Google Scholar] [CrossRef]
- Lightner, D.A.; Reisinger, M.; Landen, G.L. On the Structure of Albumin-Bound Bilirubin. Selective Binding of Intramolecularly Hydrogen-Bonded Conformational Enantiomers. J. Biol. Chem. 1986, 261, 6034–6038. [Google Scholar] [CrossRef] [PubMed]
- Trull, F.R.; Person, R.V.; Lightner, D.A. Conformational Analysis of Symmetric Bilirubin Analogues with Varying Length Alkanoic Acids. Enantioselectivity by Human Serum Albumin. J. Chem. Soc. Perkin Trans. 2 1997, 1241–1250. [Google Scholar] [CrossRef]
- Goncharova, I.; Orlov, S.; Urbanová, M. Chiroptical Properties of Bilirubin-Serum Albumin Binding Sites: Chiroptical Properties Of Bilirubin-Serum Albumin Binding Sites. Chirality 2013, 25, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.; Goncharova, I.; Urbanová, M. Circular Dichroism Study of the Interaction between Mutagens and Bilirubin Bound to Different Binding Sites of Serum Albumins. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 126, 68–75. [Google Scholar] [CrossRef]
- Zsila, F. Circular Dichroism Spectroscopy Is a Sensitive Tool for Investigation of Bilirubin−Enzyme Interactions. Biomacromolecules 2011, 12, 221–227. [Google Scholar] [CrossRef]
- Zsila, F.; Mády, G. Biliverdin Is the Endogenous Ligand of Human Serum A1-Acid Glycoprotein. Biochem. Biophys. Res. Commun. 2008, 372, 503–507. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Ghuman, J.; McDonagh, A.F.; Curry, S. Crystallographic Analysis of Human Serum Albumin Complexed with 4Z,15E-Bilirubin-IXα. J. Mol. Biol. 2008, 381, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the General Anesthetics Propofol and Halothane to Human Serum Albumin. J. Biol. Chem. 2000, 275, 38731–38738. [Google Scholar] [CrossRef] [Green Version]
- Goncharova, I.; Orlov, S.; Urbanová, M. The Location of the High- and Low-Affinity Bilirubin-Binding Sites on Serum Albumin: Ligand-Competition Analysis Investigated by Circular Dichroism. Biophys. Chem. 2013, 180–181, 55–65. [Google Scholar] [CrossRef]
- Minomo, A.; Ishima, Y.; Kragh-Hansen, U.; Chuang, V.T.G.; Uchida, M.; Taguchi, K.; Watanabe, H.; Maruyama, T.; Morioka, H.; Otagiri, M. Biological Characteristics of Two Lysines on Human Serum Albumin in the High-Affinity Binding of 4Z,15Z-Bilirubin-IXα Revealed by Phage Display: Bilirubin-Binding Site on Human Serum Albumin. FEBS J. 2011, 278, 4100–4111. [Google Scholar] [CrossRef] [Green Version]
- Goncharova, I.; Jašprová, J.; Vítek, L.; Urbanová, M. Photo-Isomerization and Oxidation of Bilirubin in Mammals Is Dependent on Albumin Binding. Anal. Biochem. 2015, 490, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Lemli, B.; Lomozová, Z.; Huber, T.; Lukács, A.; Poór, M. Effects of Heme Site (FA1) Ligands Bilirubin, Biliverdin, Hemin, and Methyl Orange on the Albumin Binding of Site I Marker Warfarin: Complex Allosteric Interactions. Int. J. Mol. Sci. 2022, 23, 14007. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Pu, Y.-M.; Lightner, D.A. Effect of Volatile Anesthetics on the Circular Dichroism of Bilirubin Bound to Human Serum Albumin. Experientia 1992, 48, 246–248. [Google Scholar] [CrossRef]
- Chen, R.F. Fluorescence Stopped-Flow Study of Relaxation Processes in the Binding of Bilirubin to Serum Albumins. Arch. Biochem. Biophys. 1974, 160, 106–112. [Google Scholar] [CrossRef]
- Cu, A.; Bellah, G.G.; Lightner, D.A. Fluorescence of Bilirubin. J. Am. Chem. Soc. 1975, 97, 2579–2580. [Google Scholar] [CrossRef]
- Sawas, A.H.; Pentyala, S.N.; Rebecchi, M.J. Binding of Volatile Anesthetics to Serum Albumin: Measurements of Enthalpy and Solvent Contributions. Biochemistry 2004, 43, 12675–12685. [Google Scholar] [CrossRef] [PubMed]
- Streiff, J.H.; Allen, T.W.; Atanasova, E.; Juranic, N.; Macura, S.; Penheiter, A.R.; Jones, K.A. Prediction of Volatile Anesthetic Binding Sites in Proteins. Biophys. J. 2006, 91, 3405–3414. [Google Scholar] [CrossRef] [Green Version]
- Johansson, J.S.; Zou, H.; Tanner, J.W. Bound Volatile General Anesthetics Alter Both Local Protein Dynamics and Global Protein Stability. Anesthesiology 1999, 90, 235–245. [Google Scholar] [CrossRef]
- Zhang, T.; Johansson, J.S. An Isothermal Titration Calorimetry Study on the Binding of Four Volatile General Anesthetics to the Hydrophobic Core of a Four-α-Helix Bundle Protein. Biophys. J. 2003, 85, 3279–3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loll, P.J. Structural Analysis of Anesthetics in Complex with Soluble Proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 603, pp. 3–20. ISBN 978-0-12-814574-6. [Google Scholar]
- Wang, H.-J.; Kleinhammes, A.; Tang, P.; Xu, Y.; Wu, Y. Critical Role of Water in the Binding of Volatile Anesthetics to Proteins. J. Phys. Chem. B 2013, 117, 12007–12012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.K.; Terraneo, G.; Piacevoli, Q.; Bertolotti, F.; Scilabra, P.; Brown, J.T.; Rosokha, S.V.; Resnati, G. Molecular Bases for Anesthetic Agents: Halothane as a Halogen- and Hydrogen-Bond Donor. Angew. Chem. Int. Ed. 2019, 58, 12456–12459. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F. The Un(f)Told Story of General Anesthesia. ChemBioChem 2018, 19, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Tramarin, A.; Tedesco, D.; Naldi, M.; Baldassarre, M.; Bertucci, C.; Bartolini, M. New Insights into the Altered Binding Capacity of Pharmaceutical-Grade Human Serum Albumin: Site-Specific Binding Studies by Induced Circular Dichroism Spectroscopy. J. Pharm. Biomed. Anal. 2019, 162, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Eckenhoff, R.G. Weak Polar Interactions Confer Albumin Binding Site Selectivity for Haloether Anesthetics. Anesthesiology 2005, 102, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Fischer, A.; Su, K.-P.; Hussein, A.A.; Dandekar, T.; Broscheit, J. Novel Approach for Characterizing Propofol Binding Affinities to Serum Albumins from Different Species. ACS Omega 2020, 5, 25543–25551. [Google Scholar] [CrossRef]
- Gligorijević, N.; Minić, S. Bilirubin Interactions with Different Proteins and Implications of These Interactions. In Advances in Biology; Nova Science Publishers, Inc.: New York, NY, USA, 2022; Volume 1, pp. 85–122. ISBN 979-888697201-6. [Google Scholar]
- Gazzin, S.; Vitek, L.; Watchko, J.; Shapiro, S.M.; Tiribelli, C. A Novel Perspective on the Biology of Bilirubin in Health and Disease. Trends Mol. Med. 2016, 22, 758–768. [Google Scholar] [CrossRef]
- Gligorijević, N.; Minić, S.; Robajac, D.; Nikolić, M.; Ćirković Veličković, T.; Nedić, O. Characterisation and the Effects of Bilirubin Binding to Human Fibrinogen. Int. J. Biol. Macromol. 2019, 128, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D. Bilirubin Binding to PPARα Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, D.M.; Hong, S.H.; Kipp, Z.A.; Hinds, T.D. Identification of Binding Regions of Bilirubin in the Ligand-Binding Pocket of the Peroxisome Proliferator-Activated Receptor-A (PPARalpha). Molecules 2021, 26, 2975. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.A.; Wooldridge, T.A.; McDonagh, A.F. Photobilirubin: An Early Bilirubin Photoproduct Detected by Absorbance Difference Spectroscopy. Proc. Natl. Acad. Sci. USA 1979, 76, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lightner, D.A.; Wooldridge, T.A.; McDonagh, A.F. Configurational Isomerization of Bilirubin and the Mechanism of Jaundice Phototherapy. Biochem. Biophys. Res. Commun. 1979, 86, 235–243. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.F.; Palma, L.A.; Lightner, D.A. Phototherapy for Neonatal Jaundice. Stereospecific and Regioselective Photoisomerization of Bilirubin Bound to Human Serum Albumin and NMR Characterization of Intramolecularly Cyclized Photoproducts. J. Am. Chem. Soc. 1982, 104, 6867–6869. [Google Scholar] [CrossRef]
- Jasprova, J.; Dal Ben, M.; Vianello, E.; Goncharova, I.; Urbanova, M.; Vyroubalova, K.; Gazzin, S.; Tiribelli, C.; Sticha, M.; Cerna, M.; et al. The Biological Effects of Bilirubin Photoisomers. PLoS ONE 2016, 11, e0148126. [Google Scholar] [CrossRef]
- Pu, R.; Wang, Z.; Zhu, R.; Jiang, J.; Weng, T.-C.; Huang, Y.; Liu, W. Investigation of Ultrafast Configurational Photoisomerization of Bilirubin Using Femtosecond Stimulated Raman Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.; Ando, R.; Miyatake, H.; Greimel, P.; Kobayashi, T.; Hirabayashi, Y.; Shimogori, T.; Miyawaki, A. A Bilirubin-Inducible Fluorescent Protein from Eel Muscle. Cell 2013, 153, 1602–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Lee, S.; Lee, D.Y.; Yu, B.; Miao, W.; Jon, S. Multistimuli-Responsive Bilirubin Nanoparticles for Anticancer Therapy. Angew. Chem. Int. Ed. 2016, 55, 10676–10680. [Google Scholar] [CrossRef]
- Fathi, P.; Knox, H.J.; Sar, D.; Tripathi, I.; Ostadhossein, F.; Misra, S.K.; Esch, M.B.; Chan, J.; Pan, D. Biodegradable Biliverdin Nanoparticles for Efficient Photoacoustic Imaging. ACS Nano 2019, 13, 7690–7704. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhi, G.; Ghidinelli, S.; Abbate, S.; Mazzeo, G.; Fusè, M.; Boiadjiev, S.E.; Lightner, D.A. Insights into the Structures of Bilirubin and Biliverdin from Vibrational and Electronic Circular Dichroism: History and Perspectives. Molecules 2023, 28, 2564. https://doi.org/10.3390/molecules28062564
Longhi G, Ghidinelli S, Abbate S, Mazzeo G, Fusè M, Boiadjiev SE, Lightner DA. Insights into the Structures of Bilirubin and Biliverdin from Vibrational and Electronic Circular Dichroism: History and Perspectives. Molecules. 2023; 28(6):2564. https://doi.org/10.3390/molecules28062564
Chicago/Turabian StyleLonghi, Giovanna, Simone Ghidinelli, Sergio Abbate, Giuseppe Mazzeo, Marco Fusè, Stefan E. Boiadjiev, and David A. Lightner. 2023. "Insights into the Structures of Bilirubin and Biliverdin from Vibrational and Electronic Circular Dichroism: History and Perspectives" Molecules 28, no. 6: 2564. https://doi.org/10.3390/molecules28062564
APA StyleLonghi, G., Ghidinelli, S., Abbate, S., Mazzeo, G., Fusè, M., Boiadjiev, S. E., & Lightner, D. A. (2023). Insights into the Structures of Bilirubin and Biliverdin from Vibrational and Electronic Circular Dichroism: History and Perspectives. Molecules, 28(6), 2564. https://doi.org/10.3390/molecules28062564






