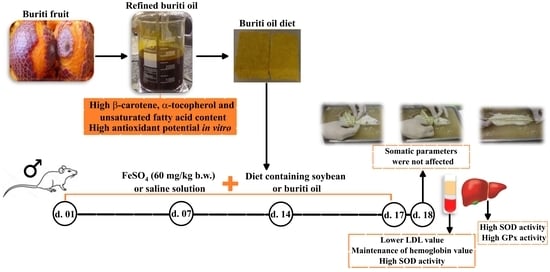
Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats
Abstract
:1. Introduction
2. Results
2.1. Oil Characterization
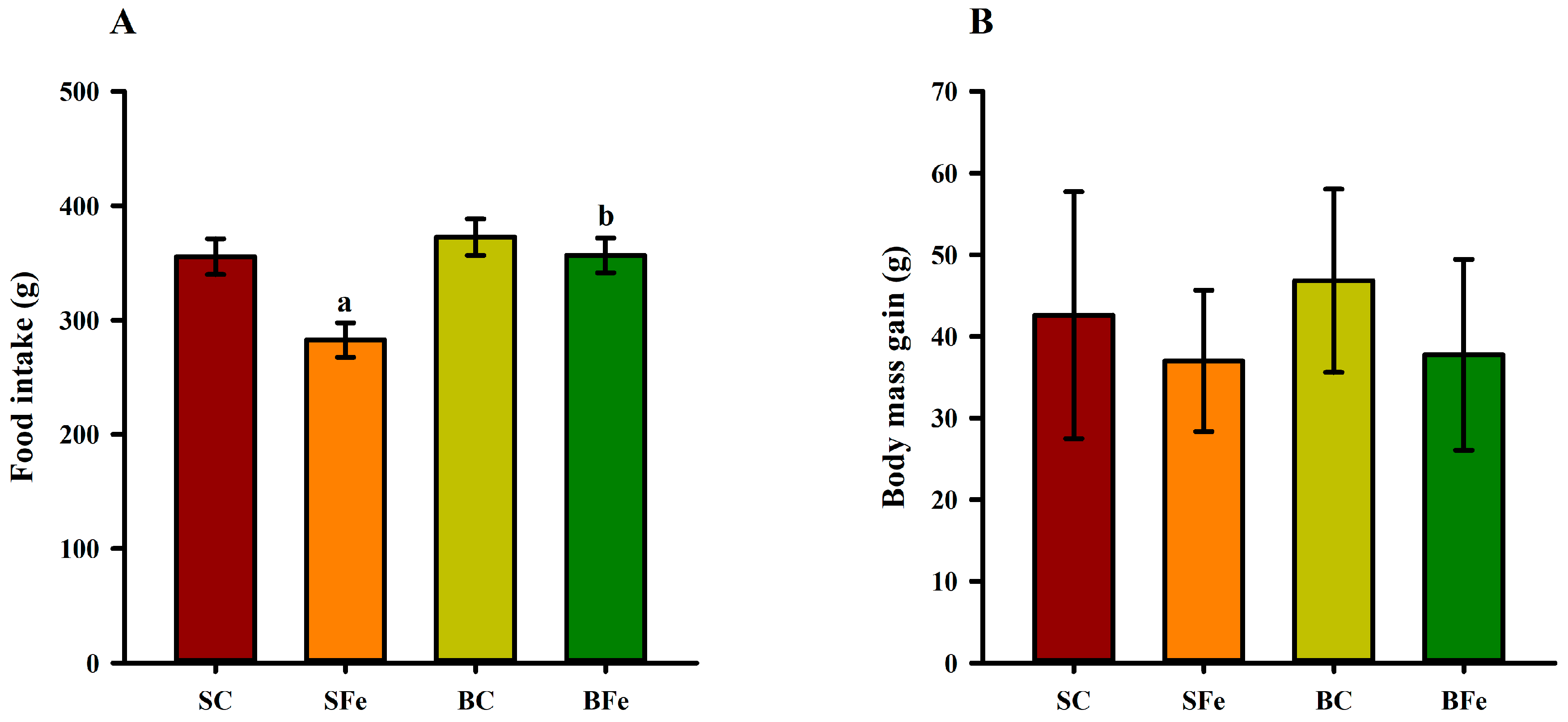
2.2. Evaluation of Somatic Parameters and Food Intake
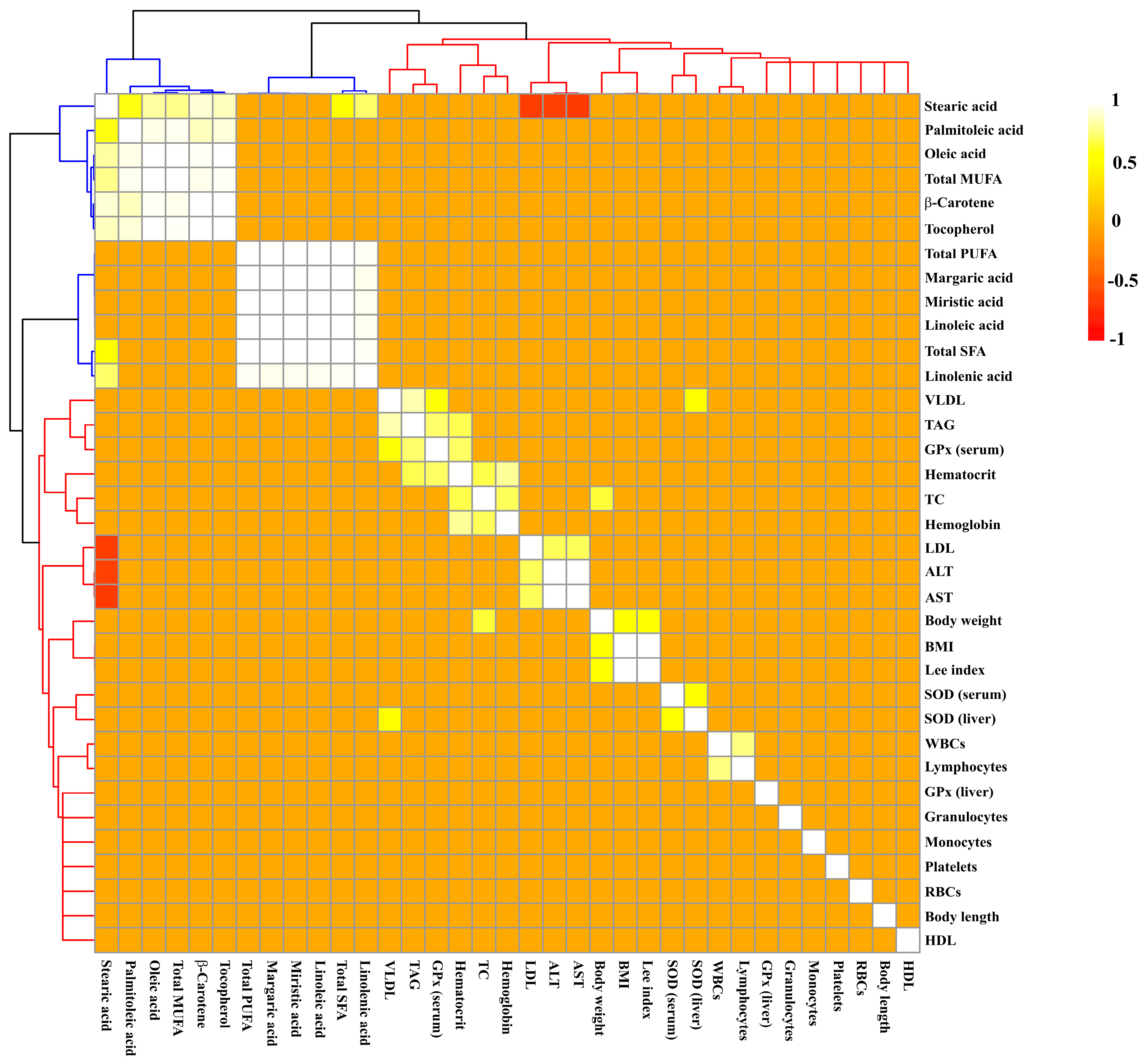
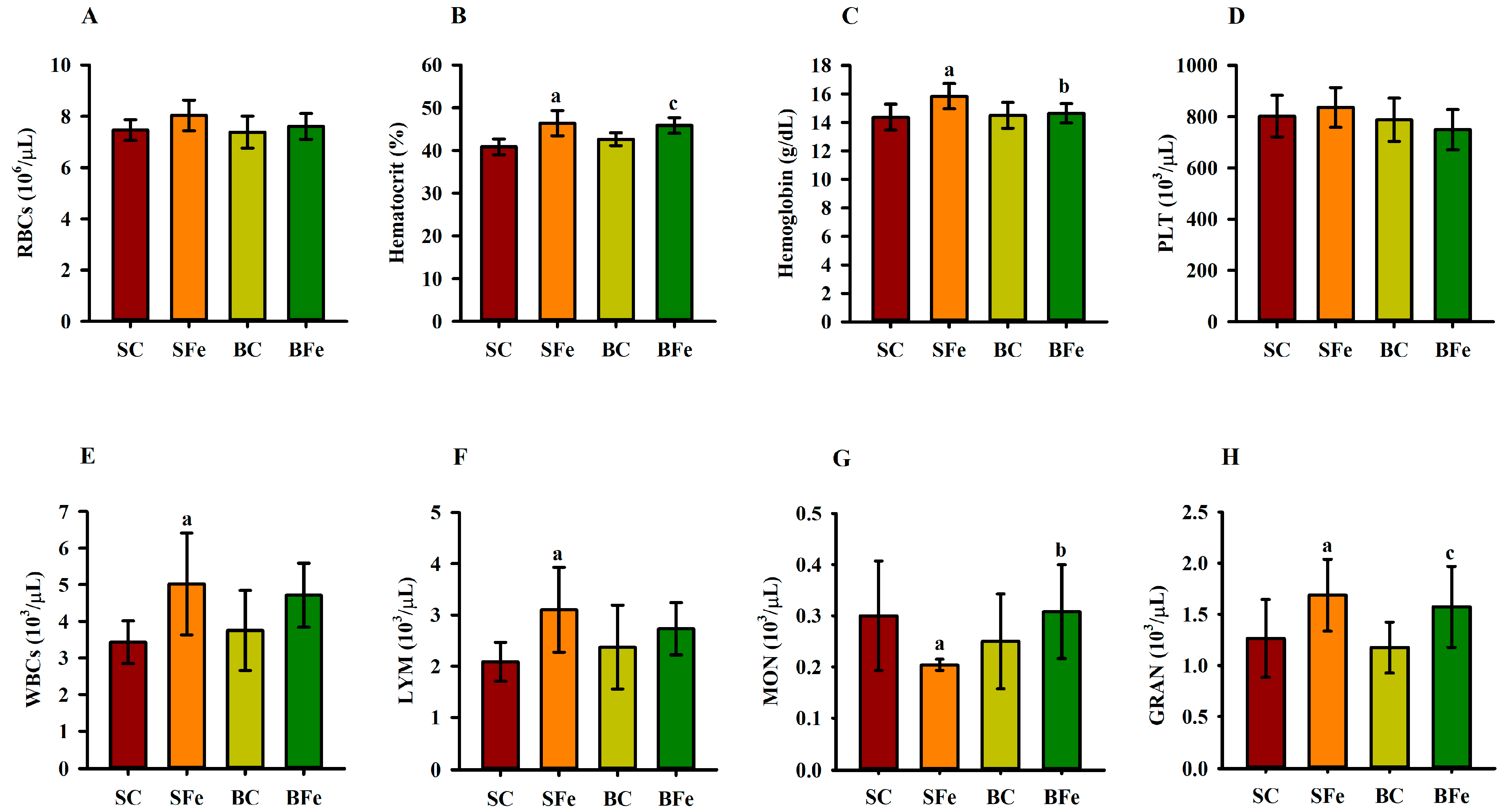
2.3. Effect of Buriti Oil on Lipid Profile, Aminotransferases, and Hematological Parameters
2.4. Effect of Buriti Oil on Antioxidant Enzyme Activity in Serum and Liver
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Oil Samples
4.3. Oil Chemical Characterization
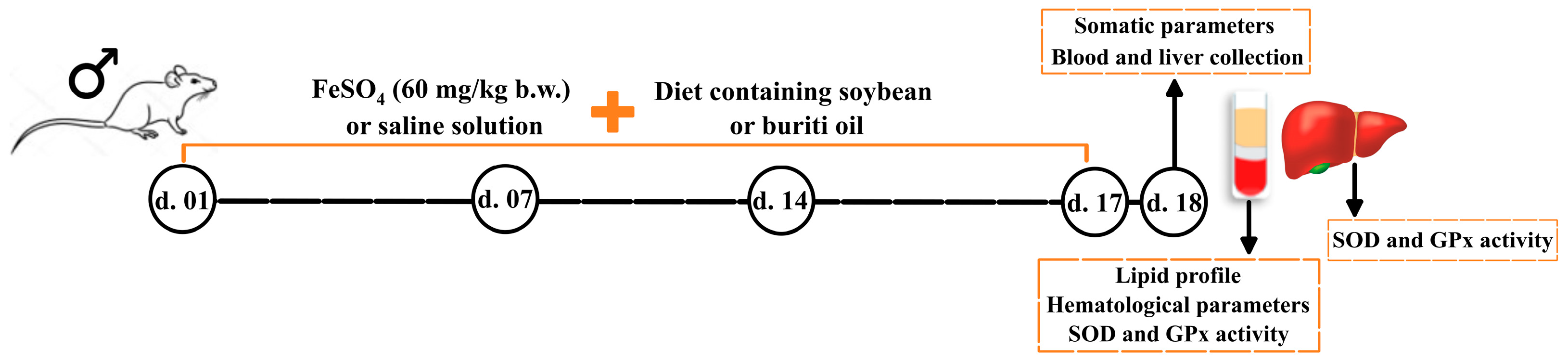
4.4. Animals, Diet, and Induction of Iron Overload
4.5. Food Intake, Weight Monitoring and Somatic Parameters
4.6. Buriti oil Effects on Lipid Profile and Hematological Biochemical Parameters
4.7. Antioxidant Activity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 100866, 1–9. [Google Scholar] [CrossRef]
- Cristina Sanz, A.G.-C.; Arturo, P. Kinetics of Iron Depletion in Hereditary Hemochromatosis. Blood 2018, 132, 3630. [Google Scholar] [CrossRef]
- Schaefer, B.; Meindl, E.; Wagner, S.; Tilg, H.; Zoller, H. Intravenous iron supplementation therapy. Mol. Asp. Med. 2020, 75, 100862. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Zhuo, Z.; Fang, S.; Hu, Q.; Huang, D.; Feng, J. Digital gene expression profiling analysis of duodenum transcriptomes in SD rats administered ferrous sulfate or ferrous glycine chelate by gavage. Sci. Rep. 2016, 6, 37923. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, L.; Oliveira, M.M.; Pessôa, M.T.C.; Barbosa, L.A. Iron overload: Effects on cellular biochemistry. Clin. Chim. Acta 2020, 504, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.L.; de Meneses, A.-A.P.M.; de Aguiar, R.P.S.; de Castro e Sousa, J.M.; de Carvalho Melo Cavalcante, A.A.; Maluf, S.W. Oxidative stress, antioxidant defense and depressive disorders: A systematic review of biochemical and molecular markers. Neurol. Psychiatry Brain Res. 2020, 36, 65–72. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Ghate, N.B.; Panja, S.; Das, A.; Mandal, N. Wild Edible Fruit of Prunus nepalensis Ser. (Steud), a Potential Source of Antioxidants, Ameliorates Iron Overload-Induced Hepatotoxicity and Liver Fibrosis in Mice. PLoS ONE 2015, 10, e0144280. [Google Scholar] [CrossRef] [Green Version]
- Nadir, S. The effect of ferrous sulphate on the gross and histological changes in the body of gastric mucosa of adult albino rats. J. Rawalpindi Med. Coll. 2015, 19, 275–279. [Google Scholar]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- Hussien, A.-M.A.; Hussein, M.A.; Mageed, A.D.A.E.; Abdel-Baky, A.M. Cranberry Extract as a Functional Food in Treatment of Oxidative Stress in Iron- Induced Hepatic Toxicity in Rats. J. Drug Metab. Toxicol. 2015, 6, 1000191. [Google Scholar] [CrossRef] [Green Version]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydinok, Y. Iron Chelation Therapy as a Modality of Management. Hematol. Oncol. Clin. 2018, 32, 261–275. [Google Scholar] [CrossRef]
- Kumfu, S.; Chattipakorn, S.; Chattipakorn, N. Chapter 11—Antioxidant and chelator cocktails to prevent oxidative stress under iron-overload conditions. In Pathology; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 117–126. [Google Scholar]
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef]
- Kramer, J.H.; Murthi, S.B.; Wise, R.M.; Mak, I.T.; Weglicki, W.B. Antioxidant and lysosomotropic properties of acute d-propranolol underlies its cardioprotection of postischemic hearts from moderate iron-overloaded rats. Exp. Biol. Med. 2006, 231, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Delaviz, H.; Mirzaei, M.; Tolooei, M. The effects of Medicago sativa and Allium porrum on iron overload in rats. Glob. J. Health Sci. 2015, 7, 137–142. [Google Scholar] [CrossRef] [Green Version]
- El-Shanshory, M.; Hablas, N.M.; Aboonq, M.S.; Fakhreldin, A.R.; Attia, M.; Arafa, W.; Mariah, R.A.; Baghdadi, H.; Ayat, M.; Zolaly, M.; et al. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J. Herb. Med. 2019, 16, 100245. [Google Scholar] [CrossRef]
- Hazra, B.; Sarkar, R.; Mandal, N. Spondias pinnata stem bark extract lessens iron overloaded liver toxicity due to hemosiderosis in Swiss albino mice. Ann. Hepatol. 2013, 12, 123–129. [Google Scholar] [CrossRef]
- Aquino, J.D.S.; Soares, J.K.B.; Magnani, M.; Stamford, T.C.M.; Mascarenhas, R.D.J.; Tavares, R.L.; Stamford, T.L.M. Effects of dietary Brazilian palm oil (Mauritia flexuosa L.) on cholesterol profile and vitamin A and E status of rats. Molecules 2015, 20, 9054–9070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Bailão, E.F.; Devilla, I.A.; Da Conceição, E.C.; Borges, L.L. Bioactive compounds found in Brazilian Cerrado fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.C.; Aquino, J.S.; Soares, J.; Figueiroa, E.B.; Mesquita, H.M.; Pessoa, D.C.; Stamford, T.M. Buriti oil (Mauritia flexuosa L.) negatively impacts somatic growth and reflex maturation and increases retinol deposition in young rats. Int. J. Dev. Neurosci. 2015, 46, 7–13. [Google Scholar] [CrossRef]
- Watkins, J.L.; Pogson, B.J. Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci. 2020, 25, 501–512. [Google Scholar] [CrossRef]
- Barboza, N.L.; Cruz, J.M.d.A.; Corrêa, R.F.; Lamarão, C.V.; Lima, A.R.; Inada, N.M.; Sanches, E.A.; Bezerra, J.d.A.; Campelo, P.H. Buriti (Mauritia flexuosa L. f.): An Amazonian fruit with potential health benefits. Food Res. Int. 2022, 159, 111654. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.N.; de Sá, É.R.A.; Bezerra, R.D.S.; Souza, J.L.; Lima, F.d.C.A. Constituents of buriti oil (Mauritia flexuosa L.) like inhibitors of the SARS-Coronavirus main peptidase: An investigation by docking and molecular dynamics. J. Biomol. Struct. Dyn. 2021, 39, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Morais, N.d.S.; Passos, T.S.; Ramos, G.R.; Ferreira, V.A.F.; Moreira, S.M.G.; Chaves Filho, G.P.; Barreto, A.P.G.; Leite, P.I.P.; Almeida, R.S.d.; Paulo, C.L.R.; et al. Nanoencapsulation of buriti oil (Mauritia flexuosa L.f.) in porcine gelatin enhances the antioxidant potential and improves the effect on the antibiotic activity modulation. PLoS ONE 2022, 17, e0265649. [Google Scholar] [CrossRef]
- Aquino, J.d.S.; Pessoa, D.C.N.d.P.; Araújo, K.d.L.G.V.; Epaminondas, P.S.; Schuler, A.R.P.; Souza, A.G.d.; Stamford, T.L.M. Refining of buriti oil (Mauritia flexuosa) originated from the Brazilian Cerrado: Physicochemical, thermal-oxidative and nutritional implications. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; Darnet, S.; Silva, L.H. Fatty acid profiles and tocopherol contents of buriti (Mauritia flexuosa), patawa (Oenocarpus bataua), tucuma (Astrocaryum vulgare), mari (Poraqueiba paraensis) and inaja (Maximiliana maripa) fruits. J. Braz. Chem. Soc. 2010, 21, 2000–2004. [Google Scholar] [CrossRef]
- Santos, M.d.F.G.d.; Alves, R.E.; Brito, E.S.D.; Silva, S.D.M.; Silveira, M.R.S.D.A. Quality characteristis of fruits and oils of palms native to the Brazilian Amazon. Rev. Bras. Frutic. 2017, 39, e-305. [Google Scholar] [CrossRef] [Green Version]
- Serra, J.L.; Rodrigues, A.M.d.C.; de Freitas, R.A.; Meirelles, A.J.d.A.; Darnet, S.H.; Silva, L.H.M.d. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef]
- De Souza, F.G.; Náthia-Neves, G.; de Araújo, F.F.; Dias Audibert, F.L.; Delafiori, J.; Neri-Numa, I.A.; Catharino, R.R.; de Alencar, S.M.; de Almeida Meireles, M.A.; Pastore, G.M. Evaluation of antioxidant capacity, fatty acid profile, and bioactive compounds from buritirana (Mauritiella armata Mart.) oil: A little-explored native Brazilian fruit. Food Res. Int. 2021, 142, 110260. [Google Scholar] [CrossRef]
- Lázaro, E.; Santas, J.; Rafecas, M. Recovery from dietary iron deficiency anaemia in rats by the intake of microencapsulated ferric saccharate. J. Food Sci. Technol. 2017, 54, 2913–2918. [Google Scholar] [CrossRef]
- Farias Machado, N.A.; de Oliveira Maia Parente, M.; Parente, H.N.; de Moura Zanine, A.; Moreira Filho, M.A.; da Cunha, I.A.L.; Santos Sousa, J.M.; dos Anjos, L.F.; de Jesus Ferreira, D.; Santos Araújo, J.d. The physiological response, feeding behaviour and water intake of feedlot lambs supplemented with babassu oil or buriti oil. Biol. Rhythm. Res. 2020, 51, 213–224. [Google Scholar] [CrossRef]
- Aquino, J.d.S.; Vasconcelos, M.H.d.A.; Pessoa, D.C.N.d.P.; Soares, J.K.B.; Prado, J.P.d.S.; Mascarenhas, R.d.J.; Magnani, M.; Stamford, T.L.M. Intake of cookies made with buriti oil (Mauritia flexuosa) improves vitamin A status and lipid profiles in young rats. Food Funct. 2016, 7, 4442–4450. [Google Scholar] [CrossRef]
- Choi, J.S.; Koh, I.-U.; Lee, H.J.; Kim, W.H.; Song, J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J. Nutr. Biochem. 2013, 24, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.R.; Haub, M.D.; Teeman, C.S.; Kurti, S.P.; Rosenkranz, S.K. Summation of blood glucose and TAG to characterise the ‘metabolic load index’. Br. J. Nutr. 2016, 116, 1553–1563. [Google Scholar] [CrossRef] [Green Version]
- Van Rooijen, M.A.; Plat, J.; Blom, W.A.M.; Zock, P.L.; Mensink, R.P. Dietary stearic acid and palmitic acid do not differently affect ABCA1-mediated cholesterol efflux capacity in healthy men and postmenopausal women: A randomized controlled trial. Clin. Nut. 2021, 40, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D. Dietary n-6 polyunsaturated fatty acids and cardiovascular disease: Epidemiologic evidence. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 5–9. [Google Scholar] [CrossRef]
- Yamagata, K. Chapter 7—Effects of dietary n-3 polyunsaturated fatty acids on cardiovascular disease. In Studies in Natural Products Chemistry; Atta Ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 217–240. [Google Scholar]
- Ye, Z.; Cao, C.; Li, Q.; Xu, Y.-J.; Liu, Y. Different dietary lipid consumption affects the serum lipid profiles, colonic short chain fatty acid composition and the gut health of Sprague Dawley rats. Food Res. Int. 2020, 132, 109117. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Barrera, C.; Valenzuela, R.; Rincón, M.Á.; Espinosa, A.; Echeverria, F.; Romero, N.; Gonzalez-Mañan, D.; Videla, L.A. Molecular mechanisms related to the hepatoprotective effects of antioxidant-rich extra virgin olive oil supplementation in rats subjected to short-term iron administration. Free. Rad. Biol. Med. 2018, 126, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Granado-Lorencio, F.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Hernández-Álvarez, E. Biomarkers of carotenoid bioavailability. Food Res. Int. 2017, 99, 902–916. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, J.; Ham, H.J.; Choue, R. Effects of d-α-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model. Nutr. Res. Pract. 2013, 7, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Qianchun, D.; Jiqu, X.; Fenghong, H.; Qingde, H.; Zhihua, Y.; Jine, Y. Effects of cold-pressed and vitamin E-enriched flaxseed oils on lipid profile and antioxidant status in high-fat fed rats. Eur. J. Lipid Sci. Technol. 2012, 114, 461–468. [Google Scholar] [CrossRef]
- Plaa, G.L. Evaluation of hepatotoxicity: Physiological and biochemical measures of hepatic function in animals. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 129–140. [Google Scholar]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular biomarkers in drug-induced liver injury: Challenges and future perspectives. Front. Pharmacol. 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE 2015, 10, e0134156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cunha, M.d.S.B.; Campos Hankins, N.A.; Arruda, S.F. Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Rajčević, N.; Bukvički, D.; Dodoš, T.; Marin, P.D. Interactions between natural products—A review. Metabolites 2022, 12, 1256. [Google Scholar] [CrossRef]
- Brissot, E.; Bernard, D.G.; Loréal, O.; Brissot, P.; Troadec, M.-B. Too much iron: A masked foe for leukemias. Blood Rev. 2020, 39, 100617. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.-K.; Wang, S.-C.; Ho, L.-W.; Huang, S.-W.; Lee, C.-H.; Lee, M.-S.; Yang, R.-C.; Shieh, J.-J. M2-like polarization of THP-1 monocyte-derived macrophages under chronic iron overload. Ann. Hematol. 2020, 99, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Ghozali, M.; Praptama, S.; Rini, W.; Ramdan, P.; Setiabudiawan, B.; Reniarti, L.; Syamsunarno, M.R.A.A. Decrease of peripheral monocyte relative number and mean platelet volume in iron overloaded mice. Biomed. Pharmacol. J. 2019, 12, 443–451. [Google Scholar] [CrossRef]
- Paolini, M.; Antelli, A.; Pozzetti, L.; Spetlova, D.; Perocco, P.; Valgimigli, L.; Pedulli, G.F.; Cantelli-Forti, G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis 2001, 22, 1483–1495. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasperczyk, S.; Dobrakowski, M.; Kasperczyk, J.; Ostałowska, A.; Zalejska-Fiolka, J.; Birkner, E. Beta-carotene reduces oxidative stress, improves glutathione metabolism and modifies antioxidant defense systems in lead-exposed workers. Toxicol. Appl. Pharmacol. 2014, 280, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Latief, U.; Husain, H.; Ahmad, R. β-Carotene supplementation ameliorates experimental liver fibrogenesis via restoring antioxidant status and hepatic stellate cells activity. J. Funct. Foods 2018, 49, 168–180. [Google Scholar] [CrossRef]
- Fasciolo, G.; Napolitano, G.; Aprile, M.; Cataldi, S.; Costa, V.; Ciccodicola, A.; Di Meo, S.; Venditti, P. Hepatic insulin resistance in hyperthyroid rat liver: Vitamin E supplementation highlights a possible role of ROS. Antioxidants 2022, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Sousa, A.; Nicola, P.; Ferreira de Oliveira, J.M.P.; Rufino, A.T.; Silva, M.; Freitas, M.; Carvalho, F.; Fernandes, E. β-Carotene and its physiological metabolites: Effects on oxidative status regulation and genotoxicity in in vitro models. Food Chem. Toxicolo. 2020, 141, 111392. [Google Scholar] [CrossRef]
- Pabón, M.L.; Lönnerdal, B. Effects of type of fat in the diet on iron bioavailability assessed in suckling and weanling rats. Trace Elem. Med. l 2001, 15, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yvonne, V.Y.; David, D.K. Dietary (n-3) fat and cholesterol alter tissue antioxidant enzymes and susceptibility to oxidation in SHR and WKY rats. J. Nutr. 2003, 133, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Arai, H. Oxidative Modification of Lipoproteins. In Lipid Hydroperoxide-Derived Modification of Biomolecules; Kato, Y., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 103–114. [Google Scholar]
- Zhang, X.; Xing, X.; Liu, H.; Feng, J.; Tian, M.; Chang, S.; Liu, P.; Zhang, H. Ionizing radiation induces ferroptosis in granulocyte-macrophage hematopoietic progenitor cells of murine bone marrow. Int. J. Radiat. Biol. 2020, 96, 584–595. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione peroxidase 4: A new player in neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; Rome/OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Pari, L.; Karthikeyan, A.; Karthika, P.; Rathinam, A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015, 2, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrous Sulfate CASRN: 7720-78-7. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/24393#section=Non-Human-Toxicity-Values (accessed on 5 June 2019).
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]


| Parameters | Soybean Oil | Buriti Oil | p |
|---|---|---|---|
| Antioxidant compounds (mg/kg) | |||
| β-carotene | 417.07 ± 1.75 | 787.05 ± 1.55 a | ≤0.001 |
| α-tocopherol | 302± 1.98 | 689.02 ± 2.11 | ≤0.001 |
| Fatty acids (g/100 g) | |||
| Miristic acid—C14:0 | 16.95 ± 0.04 a | 0.70 ± 0.01 | ≤0.001 |
| Margaric acid—C17:0 | 10.89 ± 0.30 a | 0.20 ± 0.01 | ≤0.001 |
| Stearic acid—C18:0 | 3.04 ± 0.20 | 3.30 ± 0.03 | 0.09 |
| Total SFA | 30.88 ± 0.90 a | 4.20 ± 0.05 | ≤0.001 |
| Palmitoleic acid—C16:1 | – | 19.0 ± 0.57 | |
| Oleic acid—C18:1 | 24.02 ± 0.55 | 72.30 ± 0.80 a | ≤0.001 |
| Total MUFA | 24.02 ± 0.55 | 91.30 ± 1.37 a | ≤0.001 |
| Linoleic acid—C18:2 | 40.00 ± 0.15 a | 2.40 ± 0.09 | ≤0.001 |
| Linolenic acid—C18:3 | 5.01 ± 0.15 a | 1.60 ± 0.03 | ≤0.001 |
| Total PUFA | 45.01 ± 0.30 a | 4.00 ± 1.20 | ≤0.001 |
| Parameters | SC | SFe | BC | BFe | p |
|---|---|---|---|---|---|
| FBW (g) | 369.2 ± 32.5 | 395.3 ± 27.3 | 404.7 ± 27.5 | 387.0 ± 21.4 | 0.308 |
| FBL (cm) | 33.1 ± 1.5 | 33.3 ± 1.1 | 33.0 ± 1.9 | 33.8 ± 1.7 | 0.774 |
| BMI (g/cm2) | 0.33 ± <0.1 | 0.34 ± <0.1 | 0.36 ± <0.1 | 0.34 ± <0.1 | 0.314 |
| Lee index | 0.22 ± <0.1 | 0.22 ± <0.1 | 0.22 ± <0.1 | 0.22 ± <0.1 | 0.586 |
| CC (cm) | 15.1 ± 1.0 | 15.7 ± 0.9 | 15.9 ± 1.0 | 15.3 ± 0.9 | 0.336 |
| AC (cm) | 16.8 ± 1.0 | 16.9 ± 0.6 | 17.2 ± 1.1 | 17.0 ± 1.2 | 0.826 |
| AC/CC ratio | 1.1 ± 1.0 | 1.0 ± 0.7 | 1.0 ± 1.0 | 1.1 ± 1.1 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Aquino, J.; Batista, K.S.; Araujo-Silva, G.; dos Santos, D.C.; de Brito, N.J.N.; López, J.A.; da Silva, J.A.; das Graças Almeida, M.; Pincheira, C.G.; Magnani, M.; et al. Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats. Molecules 2023, 28, 2585. https://doi.org/10.3390/molecules28062585
de Souza Aquino J, Batista KS, Araujo-Silva G, dos Santos DC, de Brito NJN, López JA, da Silva JA, das Graças Almeida M, Pincheira CG, Magnani M, et al. Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats. Molecules. 2023; 28(6):2585. https://doi.org/10.3390/molecules28062585
Chicago/Turabian Stylede Souza Aquino, Jailane, Kamila Sabino Batista, Gabriel Araujo-Silva, Darlan Coutinho dos Santos, Naira Josele Neves de Brito, Jorge A. López, João Andrade da Silva, Maria das Graças Almeida, Carla Guzmán Pincheira, Marciane Magnani, and et al. 2023. "Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats" Molecules 28, no. 6: 2585. https://doi.org/10.3390/molecules28062585
APA Stylede Souza Aquino, J., Batista, K. S., Araujo-Silva, G., dos Santos, D. C., de Brito, N. J. N., López, J. A., da Silva, J. A., das Graças Almeida, M., Pincheira, C. G., Magnani, M., de Pontes Pessoa, D. C. N., & Stamford, T. L. M. (2023). Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats. Molecules, 28(6), 2585. https://doi.org/10.3390/molecules28062585