Strong External Electric Fields Reduce Explosive Sensitivity: A Theoretical Investigation into the Reaction Selectivity in NH2NO2∙∙∙NH3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cooperativity of H-Bonds in Reactant under External Electric Field
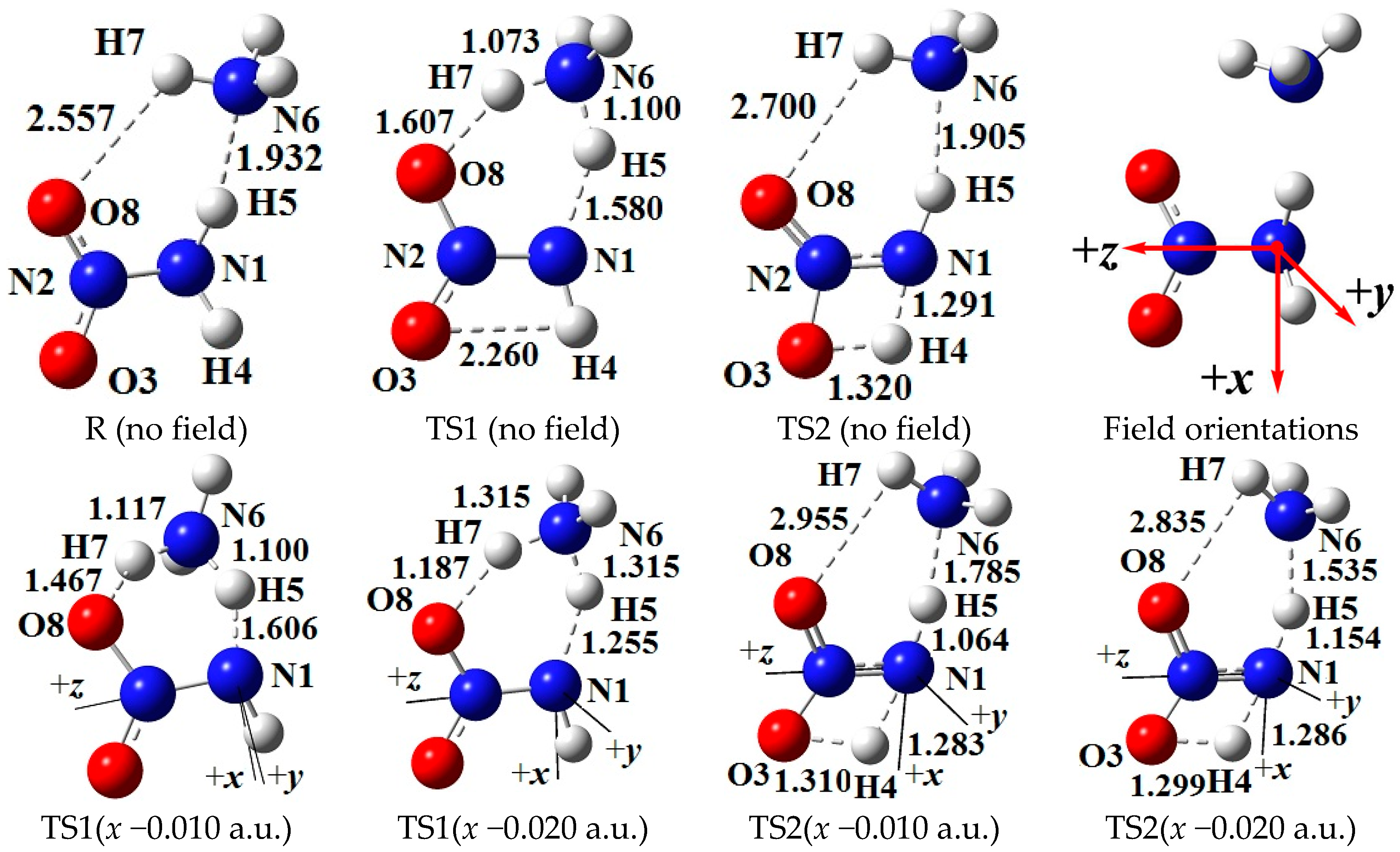
2.2. Concerted Effect of Intermolecular Hydrogen Exchange in External Electric Field
- (1)
- Structures of TS1
- (2)
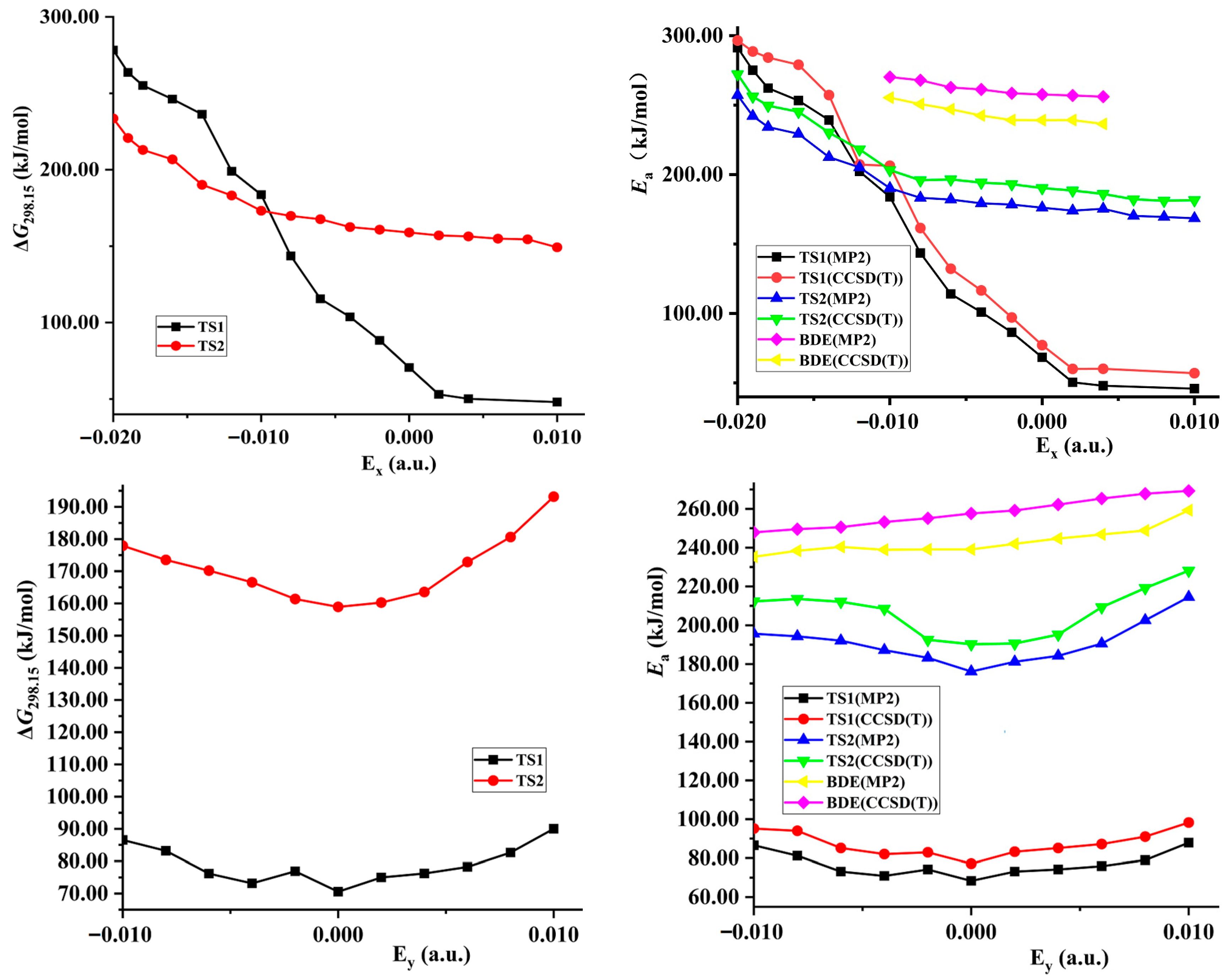
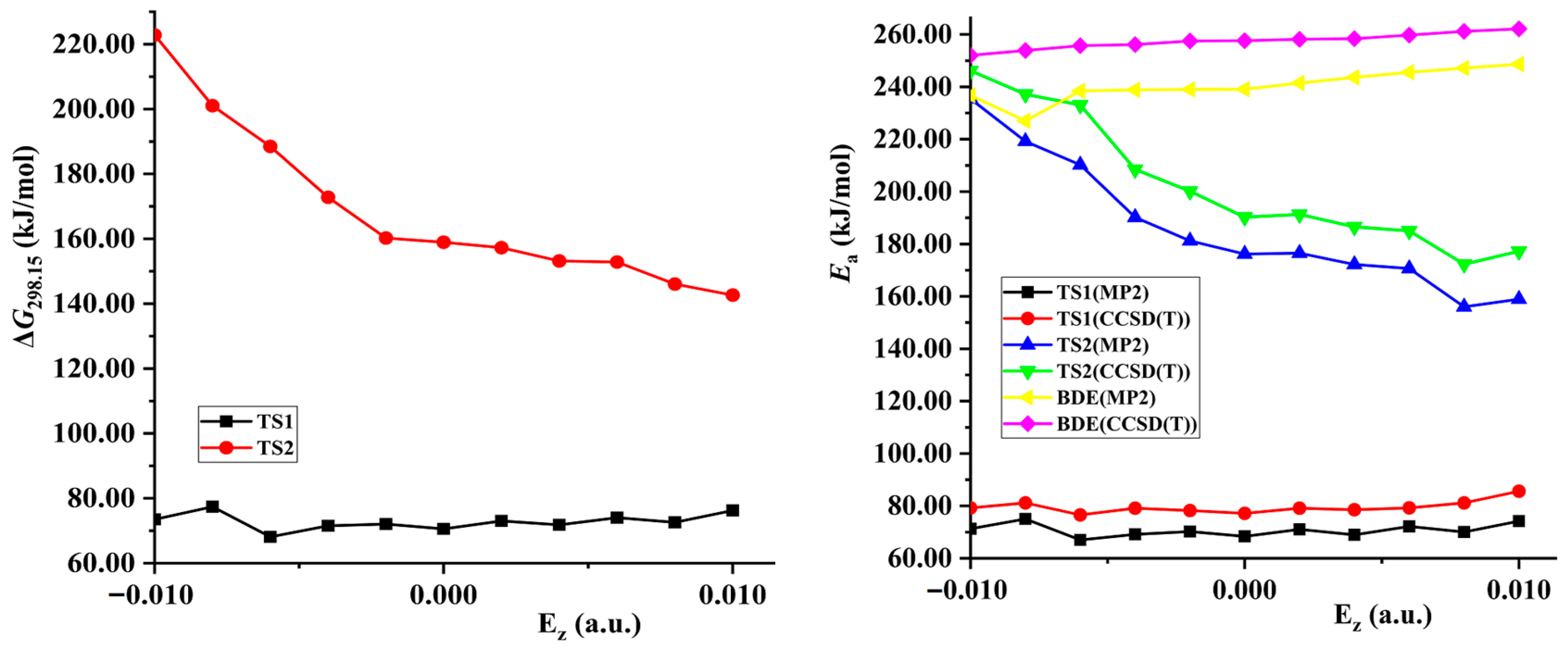
- Barrier, imaginary vibration, and rate constant of hydrogen exchange
- (3)
- Concerted reaction and reaction axis of hydrogen exchange
2.3. 1,3-Intramolecular Hydrogen Transfer in External Electric Field
2.4. Prediction of Explosive Sensitivity under External Electric Field
2.5. Surface Electrostatic Potentials of TS1 under the Field along −x-Orientation
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulazeem, M.S.; Alhasan, A.M.; Abdulrahmann, S. Initiation of solid explosives by laser. Int. J. Therm. Sci. 2011, 50, 2117–2121. [Google Scholar] [CrossRef]
- Zhao, L.; Yi, T.; Zhu, H.; Fu, Q.; Sun, X.; Yang, S.; Zheng, W.; Jiang, S. Electromagnetic pulse effect during the bridge wire electric explosion. Chin. J. Energ. Mater. 2019, 27, 481–486. [Google Scholar]
- Borisenok, V.A.; Mikhailov, A.S.; Bragunets, V.A. Investigation of the polarization of explosives during impact and the influence of an external electric field on the impact sensitivity of superfine PETN. Russ. J. Phys. Chem. B 2011, 5, 628–639. [Google Scholar] [CrossRef]
- Rodzevich, A.P.; Gazenaur, E.G.; Kuzmina, L.V.; Krasheninin, V.I.; Gazenaur, N.V. The effect of electric field in the explosive sensitivity of silver azide. J. Phys. Conf. Ser. 2017, 830, 012131. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.J.; Sun, X.J.; Zhang, L.; Lei, F.; Guo, F.; Yang, S.; Fu, Q.B. Sub-microsecond interferometry diagnostic and 3D dynamic simulation of the bridgewire electrical explosion. Chin. J. Energ. Mater. 2019, 27, 473–480. [Google Scholar]
- Politzer, P.; Murray, J.S.; Concha, M.C.; Lane, P. Effects of electric fields upon energetic molecules: Nitromethane and dimethylnitramine. Cent. Eur. J. Energ. Mat. 2007, 4, 3–21. [Google Scholar]
- Politzer, P.; Murray, J.S.; Lane, P. Computational determination of effects of electric fields upon “trigger linkages” of prototypical energetic molecules. Int. J. Quant. Chem. 2009, 109, 534–539. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Computed effects of electric fields upon the C–NO2 and N–NO2 bonds of nitromethane and dimethylnitramine. Int. J. Quant. Chem. 2009, 109, 3–7. [Google Scholar]
- Zhou, Z.J.; Li, X.P.; Liu, Z.B.; Li, Z.R.; Huang, X.R.; Sun, C.C. Electric field-driven acid-base chemistry: Proton transfer from acid (HCl) to Base (NH3/H2O). J. Phys. Chem. A 2011, 115, 1418–1422. [Google Scholar]
- Venelin, E.; Valentin, M.; Nadezhda, M.; Marin, R.; Silvia, A.; Milena, S. A model system with intramolecular hydrogen bonding: Effect of external electric field on the tautomeric conversion and electronic structures. Comput. Theor. Chem. 2013, 1006, 113–122. [Google Scholar]
- Cerón-Carrasco, J.P.; Jacquemin, D. Electric field induced DNA damage: An open door for selective mutations. Chem. Commun. 2013, 49, 7578–7580. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; de Visser, S.P.; Kumar, D. External electric field will control the selectivity of enzymatic-like bond activations. J. Am. Chem. Soc. 2004, 126, 11746–11749. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Mandal, D.; Ramanan, R. Oriented electric fields as future smart reagents in chemistry. Nat. Chem. 2016, 8, 1091–1098. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Joy, J.; Wang, Z.; Stuyver, T. Electric-field mediated chemistry: Uncovering and exploiting the potential of (oriented) electric fields to exert chemical catalysis and reaction control. J. Am. Chem. Soc. 2020, 142, 12551–12562. [Google Scholar] [CrossRef]
- Welborn, V.V.; Head-Gordon, T. Computational design of synthetic enzymes. Chem. Rev. 2019, 119, 6613–6630. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Danovich, D.; Chen, H.; Shaik, S. Oriented external electric fields: Tweezers and catalysts for reactivity in halogen-bond complexes. J. Am. Chem. Soc. 2019, 141, 7122–7136. [Google Scholar] [CrossRef]
- Laconsay, C.J.; Tsui, K.Y.; Tantillo, D.J. Tipping the balance: Theoretical interrogation of divergent extended heterolytic fragmentations. Chem. Sci. 2020, 11, 2231–2242. [Google Scholar] [CrossRef] [Green Version]
- Zang, Y.; Zou, Q.; Fu, T.; Ng, F.; Fowler, B.; Yang, J.; Li, H.; Steigerwald, M.L.; Nuckolls, C.; Venkataraman, L. Directing isomerization reactions of cumulenes with electric fields. Nat. Commun. 2019, 10, 4482. [Google Scholar] [CrossRef] [Green Version]
- Dubey, K.D.; Stuyver, T.; Kalita, S.; Shaik, S. Solvent-organization and rate-regulation of a menshutkin reaction by oriented-external electric fields are revealed by combined MD and QM/MM calculations. J. Am. Chem. Soc. 2020, 142, 9955–9965. [Google Scholar] [CrossRef]
- Joy, J.; Stuyver, T.; Shaik, S. Oriented external electric fields and ionic additives elicit catalysis and mechanistic crossover in oxidative addition reactions. J. Am. Chem. Soc. 2020, 142, 3836–3850. [Google Scholar] [CrossRef]
- Starr, R.L.; Fu, T.; Doud, E.A.; Stone, I.; Roy, X.; Venkataraman, L. Gold-carbon contacts from addition of aryl Iodides. J. Am. Chem. Soc. 2020, 142, 7128–7133. [Google Scholar] [CrossRef]
- Stuyver, T.; Huang, J.; Mallick, D.; Danovich, D.; Shaik, S. TITAN: A code for modeling and generating electric fields features and applications to enzymatic reactivity. J. Comput. Chem. 2020, 41, 74–82. [Google Scholar] [CrossRef]
- Stuyver, T.; Danovich, D.; De Proft, F.; Shaik, S. Electrophilic aromatic substitution reactions: Mechanistic landscape, electrostatic and electric-field control of reaction rates, and mechanistic crossovers. J. Am. Chem. Soc. 2019, 141, 9719–9730. [Google Scholar] [CrossRef]
- Yu, L.-J.; Coote, M.L. Electrostatic switching between SN1 and SN2 pathways. J. Phys. Chem. A 2019, 123, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Blyth, M.T.; Noble, B.B.; Russell, I.C.; Coote, M.L. Oriented internal electrostatic fields cooperatively promote ground- and excited-state reactivity: A case study in photochemical CO2 capture. J. Am. Chem. Soc. 2020, 142, 606–613. [Google Scholar] [CrossRef]
- Shaik, S.; Ramanan, R.; Danovich, D.; Mandal, D. Structure and reactivity/selectivity control by oriented-external electric fields. Chem. Soc. Rev. 2018, 47, 5125–5145. [Google Scholar] [CrossRef]
- Stuyver, T.; Danovich, D.; Joy, J.; Shaik, S. External electric field effects on chemical structure and reactivity. WIRes Comput. Mol. Sci. 2020, 10, e1438. [Google Scholar] [CrossRef]
- Alemani, M.; Peters, M.V.; Hecht, S.; Rieder, K.H.; Moresco, F.; Grill, L. Electric field-induced isomerization of azobenzene by STM. J. Am. Chem. Soc. 2006, 128, 14446–14447. [Google Scholar] [CrossRef]
- Meir, R.; Chen, H.; Lai, W.; Shaik, S. Oriented electric fields accelerate diels−alder reactions and control the endo/exo selectivity. Chem. Phys. Chem. 2010, 11, 301–310. [Google Scholar] [CrossRef]
- Ren, F.-D.; Shi, W.-J.; Cao, D.-L.; Li, Y.-X.; Zhang, D.-H.; Wang, X.-F.; Shi, Z.-Y. External electric field reduces the explosive sensitivity: A theoretical investigation into the hydrogen transference kinetics of the NH2NO2∙∙∙H2O complex. J. Mol. Model. 2020, 26, 351. [Google Scholar] [CrossRef]
- Cabalo, J.; Sausa, R. Theoretical and experimental study of the C–H stretching overtones of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12 hexaazaisowurtzitane (CL-20). J. Phys. Chem. A 2013, 117, 9039–9046. [Google Scholar] [CrossRef] [PubMed]
- Macharla, A.K.; Parimi, A.; Anuj, A.V. Decomposition mechanism of hexanitrohexaazaisowurtzitane (CL-20) by coupled computational and experimental study. J. Phys. Chem. A 2019, 123, 4014–4020. [Google Scholar]
- Demske, D. The Experimental Aspects of Coupling Electrical Energy into a Dense Detonation Wave: Part 1; Defense technical information center: Fort belvoir, VA, USA, 1982; pp. 79–143. [Google Scholar]
- Wang, Y.; Ren, F.; Cao, D. A dynamic and electrostatic potential prediction of the prototropic tautomerism between imidazole 3-oxide and 1-hydroxyimidazole in external electric field. J. Mol. Model. 2019, 25, 330. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Cao, D.; Shi, W.; You, M. A dynamic prediction of stability for nitromethane in external electric field. RSC Adv. 2017, 74, 47063–47072. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Cao, D.; Shi, W. A dynamics prediction of nitromethane → methyl nitrite isomerization in external electric field. J. Mol. Model. 2016, 22, 96. [Google Scholar] [CrossRef]
- Shu, Y.J.; Dubikhin, V.V.; Nazin, G.M.; Manelis, G.B. Effect of solvents on thermal decomposition of RDX. Chin. J. Energ. Mater. 2000, 8, 108–110. [Google Scholar]
- Wang, H.B.; Shi, W.J.; Ren, F.D.; Yang, L.; Wang, J.L. A B3LYP and MP2(full) theoretical investigation into explosive sensitivity upon the formation of the intermolecular hydrogen-bonding interaction between the nitro group of RNO2(R = –CH3, –NH2, –OCH3) and HF, HCl or HBr. Comput. Theor. Chem. 2012, 994, 73–80. [Google Scholar] [CrossRef]
- Shu, Y.J.; Dubikhin, V.V.; Nazin, G.M.; Manelis, G.B. Thermal decomposition mechanism of RDX in inertial solvents. Chin. J. Explos. Propellants 2001, 4, 58–60. [Google Scholar]
- Li, B.H.; Shi, W.J.; Ren, F.D.; Wang, Y. A B3LYP and MP2(full) theoretical investigation into the strength of the C–NO2 bond upon the formation of the intermolecular hydrogen-bonding interaction between HF and the nitro group of nitrotriazole or its methyl derivatives. J. Mol. Model. 2013, 19, 511–519. [Google Scholar] [CrossRef]
- Qiu, W.; Ren, F.D.; Shi, W.J.; Wang, Y.H. A theoretical study on the strength of the C–NO2 bond and ring strain upon the formation of the intermolecular H-bonding interaction between HF and nitro group in nitrocyclopropane, nitrocyclobutane, nitrocyclopentane or nitrocyclohexane. J. Mol. Model. 2015, 21, 114–122. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Zhao, F.Q.; Ju, X.H.; Cheng, X.C.; Yi, J.H. A density functional theory study of adsorption and decomposition of nitroamine molecules on the Al(111) surface. J. Phys. Chem. C 2010, 114, 9390–9397. [Google Scholar] [CrossRef]
- Ju, G.Z.; Ju, Q. The theoretically thermodynamic and kinetic studies on the scission and rearrangement of NH2NO2. Chem. J. Chin. Univ. 1991, 12, 1669–1671. [Google Scholar]
- Ren, F.; Cao, D.; Shi, W.; You, M.; Li, M. A theoretical prediction of the possible trigger linkage of CH3NO2 and NH2NO2 in an external electric field. J. Mol. Model. 2015, 21, 145. [Google Scholar] [CrossRef]
- Zeman, S.; Atalar, T.; Friedl, Z.; Ju, X.H. Accounts of the new aspects of nitromethane initiation reactivity. Cent. Eur. J. Energ. Mat. 2009, 6, 119–133. [Google Scholar]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in noncovalent interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef]
- Zilberberg, I.; Gora, R.W.; Zhidomirov, G.M.; Leszczynski, J. Bonding in the oxo ferrous iron species: A complete active-space self-consistent-field theory verification of the molecular-oxygen-like pattern. J. Chem. Phys. 2002, 117, 7153–7161. [Google Scholar] [CrossRef]
- František, K.; Michal, O. First step in the reaction of zerovalent iron with water. J. Chem. Theory Comput. 2011, 7, 2876–2885. [Google Scholar]
- Krylov, A.I. Spin-contamination of coupled-cluster wave functions. J. Chem. Phys. 2000, 113, 6052. [Google Scholar] [CrossRef]
- Salter, E.A.; Sekino, H.; Bartlett, R.J. Property evaluation and orbital relaxation in coupled cluster methods. J. Chem. Phys. 1987, 87, 502–509. [Google Scholar] [CrossRef]
- Emine, S.; Uğur, B. Assessment of orbital-optimized MP2.5 for thermochemistry and kinetics: Dramatic failures of standard perturbation theory approaches for aromatic bond dissociation energies and barrier heights of radical reactions. J. Chem. Theory Comput. 2015, 11, 1564–1573. [Google Scholar]
- Marios-Petros, K.; Stella, S. Spin contamination in MP2 and CC2, a surprising issue. J. Chem. Phys. 2021, 154, 131101. [Google Scholar]
- Stanton, J.F. On the extent of spin contamination in open-shell coupled-cluster wave functions. J. Chem. Phys. 1994, 101, 371–374. [Google Scholar] [CrossRef]
- Szalay, P.G.; Gauss, J. Spin-restricted open-shell coupled-cluster theory for excited states. J. Chem. Phys. 2000, 112, 4027. [Google Scholar] [CrossRef]
- Glasstone, S.; Laidler, K.J.; Eyring, H. The Theory of Rate Processes, 1st ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1941. [Google Scholar]
- Tasker, D. The Properties of Condensed Explosives for Electromagnetic Energy Coupling; Defense technical information center: Fort belvoir, VA, USA, 1985; pp. 85–360. [Google Scholar]
- Piehler, T.; Hummer, C.; Benjamin, R.; Summers, E.; Mc Nesby, K.; Boyle, V. Preliminary study of coupling electrical energy to detonation reaction zone of primasheet-1000 explosive. In Proceedings of the 27th International Symposium on Ballistics, Freiburg, Germany, 22–26 April 2013; pp. 22–26. [Google Scholar]
- Arnold, K.; Hoekstra, D.; Ohki, S. Association of lysozyme to phospholipid surfacesand vesicle fusion. Biochim. Biophys. Acta 1992, 1124, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Brockman, H. Lipid monolayers: Why use half a membrane to characterize protein–membrane interactions? Curr. Opin. Struct. Biol. 1999, 9, 438–443. [Google Scholar] [CrossRef]
- Chen, J.; Reed, M.A.; Rawlett, A.M.; Tour, J.M. Large on-off ratio and negative differential resistance in a molecular electronic device. Science 1999, 286, 1550–1552. [Google Scholar] [CrossRef] [Green Version]
- Reed, M.A.; Zhou, C.; Muller, C.J.; Burgin, T.P.; Tour, J.M. Conductance of a molecular junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Sandi, G.; Kaminski, M.; Bobadilla, A.; Mertz, C.; Seminario, J.M. Electron transport in graphene-based nanosensors for Eu(III) detection. J. Phys. Chem. C 2015, 119, 12037–12046. [Google Scholar] [CrossRef]
- Benitez, L.; Cristancho, D.; Seminario, J.M.; Hoz, J.M.; Balbuena, P.B. Electron transfer through solid-electrolyteinterphase layers formed on Si anodes of Li-ion batteries. Electrochim. Acta 2014, 140, 250–257. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Rice, B.M.; Hare, J.J. A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J. Phys. Chem. A 2002, 106, 1770–1783. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Some molecular/crystalline factors that affect the sensitivities of energetic materials: Molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J. Mol. Model. 2015, 21, 25–35. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Impact sensitivity and crystal lattice compressibility/free space. J. Mol. Model. 2014, 20, 2223–2230. [Google Scholar] [CrossRef]
- Murray, J.S.; Concha, M.C.; Politzer, P. Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C–NO2/N–NO2 bond dissociation energies. Mol. Phys. 2009, 107, 89–97. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Some perspectives on sensitivity to initiation of detonation. In Green Energetic Materials; Brinck, T., Ed.; Wiley: Chichester, UK, 2014; Volume 3, pp. 45–62. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Li, J.S. A quantitative relationship for the shock sensitivities of energetic compounds based on X–NO2 (X = C, N, O) bond dissociation energy. J. Hazard. Mater. 2010, 180, 768–772. [Google Scholar] [CrossRef]
- Tan, B.S.; Long, X.P.; Peng, R.F.; Li, H.B.; Jin, B.; Chu, S.J.; Dong, H.S. Two important factors influencing shock sensitivity of nitro compounds: Bond dissociation energy of X–NO2 (X=C, N, O) and Mulliken charges of nitro group. J. Hazard. Mater. 2010, 183, 908–912. [Google Scholar] [CrossRef]
- Youn, I.S.; Cho, W.J.; Kim, K.S. Effects of an electric field on interaction of aromatic systems. J. Comput. Chem. 2016, 37, 971–975. [Google Scholar] [CrossRef]
- Baranowska, A.; Capelo, S.B.; Fernández, B. New basis sets for the evaluation of interaction energies: An ab initio study of the He–He, Ne–Ne, Ar–Ar, He–Ne, He–Ar and Ne–Ar van der Waals complex internuclear potentials and ro-vibrational spectra. Phys. Chem. Chem. Phys. 2010, 12, 13586–13596. [Google Scholar] [CrossRef]
- Hill, J.G.; Peterson, K.A.; Knizia, G.; Werner, H.-J. Extrapolating MP2 and CCSD explicitly correlated correlation energies to the complete basis set limit with first and second row correlation consistent basis sets. J. Chem. Phys. 2009, 131, 194105. [Google Scholar] [CrossRef] [PubMed]
- Holger, K.; Pavel, B.; Jiří, Š. Investigations of stacked DNA base-Pair steps: Highly accurate stacking interaction energies, energy decomposition, and many-body stacking effects. J. Chem. Theory Comput. 2019, 15, 95–115. [Google Scholar]
- Flaig, D.; Maurer, M.; Hanni, M.; Braunger, K.; Kick, L.; Thubauville, M.; Ochsenfeld, C. Benchmarking hydrogen and carbon NMR chemical shifts at HF, DFT, and MP2 levels. J. Chem. Theory Comput. 2014, 10, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Friedrich, J.; Dolg, M. Fully automated incremental evaluation of MP2 and CCSD(T) core, core-valence and valence correlation energies. Chem. Phys. 2010, 376, 36–45. [Google Scholar] [CrossRef]
- Arathala, P.; Tangtartharakul, C.B.; Sinha, A. Atmospheric ring-closure and dehydration reactions of 1,4-Hydroxycarbonyls in the gas Phase: The impact of catalysts. J. Phys. Chem. A 2021, 125, 5963–5975. [Google Scholar] [CrossRef]
- Boese, A.D. Assessment of coupled cluster theory and more approximate methods for hydrogen bonded systems. J. Chem. Theory Comput. 2013, 9, 4403–4413. [Google Scholar] [CrossRef]
- Wigner, E.P. Über das überschreiten von potential schwellen bei chemischen reaktionen. Z. Phys. Chem. B 1932, 19, 203–216. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Arabi, A.A.; Matta, C.F. Effects of external electric fields on double proton transfer kinetics in the formic acid dimer. Phys. Chem. Chem. Phys. 2011, 13, 13738–13748. [Google Scholar] [CrossRef]
- Eyring, H.; Eyring, E.M. Modern Chemical Kinetics; Reinhold Publishing Corporation: New York, NY, USA, 1963. [Google Scholar]
- Biegler-König, F.W.; Bader, R.F.W.; Tang, T.H. Calculation of the average properties of atoms in molecules. II. J. Comput. Chem. 1982, 3, 317–328. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]

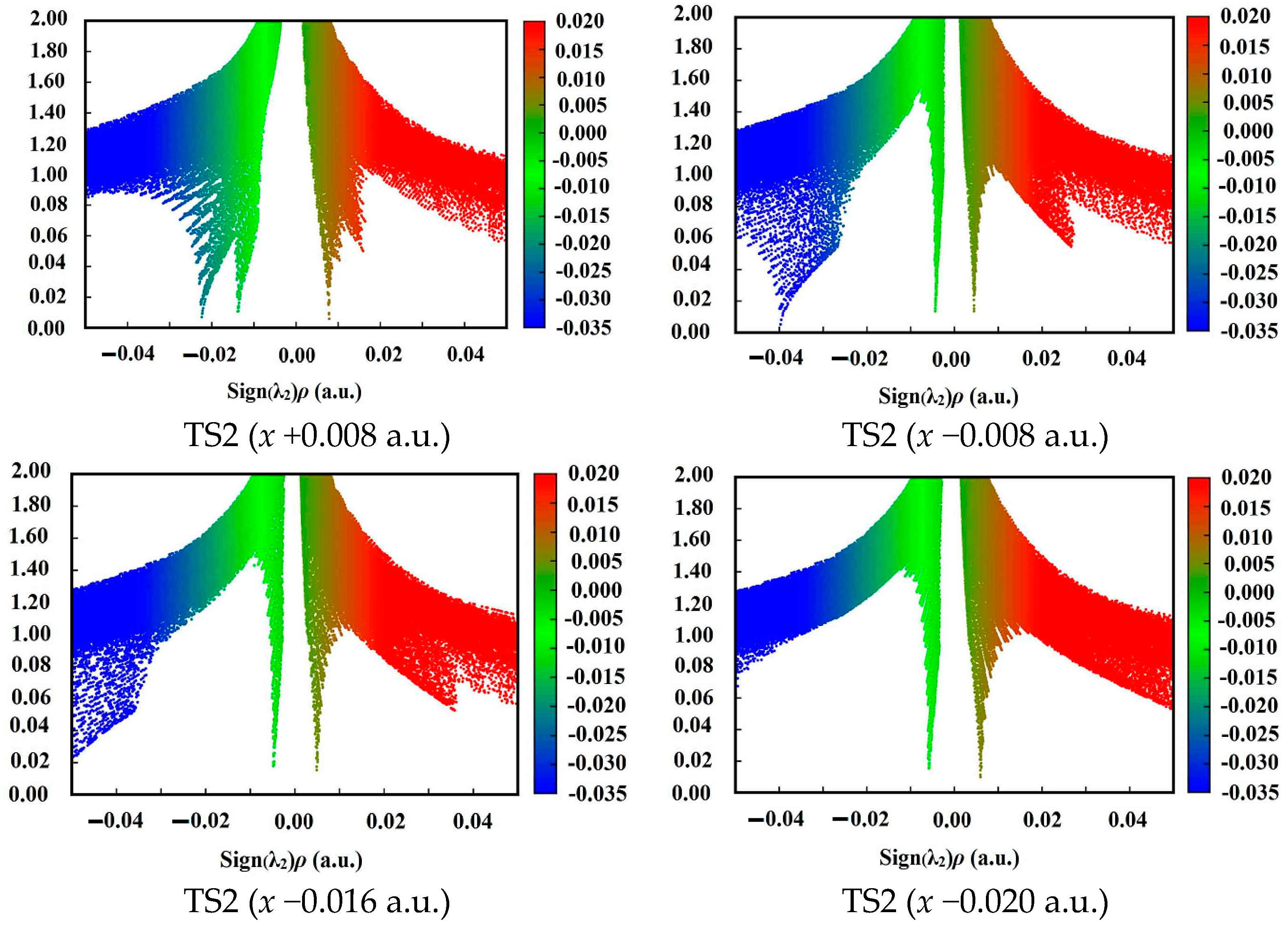
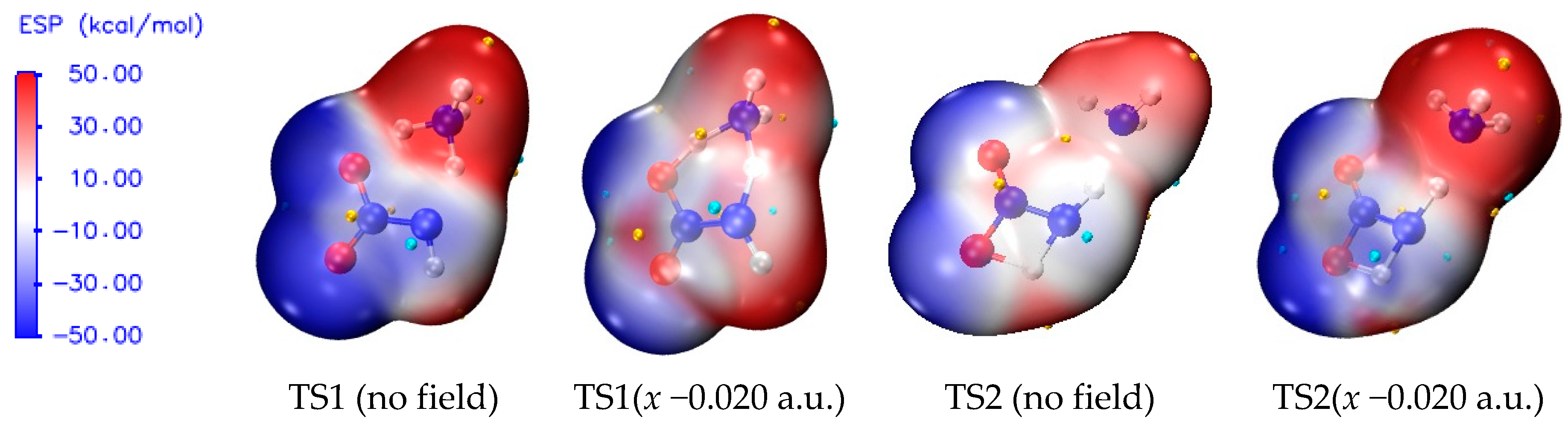
| Field | k298.15 K,C (TS1) | k688 K,C (TS1) | k298.15 K,C (TS2) | k688 K,C (TS2) |
|---|---|---|---|---|
| No field | 3.24 × 100 | 2.74 × 106 | 4.48 × 10−15 | 5.63 × 10−1 |
| z −0.010 | 1.59 × 100 | 1.55 × 106 | 2.92 × 10−26 | 1.58 × 10−6 |
| z −0.008 | 3.19 × 10−1 | 1.39 × 106 | 1.92 × 10−22 | 3.99 × 10−4 |
| z −0.006 | 9.49 × 100 | 4.73 × 106 | 2.98 × 10−20 | 2.78 × 10−3 |
| z −0.004 | 2.20 × 100 | 5.97 × 106 | 1.67 × 10−17 | 2.16 × 10−2 |
| z −0.002 | 1.78 × 100 | 4.32 × 106 | 2.64 × 10−15 | 3.65 × 10−1 |
| z +0.002 | 1.20 × 100 | 2.60 × 106 | 8.69 × 10−15 | 9.27 × 10−1 |
| z −0.004 | 1.90 × 100 | 2.00 × 106 | 4.52 × 10−14 | 1.47 × 100 |
| z −0.006 | 7.90 × 10−1 | 1.39 × 106 | 5.19 × 10−14 | 2.65 × 100 |
| z −0.008 | 1.38 × 100 | 3.39 × 106 | 7.96 × 10−13 | 3.53 × 100 |
| z −0.010 | 3.09 × 10−1 | 8.13 × 105 | 3.13 × 10−12 | 9.80 × 100 |
| y −0.010 | 5.10 × 10−3 | 6.10 × 104 | 2.14 × 10−18 | 1.12 × 10−2 |
| y −0.008 | 1.96 × 10−2 | 1.25 × 105 | 1.27 × 10−17 | 3.79 × 10−2 |
| y −0.006 | 3.33 × 10−1 | 8.95 × 105 | 4.75 × 10−17 | 4.28 × 10−2 |
| y −0.004 | 1.13 × 100 | 2.49 × 106 | 2.06 × 10−16 | 1.77 × 10−1 |
| y −0.002 | 2.51 × 10−1 | 1.43 × 106 | 1.67 × 10−15 | 2.91 × 10−1 |
| y +0.002 | 5.33 × 10−1 | 1.22 × 106 | 2.63 × 10−15 | 5.17 × 10−1 |
| y +0.004 | 3.21 × 10−1 | 8.67 × 105 | 6.98 × 10−16 | 1.60 × 10−1 |
| y +0.006 | 1.42 × 10−1 | 1.80 × 106 | 1.61 × 10−17 | 3.01 × 10−2 |
| y +0.008 | 2.45 × 10−2 | 1.23 × 105 | 7.19 × 10−19 | 6.55 × 10−3 |
| y +0.010 | 1.25 × 10−3 | 8.81 × 104 | 4.55 × 10−21 | 2.73 × 10−4 |
| x +0.010 | 2.71 × 104 | 2.76 × 108 | 2.3 × 10−13 | 6.55 × 100 |
| x +0.008 | 2.8 × 10−14 | 1.65 × 100 | ||
| x +0.006 | 2.29 × 10−14 | 1.06 × 100 | ||
| x +0.004 | 1.15 × 104 | 3.67 × 108 | 1.26 × 10−14 | 7.85 × 10−1 |
| x +0.002 | 3.77 × 103 | 2.79 × 108 | 9.51 × 10−15 | 6.83 × 10−1 |
| x −0.002 | 2.61 × 10−3 | 4.45 × 104 | 2.12 × 10−15 | 4.65 × 10−1 |
| x −0.004 | 5.80 × 10−6 | 2.55 × 103 | 1.04 × 10−15 | 2.31 × 10−1 |
| x −0.006 | 5.58 × 10−8 | 4.73 × 102 | 1.32 × 10−16 | 1.19 × 10−1 |
| x −0.008 | 8.09 × 10−13 | 3.50 × 10−1 | 5.46 × 10−17 | 4.58 × 10−2 |
| x −0.010 | 6.21 × 10−20 | 8.71 × 10−5 | 1.36 × 10−17 | 2.76 × 10−2 |
| x −0.012 | 1.06 × 10−22 | 2.09 × 10−6 | 2.39 × 10−19 | 2.69 × 10−3 |
| x −0.014 | 5.96 × 10−29 | 2.26 × 10−9 | 1.45 × 10−20 | 8.11 × 10−4 |
| x −0.016 | 1.13 × 10−30 | 5.45 × 10−13 | 1.71 × 10−23 | 1.48 × 10−5 |
| x −0.018 | 4.40 × 10−32 | 2.98 × 10−13 | 1.42 × 10−24 | 1.05 × 10−5 |
| x −0.019 | 1.56 × 10−33 | 4.11 × 10−15 | 1.07 × 10−27 | 4.52 × 10−6 |
| x −0.020 | 4.15 × 10−36 | 2.13 × 10−15 | 3.44 × 10−28 | 2.92 × 10−6 |
| Field | PSA(%) | PSA(TS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Field | −55.8 | 34.8 | −28.2 | 381.9 | 264.3 | 82.7 | −23.2 | 22.0 | −16.1 | 235.9 | 93.5 | 73.2 |
| x −0.002 | −54.4 | 33.1 | −27.5 | 346.8 | 254.8 | 81.7 | −22.4 | 21.5 | −17.3 | 231.8 | 89.5 | 72.1 |
| x −0.004 | −53.0 | 31.5 | −26.6 | 310.2 | 246.0 | 80.6 | −20.3 | 20.8 | −16.5 | 220.3 | 78.3 | 70.8 |
| x −0.006 | −51.7 | 29.8 | −25.8 | 278.7 | 223.5 | 78.3 | −21.6 | 19.6 | −16.6 | 218.1 | 65.7 | 70.3 |
| x −0.008 | −50.7 | 32.5 | −27.2 | 251.6 | 190.7 | 83.9 | −20.3 | 21.3 | −15.1 | 206.2 | 58.3 | 69.5 |
| x −0.010 | −49.6 | 34.4 | −28.2 | 279.9 | 146.2 | 90.6 | −19.7 | 19.7 | −12.2 | 223.4 | 51.4 | 68.8 |
| x −0.012 | −47.8 | 35.2 | −26.7 | 288.3 | 122.8 | 90.8 | −30.2 | 22.5 | −13.6 | 218.7 | 72.1 | 68.1 |
| x −0.013 | −29.8 | 19.3 | −12.8 | 201.3 | 63.6 | 67.3 | ||||||
| x −0.014 | −44.9 | 32.6 | −24.3 | 250.2 | 109.7 | 88.4 | −29.5 | 20.2 | −15.1 | 212.9 | 55.0 | 67.2 |
| x −0.015 | −28.2 | 21.3 | −12.7 | 197.9 | 49.8 | 65.2 | ||||||
| x −0.016 | −41.2 | 28.7 | −21.6 | 211.8 | 111.3 | 84.1 | ||||||
| x −0.018 | −37.6 | 22.0 | −19.9 | 167.6 | 102.5 | 76.5 | ||||||
| x −0.019 | −35.0 | 19.3 | −18.2 | 139.2 | 91.3 | 70.8 | ||||||
| x −0.020 | −33.1 | 16.7 | −15.6 | 116.9 | 90.1 | 68.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.-D.; Liu, Y.-Z.; Wang, X.-L.; Qiu, L.-L.; Meng, Z.-H.; Cheng, X.; Li, Y.-X. Strong External Electric Fields Reduce Explosive Sensitivity: A Theoretical Investigation into the Reaction Selectivity in NH2NO2∙∙∙NH3. Molecules 2023, 28, 2586. https://doi.org/10.3390/molecules28062586
Ren F-D, Liu Y-Z, Wang X-L, Qiu L-L, Meng Z-H, Cheng X, Li Y-X. Strong External Electric Fields Reduce Explosive Sensitivity: A Theoretical Investigation into the Reaction Selectivity in NH2NO2∙∙∙NH3. Molecules. 2023; 28(6):2586. https://doi.org/10.3390/molecules28062586
Chicago/Turabian StyleRen, Fu-De, Ying-Zhe Liu, Xiao-Lei Wang, Li-Li Qiu, Zi-Hui Meng, Xiang Cheng, and Yong-Xiang Li. 2023. "Strong External Electric Fields Reduce Explosive Sensitivity: A Theoretical Investigation into the Reaction Selectivity in NH2NO2∙∙∙NH3" Molecules 28, no. 6: 2586. https://doi.org/10.3390/molecules28062586
APA StyleRen, F.-D., Liu, Y.-Z., Wang, X.-L., Qiu, L.-L., Meng, Z.-H., Cheng, X., & Li, Y.-X. (2023). Strong External Electric Fields Reduce Explosive Sensitivity: A Theoretical Investigation into the Reaction Selectivity in NH2NO2∙∙∙NH3. Molecules, 28(6), 2586. https://doi.org/10.3390/molecules28062586