Hydrophobic Nanoporous Silver with ZIF Encapsulation for Nitrogen Reduction Electrocatalysis
Abstract
:1. Introduction
2. Results
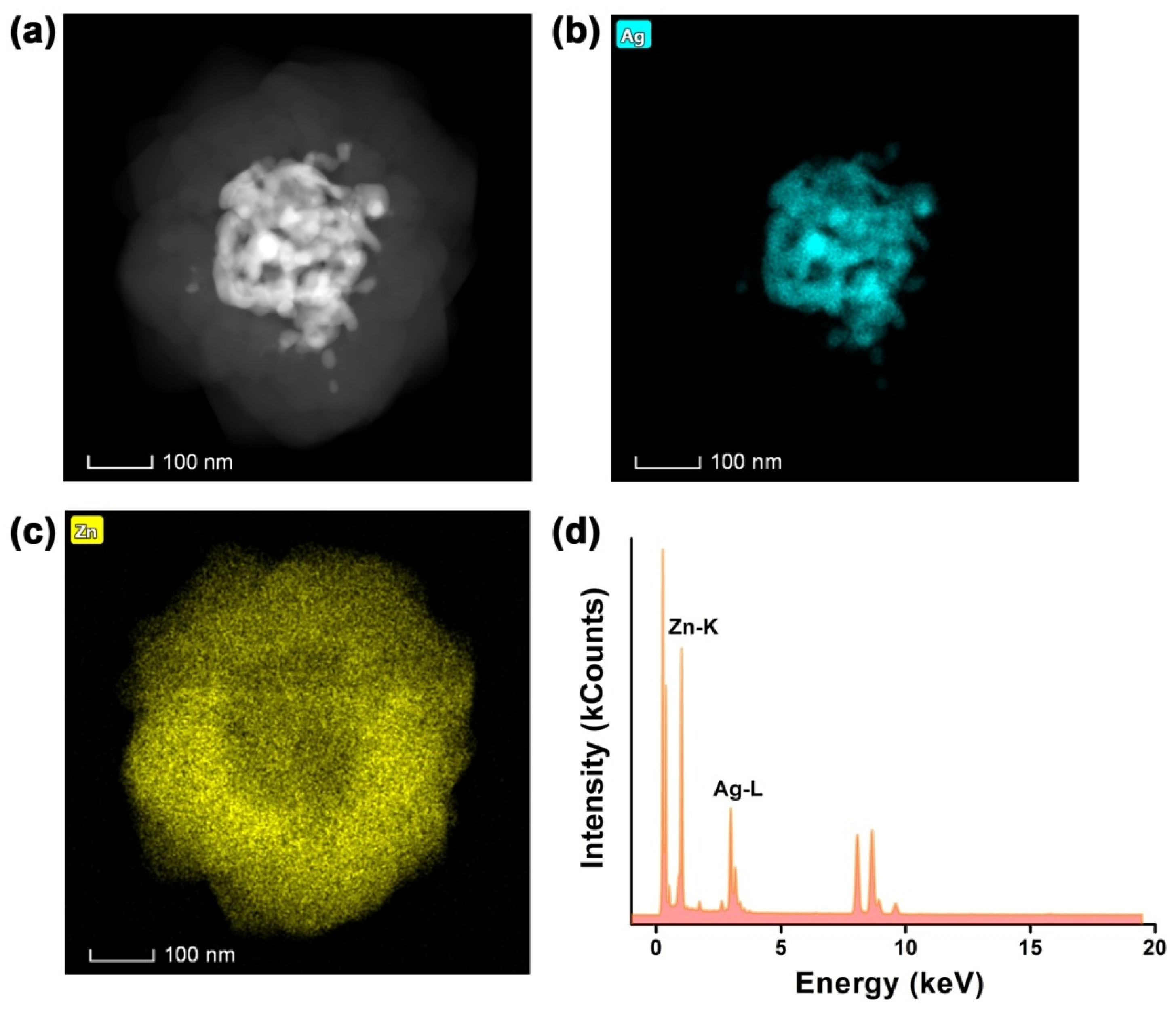
2.1. Morphological and Structural Description
2.2. ENRR Catalysis Performance
3. Materials and Methods
3.1. Materials and General Methods
3.2. Synthesis of NPS@O-ZIF
3.3. Synthesis of Ag Nanoparticles and Corresponding Ag@O-ZIF for Control Experiments
3.4. ENRR Tests
3.5. Calculation of Ammonia Yield and Faradaic Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.-P.; Smith, M.R., III; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef]
- Jiao, F.; Xu, B. Electrochemical Ammonia Synthesis and Ammonia Fuel Cells. Adv. Mater. 2019, 31, 1805173. [Google Scholar] [CrossRef]
- Seo, Y.; Han, S. Economic Evaluation of an Ammonia-Fueled Ammonia Carrier Depending on Methods of Ammonia Fuel Storage. Energies 2021, 14, 8326. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Vallana, F.M.F.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Ham, A.G.J.V.; Lefferts, L. Beyond Haber-Bosch: The renaissance of the Claude process. Int. J. Hydrogen Energy 2021, 46, 21566–21579. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J.K. Exploring the Limits: A Low-Pressure, Low-Temperature Haber–Bosch Process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Xu, H.; Ithisuphalap, K.; Li, Y.; Mukherjee, S.; Lattimer, J.; Soloveichik, G.; Wu, G. Electrochemical Ammonia Synthesis Through N2 and H2O Under Ambient Conditions: Theory, Practices, and Challenges for Catalysts and Electrolytes. Nano Energy 2020, 69, 104469. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Hu, Z.; Zheng, Y.; Tang, C.; Chen, P.; Wang, R.; Qiu, K.; Mao, J.; Ling, T.; et al. The Crucial Role of Charge Accumulation and Spin Polarization in Activating Carbon-Based Catalysts for Electrocatalytic Nitrogen Reduction. Angew. Chem. Int. Ed. 2020, 59, 4525–4531. [Google Scholar] [CrossRef]
- Choi, J.; Suryanto, B.H.R.; Wang, D.; Du, H.-L.; Hodgetts, R.Y.; Vallana, F.M.F.; MacFarlane, D.R.; Simonov, A.N. Identification and Elimination of False Positives in Electrochemical Nitrogen Reduction Studies. Nat. Commun. 2020, 11, 5546. [Google Scholar] [CrossRef]
- Lin, L.; Yan, L.; Fu, L.; He, C.; Xie, K.; Zhu, L.; Sun, J.; Zhang, Z. First Principle Investigation of W/P3C Sheet as an Efficient Single Atom Electrocatalyst for N2 and NO Electrochemical Reaction with Suppressed Hydrogen Evolution. Fuel 2022, 308, 122068. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Du, H.-L.; Wang, D.; Chen, J.; Simonov, A.N.; MacFarlane, D.R. Challenges and Prospects in the Catalysis of Electroreduction of Nitrogen to Ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Chen, T. Porous Noble Metal Electrocatalysts: Synthesis, Performance, and Development. Small 2021, 17, 2005354. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent Progress Towards the Electrosynthesis of Ammonia from Sustainable Resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Ran, J.; Vasileff, A.; Qiao, S.-Z. Rational Design of Electrocatalysts and Photo(electro)catalysts for Nitrogen Reduction to Ammonia (NH3) under Ambient Conditions. Energy Environ. Sci. 2018, 11, 45–56. [Google Scholar] [CrossRef]
- Huang, H.; Xia, L.; Shi, X.; Asiri, A.M.; Sun, X. Ag Nanosheet for Efficient Electrocatalytic N2 Fixation to NH3 at Ambient Conditions. Chem. Commun. 2018, 54, 11427–11430. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.; Panikkanvalappil, S.R.; El-Sayed, M.A. Enhancing the Rate of Electrochemical Nitrogen Reduction Reaction for Ammonia Synthesis Under Ambient Conditions Using Hollow Gold Nanocages. Nano Energy 2018, 49, 316–323. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Zhu, X.; Fang, Y.; Dong, S. Coupling Cu with Au for Enhanced Electrocatalytic Activity of Nitrogen Reduction Reaction. Nanoscale 2020, 12, 1811–1816. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, S.; Wang, H.; Li, H.; Shao, M. A Spectroscopic Study on the Nitrogen Electrochemical Reduction Reaction on Gold and Platinum Surfaces. J. Am. Chem. Soc. 2018, 140, 1496–1501. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, C.; Deng, K.; Wang, Z.; Xu, Y.; Li, X.; Xue, H.; Wang, L. One-pot Synthesis of Bi-metallic PdRu Tripods as an Efficient Catalyst for Electrocatalytic Nitrogen Reduction to Ammonia. J. Mater. Chem. A 2019, 7, 801–805. [Google Scholar] [CrossRef]
- Kim, C.; Song, J.-Y.; Choi, C.; Ha, J.P.; Lee, W.; Nam, Y.T.; Lee, D.-M.; Kim, G.; Gereige, I.; Jung, W.-B.; et al. Atomic-Scale Homogeneous Ru-Cu Alloy Nanoparticles for Highly Efficient Electrocatalytic Nitrogen Reduction. Adv. Mater. 2022, 34, 2205270. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Liu, C.; Zhao, Y.; Ren, W.; Chen, X.; Chen, S.; Zhao, C. Metal-Sulfur Linkages Achieved by Organic Tethering of Ruthenium Nanocrystals for Enhanced Electrochemical Nitrogen Reduction. Angew. Chem. Int. Ed. 2020, 59, 21465. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, K.; Ye, Y.; Zhang, S.; Liu, Y.; Wang, G.; Liang, C.; Zhang, H.; Zhao, H. Efficient Electrocatalytic Nitrogen Reduction to Ammonia with Aqueous Silver Nanodots. Commun. Chem. 2021, 4, 10. [Google Scholar] [CrossRef]
- Chen, D.; Ning, S.; Lan, J.; Peng, M.; Duan, H.; Pan, A.; Tan, Y. General Synthesis of Nanoporous 2D Metal Compounds with 3D Bicontinous Structure. Adv. Mater. 2020, 32, 2004055. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; Wang, Z.; Zhang, K.; He, J.; Zhang, W.; Guo, C.; Ding, Y. Bimodal Nanoporous Pd3Cu1 Alloy with Restrained Hydrogen Evolution for Stable and High Yield Electrochemical Nitrogen Reduction. Nano Energy 2019, 58, 834–841. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Peng, L.; Li, X.; Xu, H.T.; Wang, Y. Using Corrosion to Fabricate Various Nanoporous Metal Structures. Corros. Sci. 2015, 92, 16–31. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.-Q.; Wen, H.; Ye, T.; Chen, J.; Li, C.-P.; Du, M. Nanoporous Gold Embedded ZIF Composite for Enhanced Electrochemical Nitrogen Fixation. Angew. Chem. Int. Ed. 2019, 58, 15362–15366. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, Z.; Wang, Z. Electroreduction of Nitrogen to Ammonia on Nanoporous Gold. Nanoscale 2021, 13, 1717–1722. [Google Scholar] [CrossRef]
- Lü, F.; Zhao, S.; Guo, R.; He, J.; Peng, X.; Bao, H.; Fu, J.; Han, L.; Qi, G.; Luo, J.; et al. Nitrogen-Coordinated Single Fe Sites for Efficient Electrocatalytic N2 Fixation in Neutral Media. Nano Energy 2019, 61, 420–427. [Google Scholar] [CrossRef]
- Lazouski, N.; Chung, M.; Williams, K.; Gala, M.L.; Manthiram, K. Non-aqueous Gas Diffusion Electrodes for Rapid Ammonia synthesis from Nitrogen and Water-Splitting-Derived Hydrogen. Nat. Catal. 2020, 3, 463–469. [Google Scholar] [CrossRef]
- Chen, G.-F.; Ren, S.; Zhang, L.; Cheng, H.; Luo, Y.; Zhu, K.; Ding, L.-X.; Wang, H. Advances in Electrocatalytic N2 Reduction-Strategies to Tackle the Selectivity Challenge. Small Methods 2019, 3, 1800337. [Google Scholar] [CrossRef]
- Nazemi, M.; Ou, P.; Alabbady, A.; Soule, L.; Liu, A.; Song, J.; Sulchek, T.A.; Liu, M.; El-Sayed, M.A. Electrosynthesis of Ammonia Using Porous Bimetallic Pd−Ag Nanocatalysts in Liquid- and Gas-Phase Systems. ACS Catal. 2020, 10, 10197–10206. [Google Scholar] [CrossRef]
- Sim, H.Y.F.; Chen, J.R.T.; Koh, C.S.L.; Lee, H.K.; Han, X.; Phan-Quang, G.C.; Pang, J.Y.; Lay, C.L.; Pedireddy, S.; Phang, I.Y.; et al. ZIF-induced D-Band Modification in Bimetallic Nanocatalyst: Achieving 44% Efficiency in Ambient Nitrogen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 16997–17003. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xia, Q.; Hao, L.; Robertson, A.W.; Sun, Z. Design of Porous Core-Shell Manganese Oxides to Boost Electrocatalytic Dinitrogen Reduction. ACS Sustain. Chem. Eng. 2022, 10, 1316–1322. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, S.; Zhang, L.; Li, Y.; Carraro, C.; Maboudian, R.; Wei, W.; Liu, A.; Zhang, Y.; Liu, S. Reconstructing Hydrophobic ZIF-8 Crystal into Hydrophilic Hierarchically-Porous Nanoflowers as Catalyst Carrier for Nonenzymatic Glucose Sensing. Sensor. Actuat. B Chem. 2020, 313, 128031. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Liu, S.; Lu, C.; Liu, D.; Pan, Y.; Sakiyama, H.; Muddassir, M.; Liu, J. A New Magnetic Adsorbent of Eggshell-Zeolitic Imidazolate Framework for Highly Efficient Removal of Norfloxacin. Dalton Trans. 2021, 50, 18016–18026. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-Step Construction of a Hollow Au@Bimetal–Organic Framework Core–Shell Catalytic Nanoreactor for Selective Alcohol Oxidation Reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef]
- Katsumi, N.; Nagao, S.; Okochi, H. Addition of Polyvinyl Pyrrolidone During Density Separation with Sodium Iodide Solution Improves Recovery Rate of Small Microplastics (20–150 μm) from Soils and Sediments. Chemosphere 2022, 307, 135730. [Google Scholar] [CrossRef]
- Koh, C.S.L.; Lee, H.K.; Sim, H.Y.F.; Han, X.; Phan-Quang, G.C.; Ling, X.Y. Turning Water from a Hindrance to the Promotor of Preferential Electrochemical Nitrogen Reduction. Chem. Mater. 2020, 32, 1674–1683. [Google Scholar] [CrossRef]
- Yang, Z.; Pedireddy, S.; Lee, H.K.; Liu, Y.; Tjiu, W.W.; Phang, I.Y.; Ling, X.Y. Manipulating the D-Band Electronic Structure of Platinum Functionalized Nanoporous Gold Bowls: Synergistic Intermetallic Interactions Enhance Catalysis. Chem. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Qiu, W.; Xie, X.-Y.; Qiu, J.; Fang, W.-H.; Liang, R.; Ren, X.; Ji, X.; Cui, G.; Asiri, A.M.; Cui, G.; et al. High-performance Artificial Nitrogen Fixation at Ambient Conditions Using a Metal-free Electrocatalyst. Nat. Commun. 2018, 9, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, G.W.; Chrisp, J.D. Spectrophotometric Method for Determination of Hydrazine. Anal. Chem. 1952, 24, 2006–2008. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Liang, W.; Zhou, G.; Liang, Z.; Wang, Y.; Qu, J.; Sun, Y.; Jiang, L. Three-phase Electrolysis by Gold Nanoparticle on Hydrophobic Interface for Enhanced Electrochemical Nitrogen Reduction Reaction. Adv. Sci. 2020, 7, 2002630. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zhao, S.; Pang, Y.; Yang, Y. Hydrophobic Nanoporous Silver with ZIF Encapsulation for Nitrogen Reduction Electrocatalysis. Molecules 2023, 28, 2781. https://doi.org/10.3390/molecules28062781
Qi Y, Zhao S, Pang Y, Yang Y. Hydrophobic Nanoporous Silver with ZIF Encapsulation for Nitrogen Reduction Electrocatalysis. Molecules. 2023; 28(6):2781. https://doi.org/10.3390/molecules28062781
Chicago/Turabian StyleQi, Yating, Shulin Zhao, Yue Pang, and Yijie Yang. 2023. "Hydrophobic Nanoporous Silver with ZIF Encapsulation for Nitrogen Reduction Electrocatalysis" Molecules 28, no. 6: 2781. https://doi.org/10.3390/molecules28062781
APA StyleQi, Y., Zhao, S., Pang, Y., & Yang, Y. (2023). Hydrophobic Nanoporous Silver with ZIF Encapsulation for Nitrogen Reduction Electrocatalysis. Molecules, 28(6), 2781. https://doi.org/10.3390/molecules28062781






