Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries
Abstract
:1. Introduction
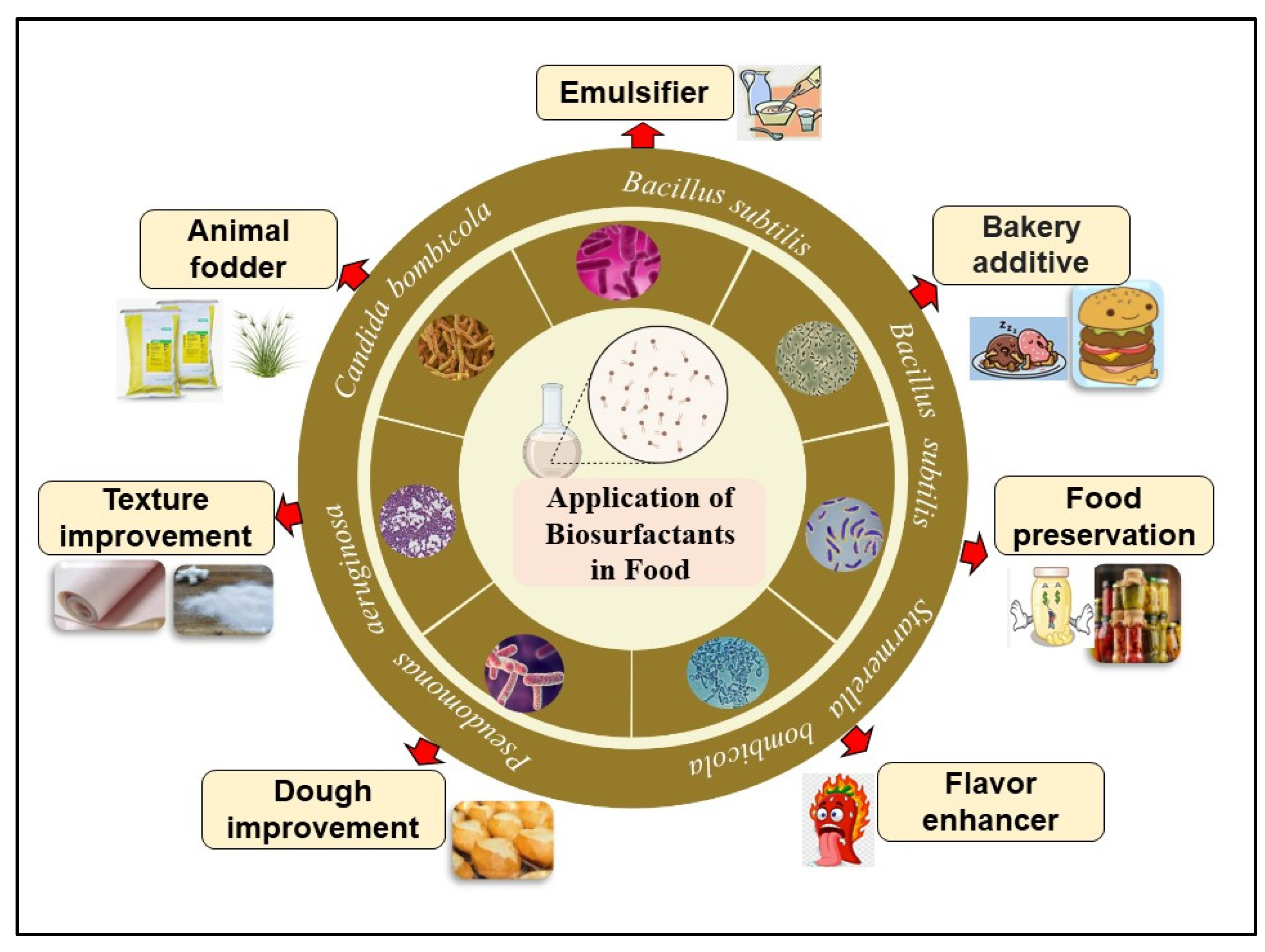
2. Biosurfactants as Food Additives
| ANTIADHESIVE | |||
| Microorganisms | Biosurfactant | Pathogens | Reference |
| Pseudomonas putida | Putisolvin I and II | Pseudomonas sp. | [19] |
| Psedofactin II | Enterobacter faecalis Proteus mirabilis, Candida sp. | [20] | |
| Bacillus subtilis | Fengycin | Salmonella enterica | [21] |
| Bacillus tequilensis | Lipopeptide | Streptococcus mutans | [22] |
| Candida spaerica | Lunasan | Streptococcus agalactiae Pseudomonas aeruginosa | [23] |
| Pseudomonas aeruginosa | Rhamnolipid | Yarrowia sp. | [24] |
| Candida lipolytica | Rufisan | Streptococcus sp. | [25] |
| Serretia marsecens | Glycolipid | Candida albicans Pseudomonas aeruginosa Bacillus pumilus | [26] |
| ANTIMICROBIAL | |||
| Microorganisms | Biosurfactant (MIC µg/mL) | Pathogens | Reference |
| Pseudomonas aeruginosa | Rhamnolipids (4–64) | Alternaria alternata Aureobasidium pullulans Aspergillus niger Candida albicans Chaetonium globosum Gliocadium virens | [27] |
| Pseudomonas aeruginosa | Rhamnolipids (20–50) | Alternaria mali Brotrytis cinerea Fusarium sp. Rhizoctonia solani | [28] |
| Pseudomonas aeruginosa | Rhamnolipids (0.5–1.70) | Brotrytis cinerea Fusarium sp. Fusarium solani Gliocadium virens Penicillium funiculosum Rhizoctonia solani | [29] |
| Pseudomonas aeruginosa | Rhamnolipids | Brotrytis cinereal | [30] |
| Pseudomonas aeruginosa | Rhamnolipids (64–256) | Brotrytis cinereal Mucor miehei Staphylococcus aureus Bacillus cereus | [31] |
| Pseudomonas sp. | Rhamnolipid | Pseudomonas aeruginosa | [32] |
| FOOD ADDITIVES | |||
| Microorganisms | Biosurfactant | Applications | Reference |
| B. subtilis | Surfactants | Emulsifier | [33] |
| C. utilis | - | Mayonnaise emulsifier | [34] |
| B. subtilis | - | Bakery additive | [35] |
| B. subtilis | Surfactin | Food preservative | [36] |
| Pseudomonas sp. | Rhamnolipid | Dough improvement | [15] |
| Pseudomonas aeruginosa | Rhamnolipid | [16] | |
| Nesterenkonia sp. | Lipopeptides | Texture improvement | [17] |
| Bacillus subtilis | - | Cookie dough | [37] |
| Bacillus subtilis | Lipopeptides | Bread improvement | [38] |
| Candida bombicola | Glycolipids | Cupcake additive | [39] |
| Starmerella bombicola | Sophorolipids | Sophorolipids + curcumin | [40] |
| Probiotic (GRAS) | - | Animal fodder | [41] |
| EMULSIFICATION | |||
| Microorganisms | Biosurfactant type | Emulsification material | Reference |
| Bacillus vallismortis | Exopolysaccharides | Essential oils | [42] |
| Pseudomonas fluorescens | Exopolysaccharides | Edible oils | [43] |
| Nesterenkonia sp. | Lipopeptide | Unsaturated hydrocarbons | [18] |
| Candida utilis | Glycolipids | Vegetable oil | [44] |
| Pseudomonas aeruginosa | Rhamnolipids | Saturated hydrocarbons | [45] |
| Kluyveromyces marxianus | Mannoprotien | Corn oil | [46] |
| Saccharomyces lipolytica | - | Cooking vegetable oil | [47] |
| Candida utilis | Glycolipids | Canola oil | [48] |
| Pseudomonas aeruginosa | Rhamnolipids | Nano-emulsion | [49] |
| Type of Biosurfactants | HLB Value | Reference |
|---|---|---|
| Rhamnolipids (RLs) | 10.17 | [56] |
| Sophorolipids (SLs) | 10–13 | [57] |
| Surfactin | 10–12 | [52] |
| Mannosylerythritol lipid (MEL) | ≥12 | [58] |
| Other glycolipids | 10–15 | [59] |
| Lipopeptides | 10–11.1 | [60] |
| Polysorbate 80 | 14.4–15.6 | [61] |
3. Sensorial Behavior
4. Food Matrix Interfaces
5. Regulations to Chartered BSs and BEs as Food Additives
6. Antimicrobial Activity of Biosurfactant
7. Challenges and Forthcomings
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, D.; Saharan, B.S. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol. Rep. 2016, 11, 27–35. [Google Scholar]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [PubMed]
- Karamchandani, B.M.; Pawar, A.A.; Pawar, S.S.; Syed, S.; Mone, N.S.; Dalvi, S.G.; Rahman, P.K.; Banat, I.M.; Satpute, S.K. Biosurfactants’ multifarious functional potential for sustainable agricultural practices. Front. Bioeng. Biotechnol. 2022, 10, 1–20. [Google Scholar]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [PubMed] [Green Version]
- Campos, J.M.; Montenegro Stamford, T.L.; Sarubbo, L.A.; de Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108. [Google Scholar]
- Campos, J.M.; Banat, I.M.; Sarubbo, L.A. Natural and Microbial Biosurfactants’ Use in the Food Industry. In Microbial Biosurfactants and Their Environmental and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 242–257. [Google Scholar]
- Glynn, A.; Igra, A.M.; Sand, S.; Ilbäck, N.G.; Hellenäs, K.E.; Rosén, J.; Aspenström-Fagerlund, B. Are additive effects of dietary surfactants on intestinal tight junction integrity an overlooked human health risk?—A mixture study on Caco-2 monolayers. Food Chem. Toxicol. 2017, 106, 314–323. [Google Scholar]
- Csáki, K.F. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med. Hypotheses 2011, 76, 676–681. [Google Scholar]
- Sharma, D. Biosurfactants: Greener Surface Active Agents for Sustainable Future; Springer: Singapore, 2021. [Google Scholar]
- da Silva, A.F.; Banat, I.M.; Giachini, A.J. Fungal biosurfactants, from nature to biotechnological product: Bioprospection, production and potential applications. Bioprocess Biosyst. Eng. 2021, 44, 2003–2034. [Google Scholar]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar]
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Rita de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar]
- Sharma, D. Applications of biosurfactants in food. Biosurfactants Food 2016, 43–80. [Google Scholar]
- Van Haesendonck, I.; Vanzeveren, E. International patent PCT/BE/2003/000186. 2005 Patent Application No. 2006. 10/533,499, 4 November 2003. [Google Scholar]
- Trummler, K.; Effenberger, F.; Syldatk, C. An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. Eur. J. Lipid Sci. Technol. 2003, 105, 563–571. [Google Scholar]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of lipopeptide biosurfactant by a marine Nesterenkonia sp. and its application in food industry. Front. Microbiol. 2017, 8, 1138. [Google Scholar]
- Stackebrandt, E.; Koch, C.; Gvozdiak, O.; Schumann, P. Taxonomic Dissection of the Genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int. J. Syst. Evolution. Microbiol. 1995, 45, 682–692. [Google Scholar]
- Kuiper, I.; Lagendijk, E.L.; Pickford, R.; Derrick, J.P.; Lamers, G.E.; Thomas-Oates, J.E.; Lugtenberg, B.J.J.; Bloemberg Bloemberg, G.V. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Molec. Microbiol. 2004, 51, 97–113. [Google Scholar]
- Janek, T.; Łukaszewicz, M.; Rezanka, T.; Krasowska, A. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour. Technol. 2010, 101, 6118–6123. [Google Scholar]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar]
- Pradhan, A.K.; Pradhan, N.; Mall, G.; Panda, H.T.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Application of lipopeptide biosurfactant isolated from a halophile: Bacillus tequilensis CH for inhibition of biofilm. Appl. Biochem. Biotechnol. 2013, 171, 1362–1375. [Google Scholar]
- Luna, J.; Rufino, R.; Campos, G.; Sarubbo, L. Properties of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Chem. Eng. 2012, 27, 67–72. [Google Scholar]
- Dusane, D.H.; Dam, S.; Nancharaiah, Y.V.; Kumar, A.R.; Venugopalan, V.P.; Zinjarde, S.S. Disruption of Yarrowia lipolytica biofilms by rhamnolipid biosurfactant. Aqua. Biosy. 2012, 8, 17. [Google Scholar]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Coll. Surf. B Biointerfaces 2011, 84, 1–5. [Google Scholar]
- Dusane, D.H.; Pawar, V.S.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27, 645–654. [Google Scholar]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8. [Google Scholar]
- Kim, B.S.; Lee, J.Y.; Hwang, B.K. In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 1029–1035. [Google Scholar]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar]
- Varnier, A.L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.H.; Kauffmann, S.; Pugin, A.; et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009, 32, 178–193. [Google Scholar]
- Nitschke, M.; Costa, S.G.; Contiero, J. Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Appl. Biochem. Biotechnol. 2010, 160, 2066–2074. [Google Scholar] [PubMed]
- Sotirova, A.; Spasova, D.; Vasileva-Tonkova, E.; Galabova, D. Effects of rhamnolipid-biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol. Res. 2009, 164, 297–303. [Google Scholar] [PubMed] [Green Version]
- Chander, C.S.; Lohitnath, T.; Kumar, D.M.; Kalaichelvan, P.T. Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio-preservative. Adv. Appl. Sci. Res. 2012, 3, 1827–1831. [Google Scholar]
- Campos, J.M.; Stamford, T.L.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Formulation of mayonnaise with the addition of a bioemulsifier isolated from Candida utilis. Toxicol. Rep. 2015, 2, 1164–1170. [Google Scholar]
- Zouari, R.; Besbes, S.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Cookies from composite wheat–sesame peels flours: Dough quality and effect of Bacillus subtilis SPB1 biosurfactant addition. Food Chem. 2016, 194, 758–769. [Google Scholar]
- Sharma, R.; Singh, J.; Verma, N. Production, characterization and environmental applications of biosurfactants from Bacillus amyloliquefaciens and Bacillus subtilis. Biocat. Agricul. Biotechnol. 2018, 16, 132–139. [Google Scholar]
- Zouari, R.; Ben Abdallah-Kolsi, R.; Hamden, K.; Feki, E.A.; Chaabouni, K.; Makni-Ayadi, F.; Sallemi, F.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Assessment of the antidiabetic and antilipidemic properties of Bacillus subtilis SPB1 biosurfactant in alloxan-induced diabetic rats. Pept. Sci. 2015, 104, 764–774. [Google Scholar]
- Mnif, I.; Besbes, S.; Ellouze, R.; Ellouze-Chaabouni, S.; Ghribi, D. Improvement of bread quality and bread shelf-life by Bacillus subtilis biosurfactant addition. Food Sci. Biotechnol. 2012, 21, 1105–1112. [Google Scholar]
- Silva, I.A.; Veras, B.O.; Ribeiro, B.G.; Aguiar, J.S.; Guerra, J.M.C.; Luna, J.M.; Sarubbo, L.A. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. Peer J. 2020, 8, e9064. [Google Scholar]
- Vasudevan, S.; Prabhune, A.A. Photophysical studies on curcumin-sophorolipid nanostructures: Applications in quorum quenching and imaging. R. Soc. Open Sci. 2008, 5, 170865. [Google Scholar]
- Konkol, D.; Szmigiel, I.; Domżał-Kędzia, M.; Kułażyński, M.; Krasowska, A.; Opaliński, S.; Korczyńskia, M.; Łukaszewicz, M. Biotransformation of rapeseed meal leading to production of polymers, biosurfactants, and fodder. Bioorganic Chem. 2019, 93, 102865. [Google Scholar]
- Song, B.; Zhu, W.; Song, R.; Yan, F.; Wang, Y. Exopolysaccharide from Bacillus vallismortis WF4 as an emulsifier for antifungal and antipruritic peppermint oil emulsion. Int. J. Biol. Macromol. 2019, 125, 436–444. [Google Scholar] [PubMed]
- Vidhyalakshmi, R.; Nachiyar, C.V.; Kumar, G.N.; Sunkar, S.; Badsha, I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of Pseudomonas fluorescens. Biocatal. Agri. Bbiotechnol. 2018, 16, 320–325. [Google Scholar]
- Campos, J.M.; Stamford, T.L.; Sarubbo, L.A. Production of a bioemulsifier with potential application in the food industry. Appl. Biochem. Biotechnol. 2014, 172, 3234–3252. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Costa, S.G.; Contiero, J. Rhamnolipid surfactants: An update on the general aspects of these remarkable biomolecules. Biotechnol. Prog. 2005, 21, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in food industry. Trends Food Sci. Technol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Lima, Á.S.; Alegre, R.M. Evaluation of emulsifier stability of biosurfactant produced by Saccharomyces lipolytica CCT-0913. Braz. Arch. Biol. Technol. 2009, 52, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.G.; Dos Santos, M.M.; Pinto, M.I.; Meira, H.M.; Durval, I.B.; Guerra, J.M. Production and optimization of the extraction conditions of a biosurfactant of Candida utilis UFPEDA1009 with potential of application in the food industry. Chem. Eng. Trans. 2019, 74, 1477–1482. [Google Scholar]
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Ely, C.M. Chick-growth stimulation produced by surfactants. Science 1951, 114, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, J.N.; Ha, J.K.; Yun, S.G.; Lee, S.S. Effects of dietary addition of surfactant Tween 80 on ruminal fermentation and nutrient digestibility of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2004, 17, 337–342. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, F.; Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef] [Green Version]
- Sagalowicz, L.; Leser, M.E. Delivery systems for liquid food products. Curr. Opin. Colloid Interface Sci. 2010, 15, 61–72. [Google Scholar] [CrossRef]
- Farheen, V.; Saha, S.B.; Pyne, S.; Chowdhury, B.R. Production of nanobiosurfactant from Pseudomonas aeruginosa and it’s application in bakery industry. Int. J. Adv. Res. Biol. Eng. Sci. Technol. 2016, 2, 67. [Google Scholar]
- Khoshdast, H.; Abbasi, H.; Sam, A.; Noghabi, K.A. Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem. Eng. J. 2012, 60, 127–134. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Behle, R.W.; Skory, C.D.; Kurtzman, C.P.; Price, N.P.J. Utilization of sophorolipids as biosurfactants for postemergence herbicides. Crop. Prot. 2004, 59, 29–34. [Google Scholar] [CrossRef]
- Randu, M.; Sylvie, H.E.R.Y.; Ravier, P.; Deprey, S. Concentrate comprising a MEL and a polyethylene glycol fatty acid ester having an HLB value greater than or equal to 12. U.S. Patent Application No. 16/085,348, 1 April 2021. [Google Scholar]
- Sekhar, K.P.; Adicherla, H.; Nayak, R.R. Impact of glycolipid hydrophobic chain length and headgroup size on self-assembly and hydrophobic guest release. Langmuir 2018, 34, 8875–8886. [Google Scholar] [CrossRef]
- De Zoysa, G.H.; Glossop, H.D.; Sarojini, V. Unexplored antifungal activity of linear battacin lipopeptides against planktonic and mature biofilms of C. albicans. European J. Med. Chem. 2018, 146, 344–353. [Google Scholar] [CrossRef]
- Braun, A.C.; Ilko, D.; Merget, B.; Gieseler, H.; Germershaus, O.; Holzgrabe, U.; Meinel, L. Predicting critical micelle concentration and micelle molecular weight of polysorbate 80 using compendial methods. Eur. J. Pharm. Biopharm. 2015, 94, 559–568. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and applications in drug delivery, biotechnology and ecotoxicology. Bioengineering 2021, 8, 115. [Google Scholar] [CrossRef]
- Durval, I.J.B.; da Silva, I.A.; Sarubbo, L.A. Application of microbial biosurfactants in the food industry. In Microbial Biosurfactants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–10. [Google Scholar]
- Ozdener, M.H.; Ashby, R.D.; Jyotaki, M.; Elkaddi, N.; Spielman, A.I.; Bachmanov, A.A.; Solaiman, D.K. Sophorolipid biosurfactants activate taste receptor type 1 member 3-mediated taste responses and block responses to bitter taste in vitro and in vivo. J. Surfact. Deter. 2019, 22, 441–449. [Google Scholar] [CrossRef]
- Magalhães, L.; Nitschke, M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control 2013, 29, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bilal, M.; Liu, S.; Zhang, J.; Lu, H.; Luo, H.; Zhao, Y. Isolation, identification and antimicrobial evaluation of bactericides secreting Bacillus subtilis natto as a biocontrol agent. Processes 2020, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Partal, P.; Guerrero, A.; Berjano, M.; Gallegos, C. Transient flow of o/w sucrose palmitate emulsions. J. Food Eng. 1999, 4, 33–41. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and characterization of short chain rhamnolipid production in a previously uninvestigated, non-pathogenic marine Pseudomonas. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, L.; Twigg, M.S.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Biosynthesis of rhamnolipid by a Marinobacter species expands the paradigm of biosurfactant synthesis to a new genus of the marine microflora. Microb. Cell Fact. 2019, 18, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeniel Biotech Inc. Home Page. Available online: https://www.jeneilbiotech.com (accessed on 27 February 2023).
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- Sharma, P.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Verma, K.S.; Verma, S.; Prasad, J.; Kothari, S.L.; Gour, V.S. An appraisal of cuticular wax of Calotropis procera (Ait.) R. Br.: Extraction, chemical composition, biosafety and application. J. Hazard. Mater. 2019, 368, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, D.; Manzoor, M.; Banpurkar, A.G.; Satpute, S.K.; Sharma, D. Characterization and cytotoxicity assessment of biosurfactant derived from Lactobacillus pentosus NCIM 2912. Braz. J. Microbiol. 2022, 53, 327–340. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; López-Prieto, A.; Lopez-Álvarez, M.; Pérez-Davila, S.; Serra, J.; González, P.; Moldes, A.B. Characterization and cytotoxic effect of biosurfactants obtained from different sources. ACS Omega 2020, 5, 31381–31390. [Google Scholar] [CrossRef]
- UNE-EN ISO 10993-5:2009; Biological evaluation of medical devices—Part 5: Tests for in Vitro Cytotoxicity. ISO (International Organization for Standardization): Geneva, Switzerland, 2022.
- Voulgaridou, G.-P.; Mantso, T.; Anestopoulos, I.; Klavaris, A.; Katzastra, C.; Kiousi, D.-E.; Mantela, M.; Galanis, A.; Gardikis, K.; Banat, I.M.; et al. toxicity profiling of biosurfactants produced by novel marine bacterial strains. Int. J. Mol. Sci. 2021, 22, 2383. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Twigg, M.S.; Naughton, N.J.; Marchant, R.; Banat, I.M. Characterization of cytotoxicity and immunomodulatory effects of glycolipid biosurfactants on human keratinocytes. Appl. Microbiol. Biotechnol. 2023, 107, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. SAVE FOOD: Global Initiative on Food Loss and Waste Reduction|Key Facts on Food Loss and Waste You should Know! 2020. Available online: http://www.fao.org/save-food/resources/keyfindings/en/ (accessed on 17 March 2023).
- Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate films encapsulating lemongrass essential oil as affected by spray calcium application. Coll. Interf. 2019, 3, 58. [Google Scholar] [CrossRef] [Green Version]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocol. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Zainal Abidin, M.; Kourmentza, C.; Karatzas, A.K.; Niranjan, K. Enzymatic hydrolysis of thermally pre-treated chitin and antimicrobial activity of N, N’-diacetylchitobiose. J. Chem. Technol. Biotechnol. 2019, 94, 2529–2536. [Google Scholar] [CrossRef]
- Meena, K.R.; Sharma, A.; Kanwar, S.S. Antitumoral and antimicrobial activity of surfactin extracted from Bacillus subtilis KLP2015. Int. J. Pept. Res. Ther. 2019, 26, 423–433. [Google Scholar] [CrossRef]
- Kourmentza, K.; Gromada, X.; Michael, N.; Degraeve, C.; Vanier, G.; Ravallec, R.; Jauregi, P. Antimicrobial activity of lipopeptide biosurfactants against foodborne pathogen and food spoilage microorganisms and their cytotoxicity. Front. Microbiol. 2021, 11, 561060. [Google Scholar] [CrossRef]
- Thimon, L.; Peypoux, F.; Wallach, J.; Michel, G. Effect of the lipopeptide antibiotic, iturin A, on morphology and membrane ultrastructure of yeast cells. FEMS Microbiol. Lett. 1995, 128, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Aranda, F.J.; Manresa, Á.; Ortiz, A. Modulation of the physical properties of dielaidoylphosphatidylethanolamine membranes by a dirhamnolipid biosurfactant produced by Pseudomonas aeruginosa. Chem. Phys. Lipids 2006, 142, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Sotirova, A.V.; Spasova, D.I.; Galabova, D.N.; Karpenko, E.; Shulga, A. Rhamnolipid–biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, D.; Aseri, G.K.; Sohal, J.S.; Vij, S.; Sharma, D. Role of lacto-fermentation in reduction of antinutrients in plant-based foods. J. Appl. Biol. Biotechnol. 2021, 9, 7–16. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Singh, D.; Sukhbir-Singh, G.M.; Karamchandani, B.M.; Aseri, G.K.; Banat, I.M.; Satpute, S.K. Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries. Molecules 2023, 28, 2823. https://doi.org/10.3390/molecules28062823
Sharma D, Singh D, Sukhbir-Singh GM, Karamchandani BM, Aseri GK, Banat IM, Satpute SK. Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries. Molecules. 2023; 28(6):2823. https://doi.org/10.3390/molecules28062823
Chicago/Turabian StyleSharma, Deepansh, Deepti Singh, Gadhwal Monika Sukhbir-Singh, Bhoomika M. Karamchandani, Gajender Kumar Aseri, Ibrahim M. Banat, and Surekha K. Satpute. 2023. "Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries" Molecules 28, no. 6: 2823. https://doi.org/10.3390/molecules28062823
APA StyleSharma, D., Singh, D., Sukhbir-Singh, G. M., Karamchandani, B. M., Aseri, G. K., Banat, I. M., & Satpute, S. K. (2023). Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries. Molecules, 28(6), 2823. https://doi.org/10.3390/molecules28062823







