Rational Fabrication of Defect-Rich and Hierarchically Porous Fe-N-C Nanosheets as Highly Efficient Oxygen Reduction Electrocatalysts for Zinc-Air Battery
Abstract
:1. Introduction
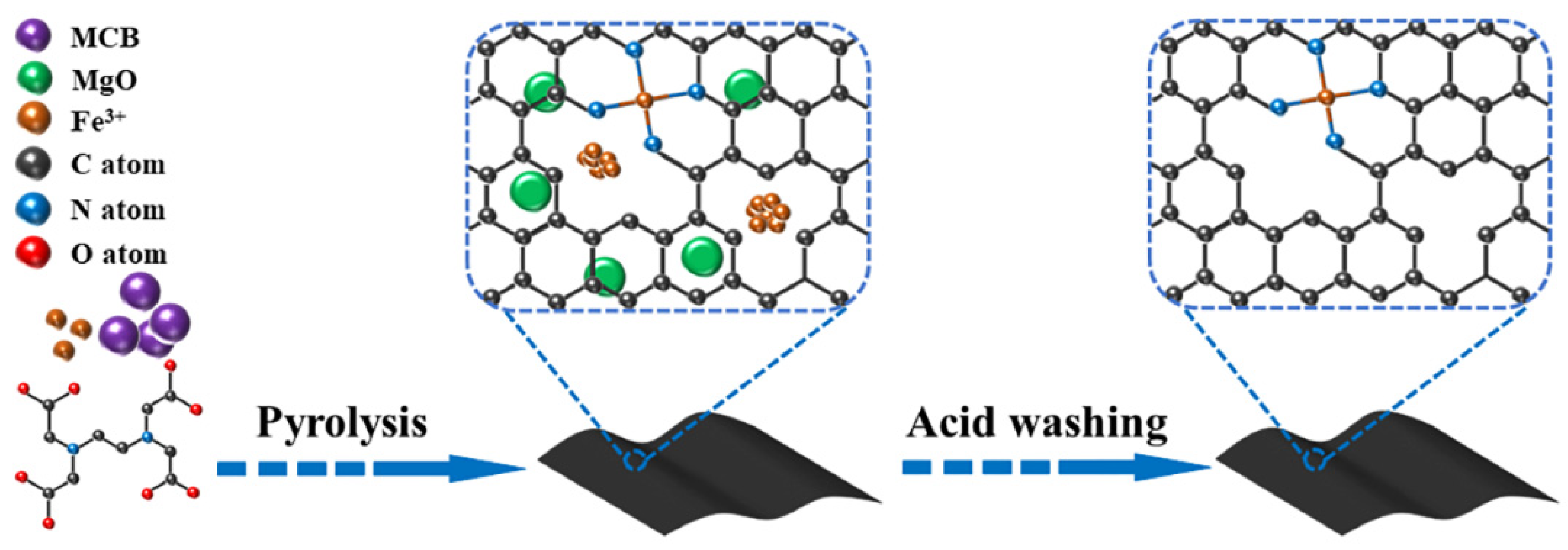
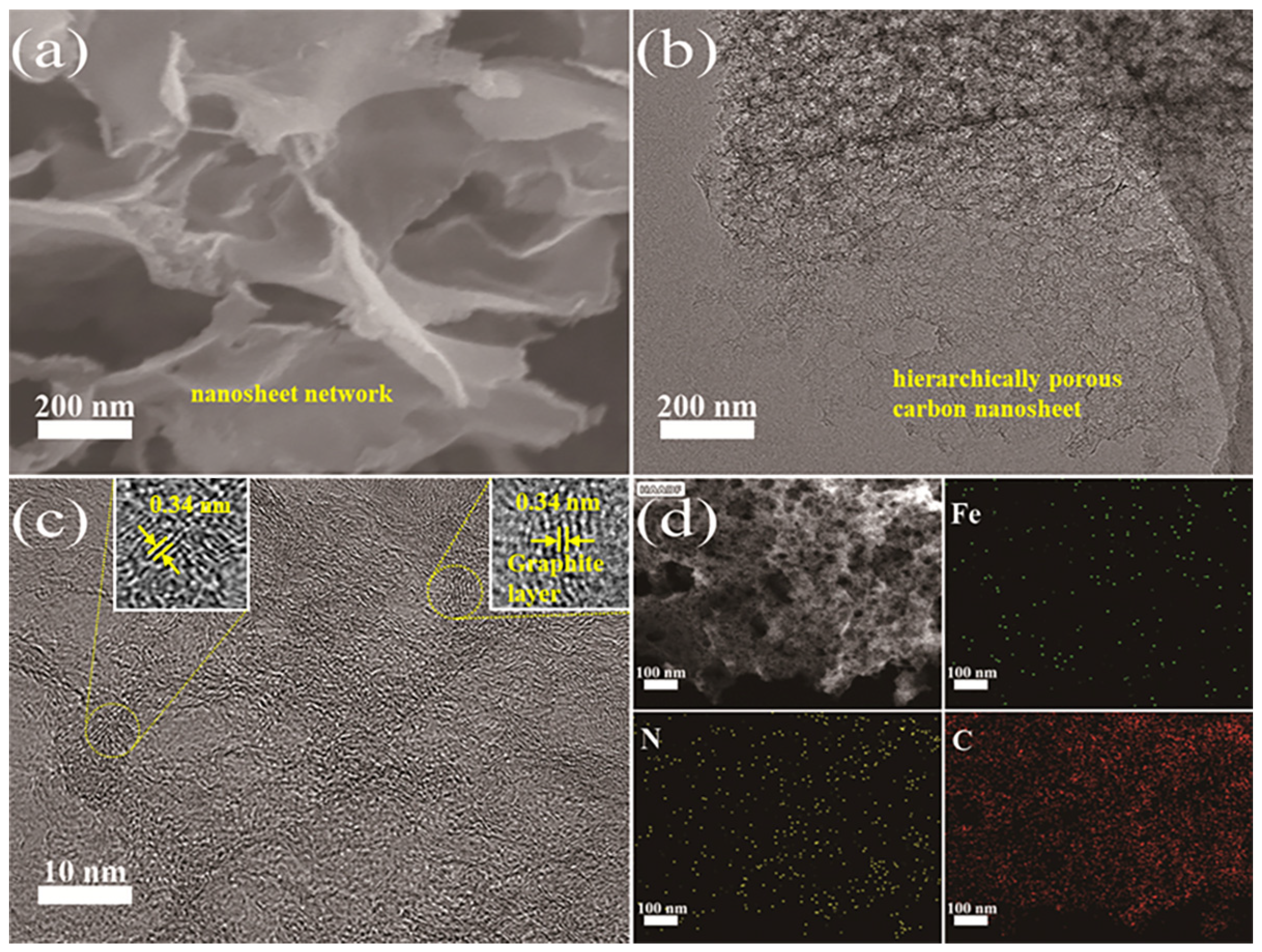
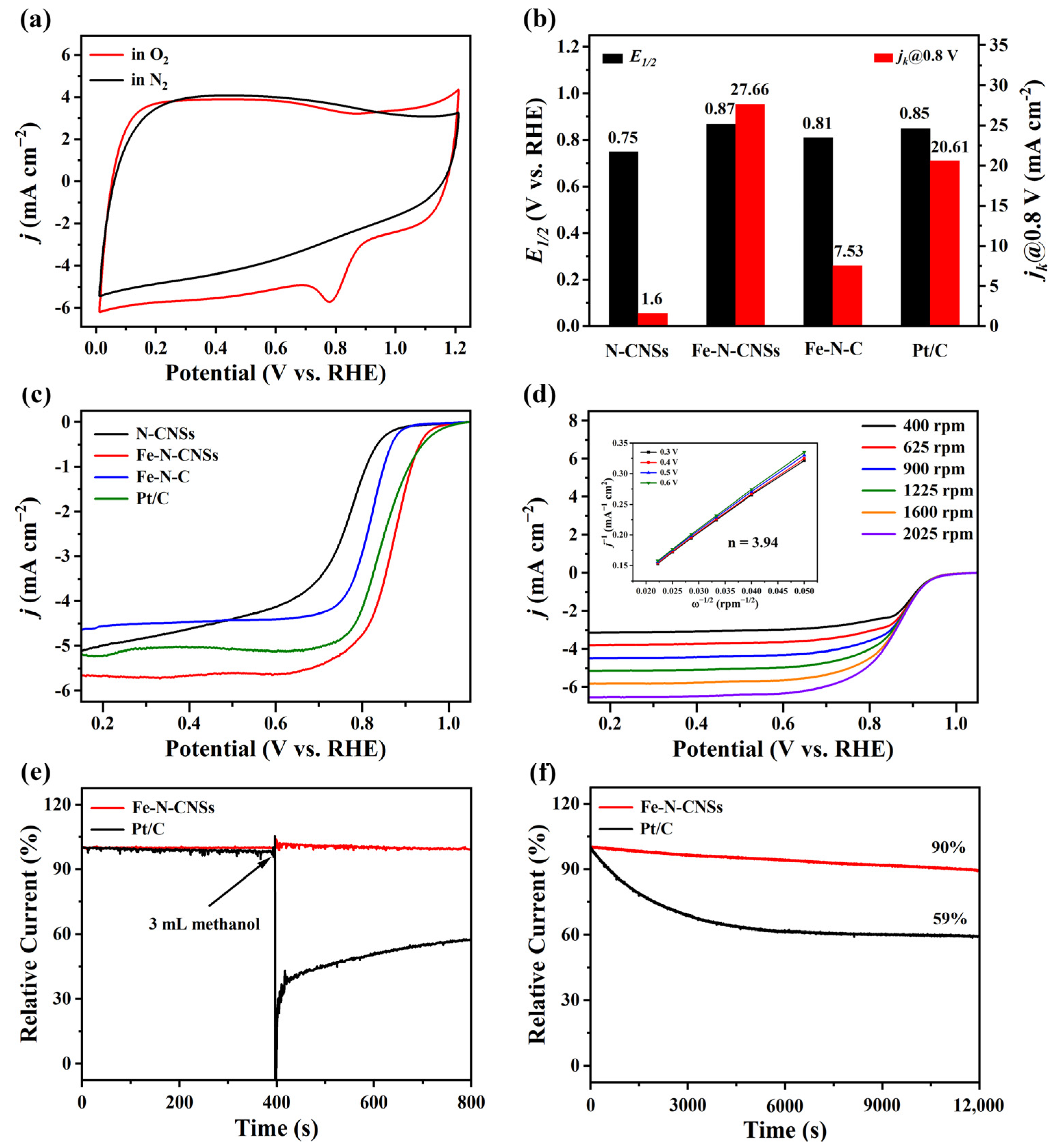
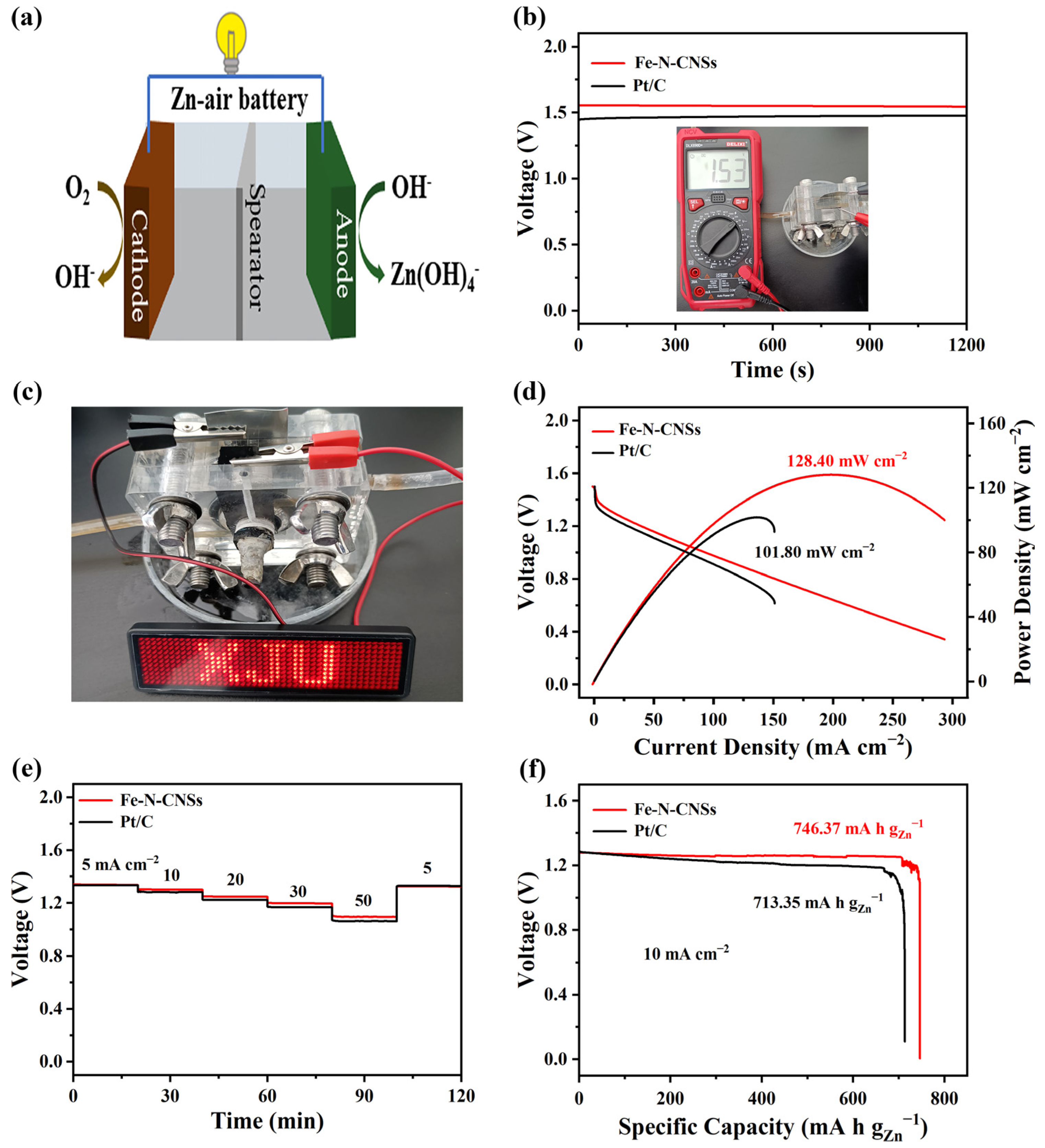
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Physical Characterizations
3.4. Electrochemical Measurements
3.5. Zn-Air Battery Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, M.; Dou, H.; Zhang, Z.; Zheng, Y.; Ren, B.; Ma, Q.; Wen, G.; Luo, D.; Yu, A.; Zhang, L. Hierarchically nanostructured solid-state electrolyte for flexible rechargeable zinc–air batteries. Angew. Chem. Int. Ed. 2022, 134, e202117703. [Google Scholar] [CrossRef]
- Xu, H.F.; Zhai, Q.G.; Jiang, Y.C.; Huang, H.; Li, S.N. Carbon foam-supported CoN nanoparticles and carbon nanotubes hybrids as bifunctional reduction electrocatalyst. Catal. Commun. 2022, 163, 106408. [Google Scholar] [CrossRef]
- Shao, Z.; Zhu, Q.; Sun, Y.; Zhang, Y.; Jiang, Y.; Deng, S.; Zhang, W.; Huang, K.; Feng, S. Phase-reconfiguration-induced NiS/NiFe2O4 composite for performance-enhanced zinc−air batteries. Adv. Mater. 2022, 34, 2110172. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, J.; Jia, J.; Hu, X.; Yang, H.; Jia, M.; Wen, Z. The fluorine-doped and defects engineered carbon nanosheets as advanced electrocatalysts for oxygen electroreduction. Appl. Catal. B Environ. 2021, 284, 119721. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Cui, J.; Qian, Z.; Liu, D. Fabrication of ORR/OER Electrocatalysts with simple one-step strategy from sustainable cornstalks. Catal. Commun. 2022, 171, 106525. [Google Scholar] [CrossRef]
- Zheng, Q.; Xiong, Y.; Tang, K.; Wu, M.; Hu, H.; Zhou, T.; Wu, Y.; Cao, Z.; Sun, J.; Yu, X. Modulation of pore-size in N, S-codoped carbon/Co9S8 hybrid for a stronger O2 affinity toward rechargable zinc-air battery. Nano Energy 2022, 92, 106750. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Liu, D.; Guo, P.; Liu, J.; Wang, R.; Guo, Y.; Tu, X.; Pan, H.; Sun, D. Alloying Co species into ordered and interconnected macroporous carbon polyhedra for efficient oxygen reduction reaction in rechargeable zinc–air batteries. Adv. Mater. 2022, 34, 2109605. [Google Scholar] [CrossRef]
- Liu, J.; He, T.; Wang, Q.; Zhou, Z.; Zhang, Y.; Wu, H.; Li, Q.; Zheng, J.; Sun, Z.; Lei, Y. Confining ultrasmall bimetallic alloys in porous N–carbon for use as scalable and sustainable electrocatalysts for rechargeable Zn–air batteries. J. Mater. Chem. A 2019, 7, 12451–12456. [Google Scholar] [CrossRef]
- Mei, Z.Y.; Cai, S.; Zhao, G.; Zou, X.; Fu, Y.; Jiang, J.; An, Q.; Li, M.; Liu, T.; Guo, H. Boosting the ORR active and Zn-air battery performance through ameliorating the coordination environment of iron phthalocyanine. Chem. Eng. J. 2022, 430, 132691. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Das, I.; Noori, M.T.; Shaikh, M.; Ghangrekar, M.M.; Ananthakrishnan, R. Synthesis and application of zirconium metal–organic framework in microbial fuel cells as a cost-effective oxygen reduction catalyst with competitive performance. ACS Appl. Energy Mater. 2020, 3, 3512–3520. [Google Scholar] [CrossRef]
- Kong, F.; Cui, X.; Huang, Y.; Yao, H.; Chen, Y.; Tian, H.; Meng, G.; Chen, C.; Chang, Z.; Shi, J. N-Doped carbon electrocatalyst: Marked ORR activity in acidic media without the contribution from metal sites? Angew. Chem. Int. Ed. 2022, 61, e202116290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Duan, L.; Zhao, Y.; Wang, L.; Zhang, J.; Bu, F.; Sun, Z.; Zhang, T.; Liu, M.; Chen, H. Constructing unique mesoporous carbon superstructures via monomicelle interface confined assembly. J. Am. Chem. Soc. 2022, 144, 11767–11777. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174. [Google Scholar] [CrossRef]
- Liang, Q.; Meng, F.; Song, K.; Wang, D.; Zhang, W. Exploiting asymmetric Co states in a Co-NC catalyst for an efficient oxygen reduction reaction. Symmetry 2022, 14, 2496. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Wang, J.; Wang, C.; Guo, X.; Li, X.-Y.; Chen, X.; Song, L.; Li, B.-Q.; Zhang, Q. A clicking confinement strategy to fabricate transition metal single-atom sites for bifunctional oxygen electrocatalysis. Sci. Adv. 2022, 8, eabn5091. [Google Scholar] [CrossRef]
- Zhou, X.; Song, K.; Feng, Y.; Jiang, C.; Chen, Z.; Wang, Z.; Yue, N.; Ge, X.; Zhang, W.; Zheng, W. Individually-atomic governing d—π* orbital interactions via Cu-promoted optimization of Fe-d band centers for high-efficiency zinc-air battery. Nano Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Xia, W.; Tang, J.; Gao, Y.; Jiang, C.; Jia, Y.; Chen, T.; Hou, Z.; Qi, R.; Jiang, D. Metal–organic framework-derived graphene mesh: A robust scaffold for highly exposed Fe–N4 active sites toward an excellent oxygen reduction catalyst in acid media. J. Am. Chem. Soc. 2022, 144, 9280–9291. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, G.L.; Sun, C.J.; Xu, M.; Wen, W.; Wang, Q.; Gu, M.; Zhu, S.; Li, Y.; Wei, Z. Nitrogen-coordinated single iron atom catalysts derived from metal organic frameworks for oxygen reduction reaction. Nano Energy 2019, 61, 60–68. [Google Scholar] [CrossRef]
- Liu, D.; Wang, B.; Srinivas, K.; Yu, B.; Chen, X.; Ma, F.; Wang, X.; Zhang, X.; Yang, D.; Chen, Y. Rich and uncovered FeNx atom clusters anchored on nitrogen-doped graphene nanosheets for highly efficient and stable oxygen reduction reaction. J. Alloys Compd. 2022, 901, 163763. [Google Scholar] [CrossRef]
- Song, A.; Cao, L.; Yang, W.; Yang, W.; Wang, L.; Ma, Z.; Shao, G. In situ construction of nitrogen-doped graphene with surface-grown carbon nanotubes as a multifactorial synergistic catalyst for oxygen reduction. Carbon 2019, 142, 40–50. [Google Scholar] [CrossRef]
- Cheng, Y.; He, S.; Lu, S.; Veder, J.P.; Johannessen, B.; Thomsen, L.; Saunders, M.; Becker, T.; De Marco, R.; Li, Q. Iron single atoms on graphene as nonprecious metal catalysts for high-temperature polymer electrolyte membrane fuel cells. Adv. Sci. 2019, 6, 1802066. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chung, H.T.; Cullen, D.A.; Wagner, S.; Kramm, U.I.; More, K.L.; Zelenay, P.; Wu, G. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 2019, 12, 2548–2558. [Google Scholar] [CrossRef]
- Bai, X.; Guan, J. MXenes for electrocatalysis applications: Modification and hybridization. Chin. J. Catal. 2022, 43, 2057–2090. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Z.; Guo, Z. Theoretical investigation on the hydrogen evolution, oxygen evolution, and oxygen reduction reactions performances of two-dimensional metal-organic frameworks Fe3(C2X)12 (X = NH, O, S). Molecules 2022, 27, 1528. [Google Scholar] [CrossRef]
- He, S.; Wu, M.; Li, S.; Jiang, Z.; Hong, H.; Cloutier, S.G.; Yang, H.; Omanovic, S.; Sun, S.; Zhang, G. Research progress on graphite-derived materials for electrocatalysis in energy conversion and storage. Molecules 2022, 27, 8644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guan, J. Applications of single-atom catalysts. Nano Res. 2022, 15, 38–70. [Google Scholar] [CrossRef]
- Bai, X.; Guan, J. Applications of MXene-based single-atom catalysts. Small Struct. 2022, 2022, 2200354. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Radhamani, A.V.; Ramakrishna, S.; Wei, Y.; Liu, Y. Recent insights on iron based nanostructured electrocatalyst and current status of proton exchange membrane fuel cell for sustainable transport. J. Energy Chem. 2022, 69, 466–489. [Google Scholar] [CrossRef]
- Zhang, X.; Truong-Phuoc, L.; Asset, T.; Pronkin, S.; Pham-Huu, C. Are Fe–N–C electrocatalysts an alternative to Pt-Based electrocatalysts for the next generation of proton exchange membrane fuel cells? ACS Catal. 2022, 12, 13853–13875. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Doyle-Davis, K.; Fu, X.; Luo, J.L.; Sun, X. Recent advances in MOF-derived single atom catalysts for electrochemical applications. Adv. Energy Mater. 2020, 10, 2001561. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Zou, J.; Yan, Y.; Xie, S.; Hu, G.; Li, Y.; Dong, A. Simple-cubic carbon frameworks with atomically dispersed iron dopants toward high-efficiency oxygen reduction. Nano Lett. 2017, 17, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Wu, Y.; Li, Y. Fabrication of single-atom catalysts with precise structure and high metal loading. Adv. Mater. 2018, 30, 1801649. [Google Scholar] [CrossRef]
- Zhan, Y.; Zeng, H.; Xie, F.; Zhang, H.; Zhang, W.; Jin, Y.; Zhang, Y.; Chen, J.; Meng, H. Templated growth of Fe/N/C catalyst on hierarchically porous carbon for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2019, 431, 31–39. [Google Scholar] [CrossRef]
- Cheng, R.; Jiang, M.; Li, K.; Guo, M.; Zhang, J.; Ren, J.; Meng, P.; Li, R.; Fu, C. Dimensional engineering of carbon dots derived sulfur and nitrogen co-doped carbon as efficient oxygen reduction reaction electrocatalysts for aluminum-air batteries. Chem. Eng. J. 2021, 425, 130603. [Google Scholar] [CrossRef]
- Dong, Y.; Fang, Z.; Ou, D.; Shi, Q.; Ma, Y.; Yang, W.; Tang, B.; Liu, Q. Rational fabrication of S-modified Fe–N–C nanosheet electrocatalysts for efficient and stable pH-universal oxygen reduction. Chem. Eng. J. 2022, 444, 136433. [Google Scholar] [CrossRef]
- Ross, R.D.; Sheng, H.; Ding, Y.; Janes, A.N.; Feng, D.; Schmidt, J.; Segre, C.U.; Jin, S. Operando elucidation of electrocatalytic and redox mechanisms on a 2D metal organic framework catalyst for efficient electrosynthesis of hydrogen peroxide in neutral media. J. Am. Chem. Soc. 2022, 144, 15845–15854. [Google Scholar] [CrossRef]
- Xu, X.; Xie, J.; Liu, B.; Wang, R.; Liu, M.; Zhang, J.; Liu, J.; Cai, Z.; Zou, J. PBA-derived FeCo alloy with core-shell structure embedded in 2D N-doped ultrathin carbon sheets as a bifunctional catalyst for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2022, 316, 121687. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: An efficient 3D electrode for overall water splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Qiu, M.; Zhang, T.; Zhuang, X.; Kim, C.S.; Yuan, C.; Feng, X. Ternary porous cobalt phosphoselenide nanosheets: An efficient electrocatalyst for electrocatalytic and photoelectrochemical water splitting. Adv. Mater. 2017, 29, 1701589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Ma, T.; Jiang, Y.; Chen, J.; Pan, H.; Sun, W. 2D metal-free nanomaterials beyond graphene and its analogues toward electrocatalysis applications. Adv. Energy Mater. 2021, 11, 2101202. [Google Scholar] [CrossRef]
- Niu, W.J.; He, J.Z.; Gu, B.N.; Liu, M.C.; Chueh, Y.L. Opportunities and challenges in precise synthesis of transition metal single-atom supported by 2D materials as catalysts toward oxygen reduction reaction. Adv. Funct. Mater. 2021, 31, 2103558. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Z.; Guan, J. 2D MOFs and their derivatives for electrocatalytic applications: Recent advances and new challenges. Coordin. Chem. Rev. 2022, 472, 214777. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, L.; Xu, S.; Lin, K.; He, W.; Ni, M.; Ruan, Q.; Zhang, P.; Liu, Y.; Zhang, W. Research progress of MXene-based catalysts for electrochemical water-splitting and metal-air batteries. Energy Storage Mater. 2021, 43, 509–530. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Y.; Ma, R.; Liu, Q.; Wang, J. Reactive template synthesis of nitrogen-doped graphene-like carbon nanosheets derived from hydroxypropyl methylcellulose and dicyandiamide as efficient oxygen reduction electrocatalysts. J. Power Sources 2017, 345, 120–130. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Li, Z.; Wang, L.; Niu, X.; Sun, P. Novel space-confinement synthesis of two-dimensional Fe, N-codoped graphene bifunctional oxygen electrocatalyst for rechargeable air-cathode. Chem. Eng. J. 2021, 411, 128492. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Chen, H.; Li, H.; Liu, P. Medulla stachyuri-derived iron and nitrogen co-doped 2D porous carbon-flakes for highly efficient oxygen reduction electrocatalysis and supercapacitors. Int. J. Hydrogen Energy 2019, 44, 21726–21737. [Google Scholar] [CrossRef]
- Cao, X.; Li, J.; Dong, X.; Song, R.; Zhou, X.; Wang, X.; Yuan, N.; Ding, J. Self-template synthesized 2D porous carbon with FeA@ Fe-NC sites as oxygen electrocatalysts for Zn-Air and membraneless methanol fuel cells. J. Alloys Compd. 2022, 928, 166932. [Google Scholar] [CrossRef]
- Wang, M.; Gao, P.; Liu, J.; Li, D.; Yang, M.; Shen, Y.; Yang, S.; Hu, X.; Liu, Z.; Liu, Y. Layered-template synthesis of graphene-like Fe-NC nanosheets for highly efficient oxygen reduction reaction. Energy Fuel 2021, 35, 20349–20357. [Google Scholar] [CrossRef]
- Gu, B.; Su, H.; Chu, X.; Wang, Q.; Huang, H.; He, J.; Wu, T.; Deng, W.; Zhang, H.; Yang, W. Rationally assembled porous carbon superstructures for advanced supercapacitors. Chem. Eng. J. 2019, 361, 1296–1303. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Kong, L.; Song, A.; Qin, X.; Shao, G. Phosphorus-doped 3D hierarchical porous carbon for high-performance supercapacitors: A balanced strategy for pore structure and chemical composition. Carbon 2018, 127, 557–567. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, G.; Zhang, X.; Chen, B.; Lu, Z.; Xu, H.; Gao, R.; Shi, C. Engineering the local coordination environment and density of FeN4 sites by Mn cooperation for electrocatalytic oxygen reduction. Small 2022, 18, 2200911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, J.; Qin, Y.-H.; Wang, C.-W. Porous N–C catalyst synthesized by pyrolyzing g-C3N4 embedded in carbon as highly effcient oxygen reduction electrocatalysts for primary Zn-air battery. Carbon 2019, 150, 475–484. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Mou, J.; Zhang, W.; Jiang, Z.; Liu, J.; Huang, J.; Liu, M. Free-standing N-self-doped carbon nanofiber aerogels for high-performance all-solid-state supercapacitors. Nano Energy 2019, 63, 103836. [Google Scholar] [CrossRef]
- Ao, X.; Ding, Y.; Nam, G.; Soule, L.; Jing, P.; Zhao, B.; Hwang, J.Y.; Jang, J.H.; Wang, C.; Liu, M. A single-atom Fe-N-C catalyst with ultrahigh utilization of active sites for efficient oxygen reduction. Small 2022, 18, 2203326. [Google Scholar] [CrossRef]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.H.; Jiang, H.L. From metal–organic frameworks to single-atom Fe implanted N-doped porous carbons: Efficient oxygen reduction in both alkaline and acidic media. Angew. Chem. Int.l Ed. 2018, 57, 8525–8529. [Google Scholar] [CrossRef]
- Subramanian, N.P.; Li, X.; Nallathambi, V.; Kumaraguru, S.P.; Colon-Mercado, H.; Wu, G.; Lee, J.W.; Popov, B.N. Nitrogen-modified carbon-based catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J. Power Sources 2009, 188, 38–44. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Shrestha, L.K.; Hossain, M.S.A.; Alothman, Z.A.; Yamauchi, Y.; Ariga, K. Activated porous carbon spheres with customized mesopores through assembly of diblock copolymers for electrochemical capacitor. ACS Appl. Mater. Inter. 2017, 9, 18986–18993. [Google Scholar] [CrossRef]

| Sample | C Content (at %) | N Content (at %) | O Content (at %) | Fe Content (at %) |
|---|---|---|---|---|
| N-CNSs | 87.53 | 3.21 | 9.26 | 0 |
| Fe-N-CNSs | 88.8 | 3.79 | 7.2 | 0.21 |
| Fe-N-C | 90.15 | 2.29 | 7.51 | 0.05 |
| Sample | Pyridinic N (at %) | Fe-N (at %) | Pyrrolic N (at %) | Graphitic N (at %) | Oxidized N (at %) |
|---|---|---|---|---|---|
| N-CNSs | 46.01 | 0 | 34.65 | 12.44 | 6.90 |
| Fe-N-CNSs | 41.34 | 9.17 | 28.12 | 11.87 | 9.50 |
| Fe-N-C | 27.36 | 6.06 | 11.64 | 35.36 | 11.58 |
| Sample | BET Surface Area (m2 g−1) | BJH Pore Volume (cm3 g−1) |
|---|---|---|
| Fe-N-CNSs-800 | 801.78 | 0.69 |
| Fe-N-CNSs-900 | 1303.43 | 1.76 |
| Fe-N-CNSs-1000 | 1178.32 | 1.69 |
| N-CNSs | 985.16 | 1.34 |
| Fe-N-C | 421.15 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Lv, Y.; Elam, S.; Zhang, X.; Yang, Z.; Wu, X.; Guo, J. Rational Fabrication of Defect-Rich and Hierarchically Porous Fe-N-C Nanosheets as Highly Efficient Oxygen Reduction Electrocatalysts for Zinc-Air Battery. Molecules 2023, 28, 2879. https://doi.org/10.3390/molecules28072879
Li S, Lv Y, Elam S, Zhang X, Yang Z, Wu X, Guo J. Rational Fabrication of Defect-Rich and Hierarchically Porous Fe-N-C Nanosheets as Highly Efficient Oxygen Reduction Electrocatalysts for Zinc-Air Battery. Molecules. 2023; 28(7):2879. https://doi.org/10.3390/molecules28072879
Chicago/Turabian StyleLi, Sensen, Yan Lv, Sawida Elam, Xiuli Zhang, Zhuojun Yang, Xueyan Wu, and Jixi Guo. 2023. "Rational Fabrication of Defect-Rich and Hierarchically Porous Fe-N-C Nanosheets as Highly Efficient Oxygen Reduction Electrocatalysts for Zinc-Air Battery" Molecules 28, no. 7: 2879. https://doi.org/10.3390/molecules28072879




