Triphenylamine-Containing Benzoic Acids: Synthesis, Liquid Crystalline and Redox Properties
Abstract
1. Introduction
2. Results and Discussion
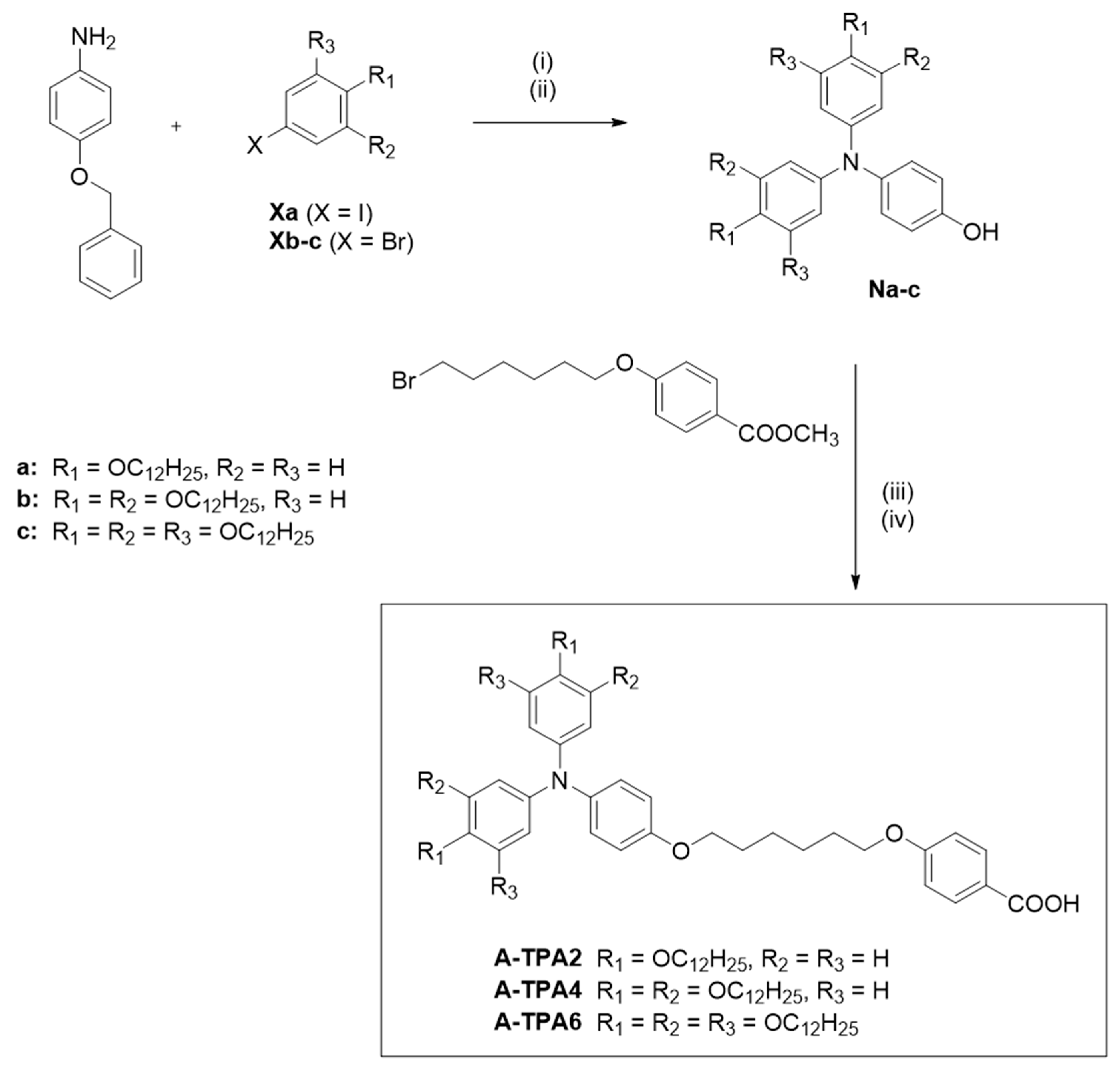
2.1. Synthesis and Characterization
2.2. Liquid Crystalline Properties
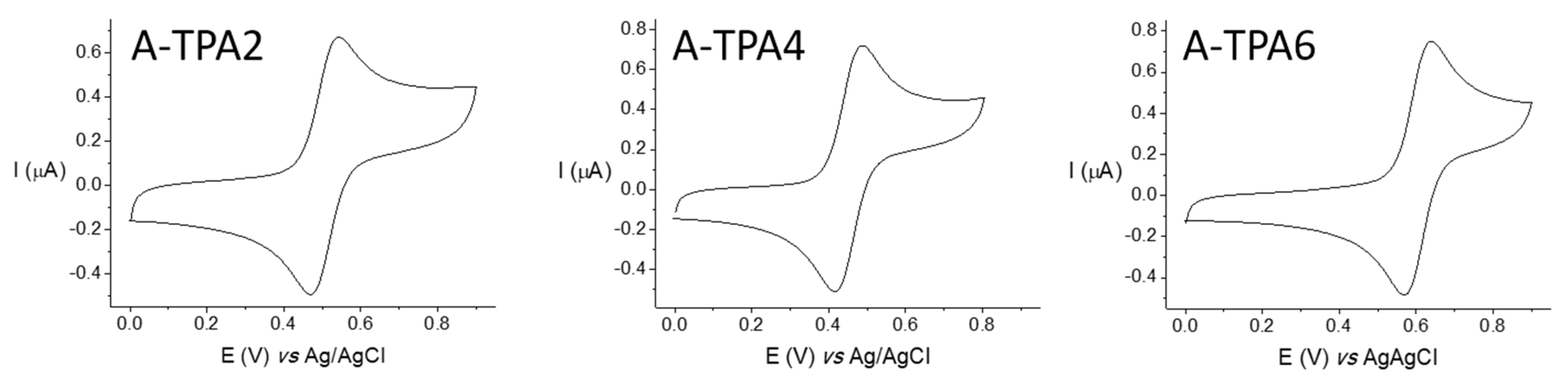
2.3. Electrochemical Properties
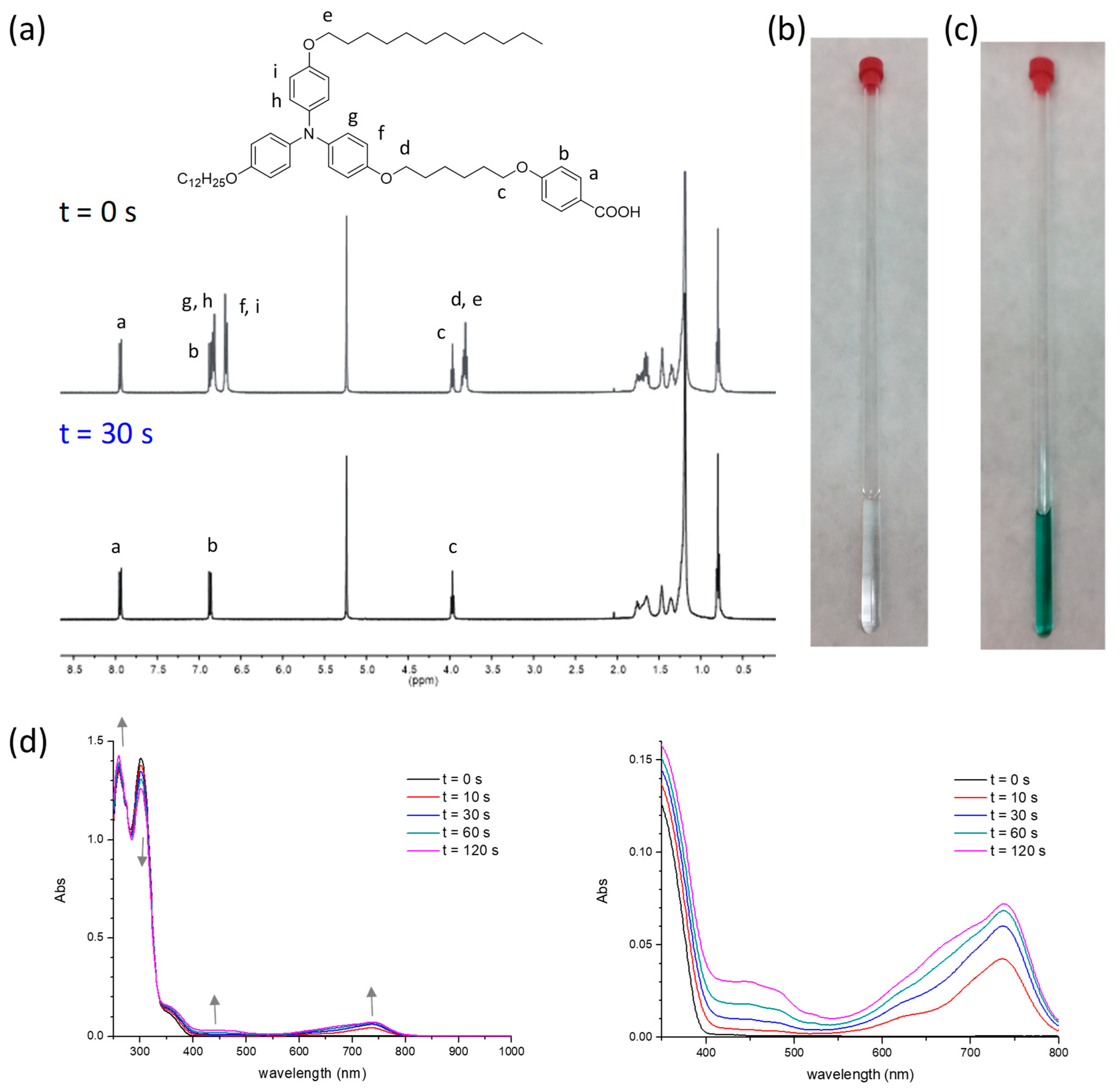
2.4. Photoactivity in Solution
3. Materials and Methods
3.1. Experimental Techniques
3.2. Synthesis of the Triphenylamine-Containing Benzoic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Thelakkat, M. Star-Shaped, Dendrimeric and Polymeric Triarylamines as Photoconductors and Hole Transport Materials for Electro-Optical Applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, K.; Ma, L.C.; Zhan, X.W. Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells. Chem. Rev. 2016, 116, 14675–14725. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Armao, J.J.; Giuseppone, N. Triarylamine-Based Supramolecular Polymers: Structures, Dynamics, and Functions. Acc. Chem. Res. 2019, 54, 975–983. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.; Forster, M.; Souharce, B.; Thiem, H.; Scherf, U. Organic semiconductors for solution-processable field-effect transistors (OFETs). Angew. Chem. Int. Ed. 2008, 47, 4070–4098. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Tian, H. Triarylamine: A promising core unit for efficient photovoltaic materials. Chem. Commun. 2009, 37, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, P.; Kabra, D. A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): Evolution and molecular engineering. J. Mater. Chem. A 2017, 5, 1348–1373. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M. Organic hole-transporting materials for efficient perovskite solar cells. Mater. Today Energy 2018, 7, 208–220. [Google Scholar] [CrossRef]
- Rodríguez-Seco, C.; Cabau, L.; Vidal-Ferran, A.; Palomares, E. Advances in the Synthesis of Small Molecules as Hole Transport Materials for Lead Halide Perovskite Solar Cells. Acc. Chem. Res. 2018, 51, 869–880. [Google Scholar] [CrossRef]
- Shirota, Y. Photo- and electroactive amorphous molecular materials—Molecular design, syntheses, reactions, properties, and applications. J. Mater. Chem. 2005, 15, 75–93. [Google Scholar] [CrossRef]
- Moulin, E.; Cid, J.-J.; Giuseppone, N. Advances in Supramolecular Electronics—From Randomly Self-assembled Nanostructures to Addressable Self-Organized Interconnects. Adv. Mater. 2013, 25, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Stupp, S.I.; Palmer, L.C. Supramolecular Chemistry and Self-Assembly in Organic Materials Design. Chem. Mater. 2014, 26, 507–518. [Google Scholar] [CrossRef]
- Pisula, W.; Zorn, M.; Chang, J.Y.; Mullen, K.; Zentel, R. Liquid Crystalline Ordering and Charge Transport in Semiconducting Materials. Macromol. Rapid Commun. 2009, 30, 1179–1202. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Ordered Materials for Organic Electronics and Photonics. Adv. Mater. 2011, 23, 566–584. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Li, Q. Self-organized Discotic Liquid Crystals as Novel Organic Semiconductors. In Self-Organized Organic Semiconductors. From Materials to Device Applications; Li, Q., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Termine, R.; Golemme, A. Charge Mobility in Discotic Liquid Crystals. Int. J. Mol. Sci. 2021, 22, 877. [Google Scholar] [CrossRef]
- De, R.; Pal, S.K. Self-assembled discotics as molecular semiconductors. Chem. Commun. 2023, in press. [Google Scholar] [CrossRef]
- Feringán, B.; Termine, R.; Golemme, A.; Granadino-Roldán, J.M.; Navarro, A.; Giménez, R.; Sierra, T. Triphenylamine- and triazine-containing hydrogen bonded complexes: Liquid crystalline supramolecular semiconductors. J. Mater. Chem. C 2021, 9, 1972–1982. [Google Scholar] [CrossRef]
- Sobolev, A.N.; Belsky, V.K.; Romm, I.P.; Chernikova, N.Y.; Guryanova, E.N. Structural Investigation of the Triaryl Derivatives of the Group V Elements. IX. Structure of Triphenylamine, C18H15N. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1985, 41, 967–971. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Mondal, S.; De, N.; Sinha, R.K.; Pal, N.; Roy, B. Synthesis and mesomorphic behaviour of new discotic liquid crystalline compounds containing triphenylamine as a core moiety via Sonogashira coupling. Tetrahedron Lett. 2010, 51, 521–524. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Pal, N.; Debnath, P.; Rao, N.V.S. A columnar mesophase from a disc-shaped molecule derived from triphenylamine: Synthesis, mesomorphic behaviour and optical properties. Tetrahedron Lett. 2007, 48, 6330–6333. [Google Scholar] [CrossRef]
- Choudhury, T.D.; Rao, N.V.S.; Tenent, R.; Blackburn, J.; Gregg, B.; Smalyukh, I.I. Homeotropic Alignment and Director Structures in Thin Films of Triphenylamine-Based Discotic Liquid Crystals Controlled by Supporting Nanostructured Substrates and Surface Confinement. J. Phys. Chem. B 2011, 115, 609–617. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Chattopadhyay, B.; Shyam, P.K.; Pal, N. A new disc-shaped mesogenic compound with olefinic linkage derived from triphenylamine: Synthesis, mesogenic behavior and fluorescence properties. Tetrahedron Lett. 2009, 50, 6901–6905. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sheu, H.S.; Lai, C.K. New star-shaped triarylamines: Synthesis, mesomorphic behavior, and photophysical properties. Tetrahedron 2007, 63, 1695–1705. [Google Scholar] [CrossRef]
- Domoto, Y.; Busseron, E.; Maaloum, M.; Moulin, E.; Giuseppone, N. Control over Nanostructures and Associated Mesomorphic Properties of Doped Self-Assembled Triarylamine Liquid Crystals. Chem. Eur. J. 2015, 21, 1938–1948. [Google Scholar] [CrossRef]
- Bennett, G.M.; Jones, B. Mesomorphism and polymorphism of some p-alkoxybenzoic and p-alkoxycinnamic acids. J. Chem. Soc. 1939, 420–425. [Google Scholar] [CrossRef]
- Abdy, M.J.; Murdoch, A.; Martínez-Felipe, A. New insights into the role of hydrogen bonding on the liquid crystal behaviour of 4-alkoxybenzoic acids: A detailed IR spectroscopy study. Liq. Cryst. 2016, 43, 2191–2207. [Google Scholar] [CrossRef]
- Seo, E.T.; Nelson, R.F.; Fritsch, J.M.; Marcoux, L.S.; Leedy, D.W.; Adams, R.N. Anodic Oxidation Pathways of Aromatic Amines. Electrochemical and Electron Paramagnetic Resonance Studies. J. Am. Chem. Soc. 1966, 88, 3498–3503. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Su, T.X.; Li, J.H.; Lin, T.H.; Liou, G.S.; Cheng, S.H. Novel trends of electrochemical oxidation of amino-substituted triphenylamine derivatives. J. Electroanal. Chem. 2005, 575, 95–101. [Google Scholar] [CrossRef]
- Jeon, N.J.; Lee, H.G.; Kim, Y.C.; Seo, J.; Noh, J.H.; Lee, J.; Seok, S.I. o-Methoxy Substituents in Spiro-OMeTAD for Efficient Inorganic-Organic Hybrid Perovskite Solar Cells. J. Am. Chem. Soc. 2014, 136, 7837–7840. [Google Scholar] [CrossRef]
- Moulin, E.; Niess, F.; Maaloum, M.; Buhler, E.; Nyrkova, I.; Giuseppone, N. The Hierarchical Self-Assembly of Charge Nanocarriers: A Highly Cooperative Process Promoted by Visible Light. Angew. Chem. Int. Ed. 2010, 49, 6974–6978. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Niess, F.; Fuks, G.; Jouault, N.; Buhler, E.; Giuseppone, N. Light-triggered self-assembly of triarylamine-based nanospheres. Nanoscale 2012, 4, 6748–6751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.J.; Churches, Q.I.; Subbiah, J.; Gupta, A.; Ali, A.; Evans, R.A.; Holmes, A.B. Enhanced photovoltaic efficiency via light-triggered self-assembly. Chem. Commun. 2013, 49, 6552–6554. [Google Scholar] [CrossRef] [PubMed]
- Busseron, E.; Cid, J.J.; Wolf, A.; Du, G.; Moulin, E.; Fuks, G.; Maaloum, M.; Polavarapu, P.; Ruff, A.; Saur, A.K.; et al. Light-controlled morphologies of self-Assembled triarylamine-fullerene conjugates. ACS Nano 2015, 9, 2760–2772. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, W.Y.; Kim, H.; Lee, S.; Lee, H.C.; Lee, Y.S.; Seo, M.; Kim, S.Y. Induction and control of supramolecular chirality by light in self-assembled helical nanostructures. Nat. Commun. 2015, 6, 6959. [Google Scholar] [CrossRef]
- Moulin, É.; Busseron, E.; Domoto, Y.; Ellis, T.; Osypenko, A.; Maaloum, M.; Giuseppone, N. Self-assembly of benzene-tris(bis(p-benzyloxy)triphenylamine)carboxamide. Comptes Rendus Chim. 2016, 19, 117–122. [Google Scholar] [CrossRef]
- Amthor, S.; Noller, B.; Lambert, C. UV/Vis/NIR spectral properties of triarylamines and their corresponding radical cations. Chem. Phys. 2005, 316, 141–152. [Google Scholar] [CrossRef]
- Feringán, B.; Romero, P.; Serrano, J.L.; Folcia, C.L.; Etxebarria, J.; Ortega, J.; Termine, R.; Golemme, A.; Giménez, R.; Sierra, T. H-Bonded Donor–Acceptor Units Segregated in Coaxial Columnar Assemblies: Toward High Mobility Ambipolar Organic Semiconductors. J. Am. Chem. Soc. 2016, 138, 12511–12518. [Google Scholar] [CrossRef]
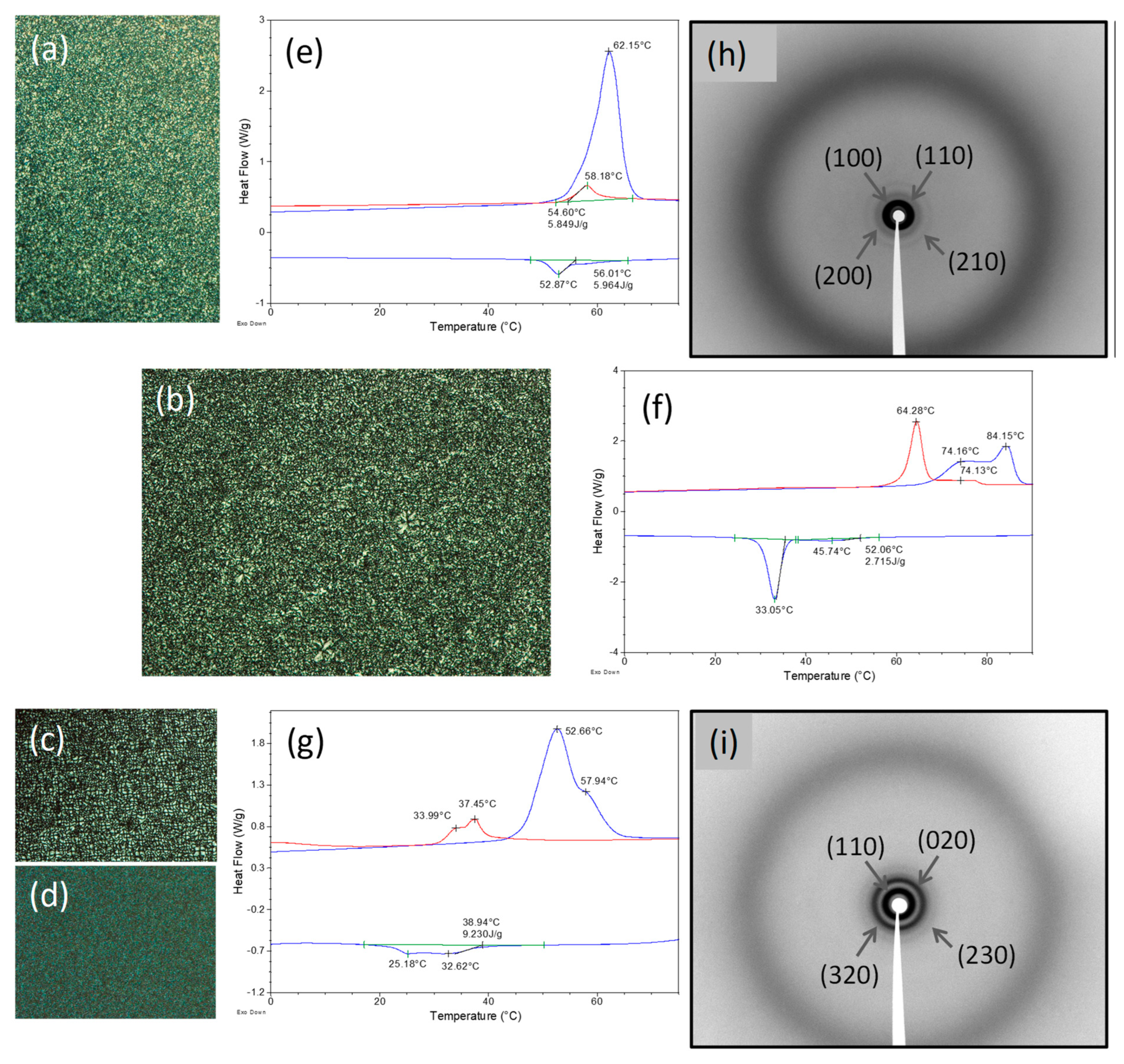

| Compound | DSC Cycle | Phase Transition 1 T (°C) (ΔH (kJ mol−1)) |
|---|---|---|
| A-TPA2 | 1st heating | Cr 62 (60.2) Iso |
| 1st cooling | Iso 53 (5.1) Colh | |
| 2nd heating | Colh 58 (5.0) Iso | |
| 2nd cooling | Iso 53 (5.1) Colh | |
| A-TPA4 | 1st heating | Cr 74 Cr’ 84 (86.8) 2 Iso |
| 1st cooling | Iso 45 (3.3) M 33 (41.8) Cr” | |
| 2nd heating | Cr” 64 Cr 74 (58.3) 2 Iso | |
| 2nd cooling | Iso 45 (3.3) M 33 (41.8) Cr” | |
| A-TPA6 | 1st heating | Cr 53 Cr’ 58 (108.7) Iso |
| 1st cooling | Iso 33 M 25 (14.6) 2 Colr | |
| 2nd heating | Colr 34 M 37 (16.2) 2 Iso | |
| 2nd cooling | Iso 33 M 25 (14.6) 2 Colr |
| Compound | Mesophase | Lattice Parameter (Å) | dobs (Å) | dcalc (Å) | Miller Index |
|---|---|---|---|---|---|
| A-TPA2 | Colh | a = 58.2 | 50.6 | 50.4 | 100 |
| 29.5 | 29.1 | 110 | |||
| 24.7 | 25.2 | 200 | |||
| 19.2 | 19 | 210 | |||
| 4.5 (diff) | - | - | |||
| A-TPA6 | Colr | a = 111.2 b = 52.8 | 47.7 | 47.7 | 110 |
| 26.4 | 26.4 | 20 | |||
| 20.9 | 21.5 | 320 | |||
| 16.9 | 16.8 | 230 | |||
| 4.5 (diff) | - | - |
| Compound | E1/2ox vs. Ag/AgCl (V) | E1/2ox vs. Fc/Fc⁺ 1 (V) | HOMO 2 (eV) |
|---|---|---|---|
| A-TPA2 | 0.51 | 0.09 | −4.89 |
| A-TPA4 | 0.46 | 0.04 | −4.84 |
| A-TPA6 | 0.60 | 0.18 | −4.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feringán, B.; Martínez-Bueno, A.; Sierra, T.; Giménez, R. Triphenylamine-Containing Benzoic Acids: Synthesis, Liquid Crystalline and Redox Properties. Molecules 2023, 28, 2887. https://doi.org/10.3390/molecules28072887
Feringán B, Martínez-Bueno A, Sierra T, Giménez R. Triphenylamine-Containing Benzoic Acids: Synthesis, Liquid Crystalline and Redox Properties. Molecules. 2023; 28(7):2887. https://doi.org/10.3390/molecules28072887
Chicago/Turabian StyleFeringán, Beatriz, Alejandro Martínez-Bueno, Teresa Sierra, and Raquel Giménez. 2023. "Triphenylamine-Containing Benzoic Acids: Synthesis, Liquid Crystalline and Redox Properties" Molecules 28, no. 7: 2887. https://doi.org/10.3390/molecules28072887
APA StyleFeringán, B., Martínez-Bueno, A., Sierra, T., & Giménez, R. (2023). Triphenylamine-Containing Benzoic Acids: Synthesis, Liquid Crystalline and Redox Properties. Molecules, 28(7), 2887. https://doi.org/10.3390/molecules28072887







