Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization
Abstract
:1. Introduction
2. Results
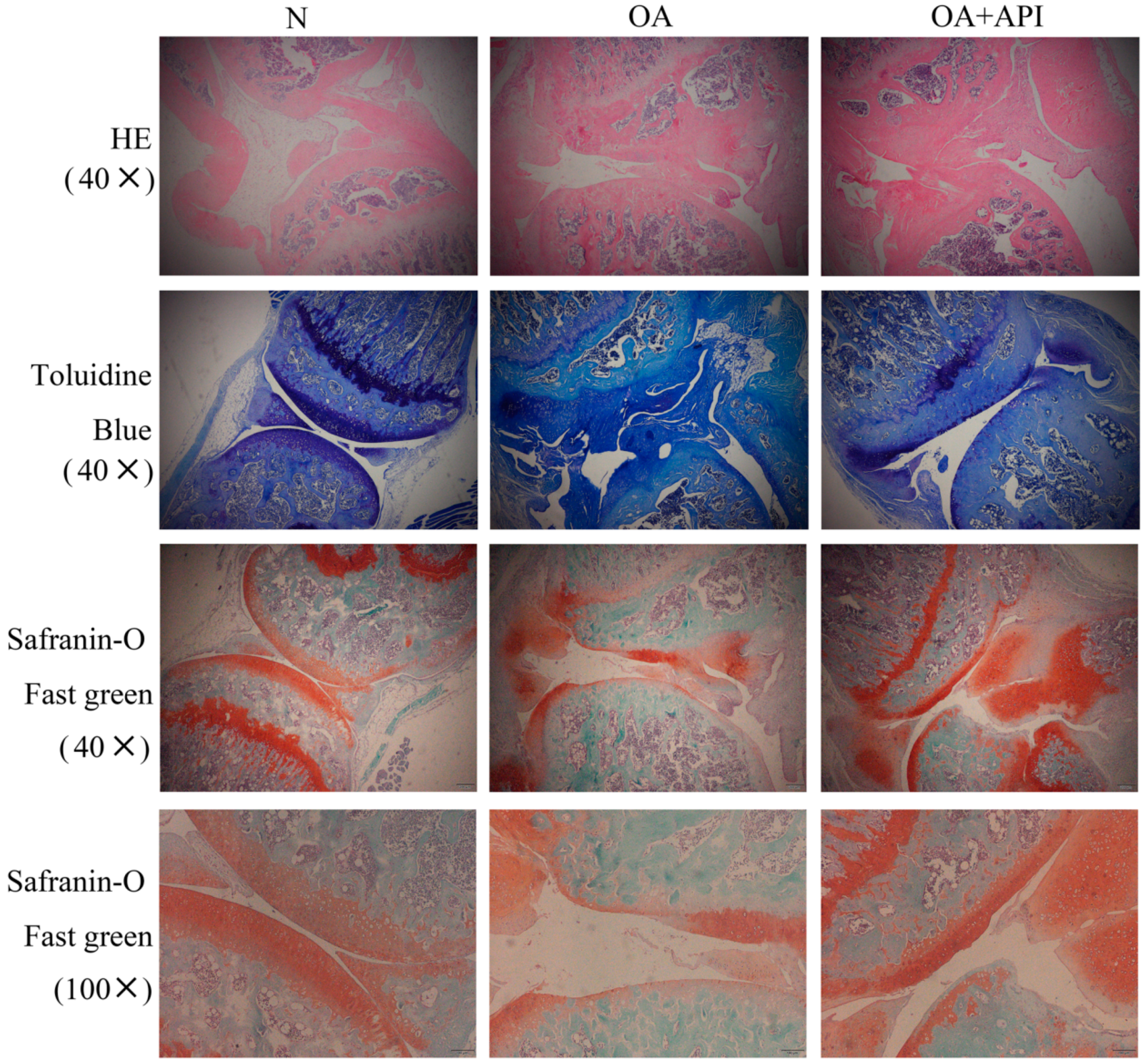
2.1. Protective Effect of Apigenin on Articular Cartilage in Modified Hulth Surgically Induced OA Mice
2.2. Apigenin Inhibits Macrophage M1 Polarization in a Macrophage–Chondrocyte Coculture System
2.3. Apigenin Promotes Macrophage M2 Polarization in a Macrophage–Chondrocyte Coculture System
2.4. Apigenin Regulates Macrophage Polarization through the TRPM7-mTOR Pathway
2.5. Apigenin Inhibits Chondrocyte Inflammation through the MAPK Pathway
2.6. Apigenin Inhibits the Apoptosis of Chondrocytes Induced by Macrophage Polarization
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture of Chondrocytes and Macrophages
4.3. Western Blot Analysis
4.4. qRT-PCR
4.5. Immunofluorescent Staining
4.6. Flow Cytometry
4.7. OA Protocol
4.8. Histological and Immunohistochemical Analyses
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2020, 268, 120555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Qu, N.; Zhang, B.; Xia, C. Chondroprotection of PPARα activation by WY14643 via autophagy involving Akt and ERK in LPS-treated mouse chondrocytes and osteoarthritis model. J. Cell. Mol. Med. 2019, 23, 2782–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, M.; Ivirico, J.L.E.; Kan, H.M.; Bordett, R.; Pandey, R.; Otsuka, T.; Nair, L.S.; Laurencin, C.T. Preparation and characterization of amnion hydrogel and its synergistic effect with adipose derived stem cells towards IL1β activated chondrocytes. Sci. Rep. 2020, 10, 18751. [Google Scholar] [CrossRef]
- Patel, J.; Ladani, A.; Sambamoorthi, N.; LeMasters, T.; Dwibedi, N.; Sambamoorthi, U. A Machine Learning Approach to Identify Predictors of Potentially Inappropriate Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Use in Older Adults with Osteoarthritis. Int. J. Environ. Res. Public Health 2020, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ming, J.; Li, Y.; Deng, M.; Chen, Q.; Ma, Y.; Chen, Z.; Zhang, Y.; Liu, S. Ligustilide attenuates nitric oxide-induced apoptosis in rat chondrocytes and cartilage degradation via inhibiting JNK and p38 MAPK pathways. J. Cell. Mol. Med. 2019, 23, 3357–3368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, X.; Yang, L.; Luo, M.; Han, L.; Lu, Y.; Shi, Q.; Wang, Y.; Liang, Q. Gentiopicroside (GENT) protects against sepsis induced by lipopolysaccharide (LPS) through the NF-κB signaling pathway. Ann. Transl. Med. 2019, 7, 731. [Google Scholar] [CrossRef]
- Nyiramana, M.M.; Cho, S.B.; Kim, E.J.; Kim, M.J.; Ryu, J.H.; Nam, H.J.; Kim, N.G.; Park, S.H.; Choi, Y.J.; Kang, S.S.; et al. Sea Hare Hydrolysate-Induced Reduction of Human Non-Small Cell Lung Cancer Cell Growth through Regulation of Macrophage Polarization and Non-Apoptotic Regulated Cell Death Pathways. Cancers 2020, 12, 726. [Google Scholar] [CrossRef] [Green Version]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Liu, T.; Yang, H.; He, F. Insights into the Role of Macrophage Polarization in the Pathogenesis of Osteoporosis. Oxidative Med. Cell. Longev. 2022, 2022, 2485959. [Google Scholar] [CrossRef]
- Sun, Y.; Zuo, Z.; Kuang, Y. An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization. Int. J. Mol. Sci. 2020, 21, 8513. [Google Scholar] [CrossRef]
- Feldt, J.; Donaubauer, A.J.; Welss, J.; Schneider, U.; Gaipl, U.S.; Paulsen, F. Anti-inflammatory effects of an autologous gold-based serum therapy in osteoarthritis patients. Sci. Rep. 2022, 12, 3560. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Lee, J.H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Estakhri, F.; Panjehshahin, M.R.; Tanideh, N.; Gheisari, R.; Mahmoodzadeh, A.; Azarpira, N.; Gholijani, N. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee 2020, 27, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Tantowi, N.; Mohamed, S.; Lau, S.F.; Hussin, P. Comparison of diclofenac with apigenin-glycosides rich Clinacanthus nutans extract for amending inflammation and catabolic protease regulations in osteoporotic-osteoarthritis rat model. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2020, 28, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 181–187. [Google Scholar] [CrossRef]
- Hong, S.; Dia, V.P.; Zhong, Q. Synergistic anti-inflammatory activity of apigenin and curcumin co-encapsulated in caseins assessed with lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 193 (Pt A), 702–712. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraden, C.A.; Huebner, J.L.; Hsueh, M.F.; Li, Y.J.; Kraus, V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther. 2019, 21, 146. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Qi, W.; Zhan, J.; Lin, Z.; Lin, J.; Xue, X.; Pan, X.; Zhou, Y. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J. Cell. Mol. Med. 2020, 24, 13046–13057. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J.; et al. Tangeretin suppresses osteoarthritis progression via the Nrf2/NF-κB and MAPK/NF-κB signaling pathways. Phytomed. Int. J. Phytother. Phytopharm. 2022, 98, 153928. [Google Scholar] [CrossRef]
- Luo, Z.; Dong, J.; Wu, J. Impact of Icariin and its derivatives on inflammatory diseases and relevant signaling pathways. Int. Immunopharmacol. 2022, 108, 108861. [Google Scholar] [CrossRef]
- Yamaura, K.; Nelson, A.L.; Nishimura, H.; Rutledge, J.C.; Ravuri, S.K.; Bahney, C.; Philippon, M.J.; Huard, J. The effects of fisetin on bone and cartilage: A systematic review. Pharmacol. Res. 2022, 185, 106504. [Google Scholar] [CrossRef]
- Schappe, M.S.; Szteyn, K.; Stremska, M.E.; Mendu, S.K.; Downs, T.K.; Seegren, P.V.; Mahoney, M.A.; Dixit, S.; Krupa, J.K.; Stipes, E.J.; et al. Chanzyme TRPM7 Mediates the Ca2+ Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation. Immunity 2018, 48, 59–74.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, H.; Pan, J.; Hu, Z.; Liu, L.; Liu, Y.; Yu, X.; Bai, X.; Cai, D.; Zhang, H. Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways. Arthritis Res. Ther. 2021, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Pellegrino, P.; Zannotti, A.; Delli Carpini, G.; Ciavattini, A.; Reis, F.M.; Ciarmela, P. Phytoprogestins: Unexplored Food Compounds with Potential Preventive and Therapeutic Effects in Female Diseases. Nutrients 2021, 13, 4326. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, J.; Pikula, S.; Suski, S.; Weremiejczyk, L.; Biesaga, M.; Strzelecka-Kiliszek, A. Apigenin Modulates AnxA6- and TNAP-Mediated Osteoblast Mineralization. Int. J. Mol. Sci. 2022, 23, 13179. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Chang, S.L.; Liu, S.C.; Achudhan, D.; Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Chang, J.W.; Fong, Y.C.; et al. Therapeutic Effects of Live Lactobacillus plantarum GKD7 in a Rat Model of Knee Osteoarthritis. Nutrients 2022, 14, 3170. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, S.; Gotterbarm, T.; Müller, T.; Baesig, A.M.; Gantz, S.; Dreher, T.; Kämmerer, P.W.; Frank, S.; Zeifang, F.; Moradi, B. The influence of bone marrow- and synovium-derived mesenchymal stromal cells from osteoarthritis patients on regulatory T cells in co-culture. Clin. Exp. Immunol. 2013, 173, 454–462. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.; Shao, X.; Wang, Z.; Du, Y.; Zhu, C.; Du, W.; Tang, D.; Ji, S. Prenylated phenolic compounds from licorice (Glycyrrhiza uralensis) and their anti-inflammatory activity against osteoarthritis. Food Funct. 2022, 13, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019, 9, 6300–6313. [Google Scholar] [CrossRef]
- Sun, A.R.; Panchal, S.K.; Friis, T.; Sekar, S.; Crawford, R.; Brown, L.; Xiao, Y.; Prasadam, I. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE 2017, 12, e0183693. [Google Scholar] [CrossRef] [Green Version]
- Dacoba, T.G.; Anfray, C.; Mainini, F.; Allavena, P.; Alonso, M.J.; Torres Andón, F.; Crecente-Campo, J. Arginine-Based Poly(I:C)-Loaded Nanocomplexes for the Polarization of Macrophages Toward M1-Antitumoral Effectors. Front. Immunol. 2020, 11, 1412. [Google Scholar] [CrossRef]
- Park, J.G.; Na, M.; Kim, M.G.; Park, S.H.; Lee, H.J.; Kim, D.K.; Kwak, C.; Kim, Y.S.; Chang, S.; Moon, K.C.; et al. Immune cell composition in normal human kidneys. Sci. Rep. 2020, 10, 15678. [Google Scholar] [CrossRef] [PubMed]








| Sequence (5′ to 3′) | |
|---|---|
| IL-1 | F: CTG CAC TAC AGG CTC CGA |
| R: GCC ACA GGT ATT TTG TCG TT | |
| IL-6 | F: TTA GCC ACT CCT TCT GTG ACT CC |
| R: ACC CCA ATT TCC AAT GCT CT | |
| IL-12a | F: GAC CTG TTT ACC ACT GGA ACT A |
| R: GAT CTG CTG ATG GTT GTG ATT C | |
| TNF-α | F: TCG TAT GAA ATG GCA AAT CG |
| R: GGT CCC AAC AAG GAG GAG | |
| MGL1 | F:TGC AAC AGC TGA GGA AGG ACT TG |
| R:AAC CAA TAG CAG CTG CCT TCA TGC | |
| MGL2 | F:GCA TGA AGG CAG CTG CTA TTG GTT |
| R:TAG GCC CAT CCA GCT AAG CAC ATT | |
| ARG-1 | F:CAT ATC TGC CAA AGA CAT CGT G |
| R:GAC ATC AAA GCT CAG GTG AAT C | |
| IL-10 | F:AGG CGC TGT CAT CGA TTT CT |
| R:TGG AGT CCA GCA GAC TCA AT | |
| MMP3 | F:GGA GGC AGC AGA GAA CCT AC |
| R:TCC AAC CCG AGG AAC TTC TG | |
| MMP13 | F:CAG TGC TGC GGT TCA CTT TG |
| R:TCA TCA TAA CTC CAC ACG TGG TT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Du, W.; Che, W.; Wang, L.; Zhao, L. Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization. Molecules 2023, 28, 2915. https://doi.org/10.3390/molecules28072915
Ji X, Du W, Che W, Wang L, Zhao L. Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization. Molecules. 2023; 28(7):2915. https://doi.org/10.3390/molecules28072915
Chicago/Turabian StyleJi, Xueyan, Wei Du, Wenqing Che, Liping Wang, and Lu Zhao. 2023. "Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization" Molecules 28, no. 7: 2915. https://doi.org/10.3390/molecules28072915
APA StyleJi, X., Du, W., Che, W., Wang, L., & Zhao, L. (2023). Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization. Molecules, 28(7), 2915. https://doi.org/10.3390/molecules28072915






