Partial Oxidation to Extend the Lifetime of Nanoporous Carbon in an Ultracapacitor with Li2SO4 Electrolyte
Abstract
1. Introduction
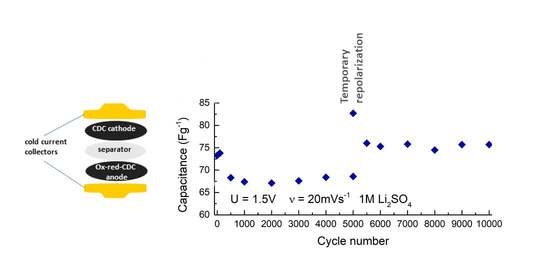
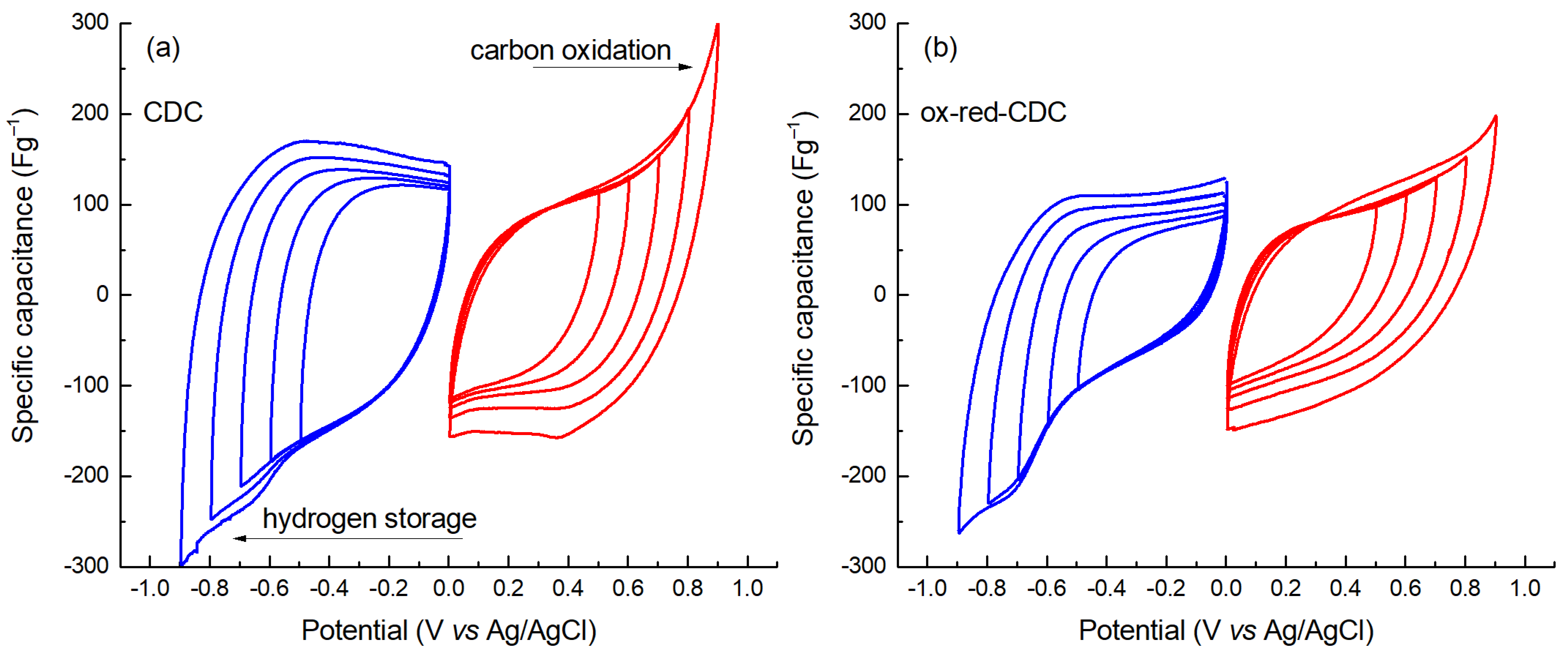
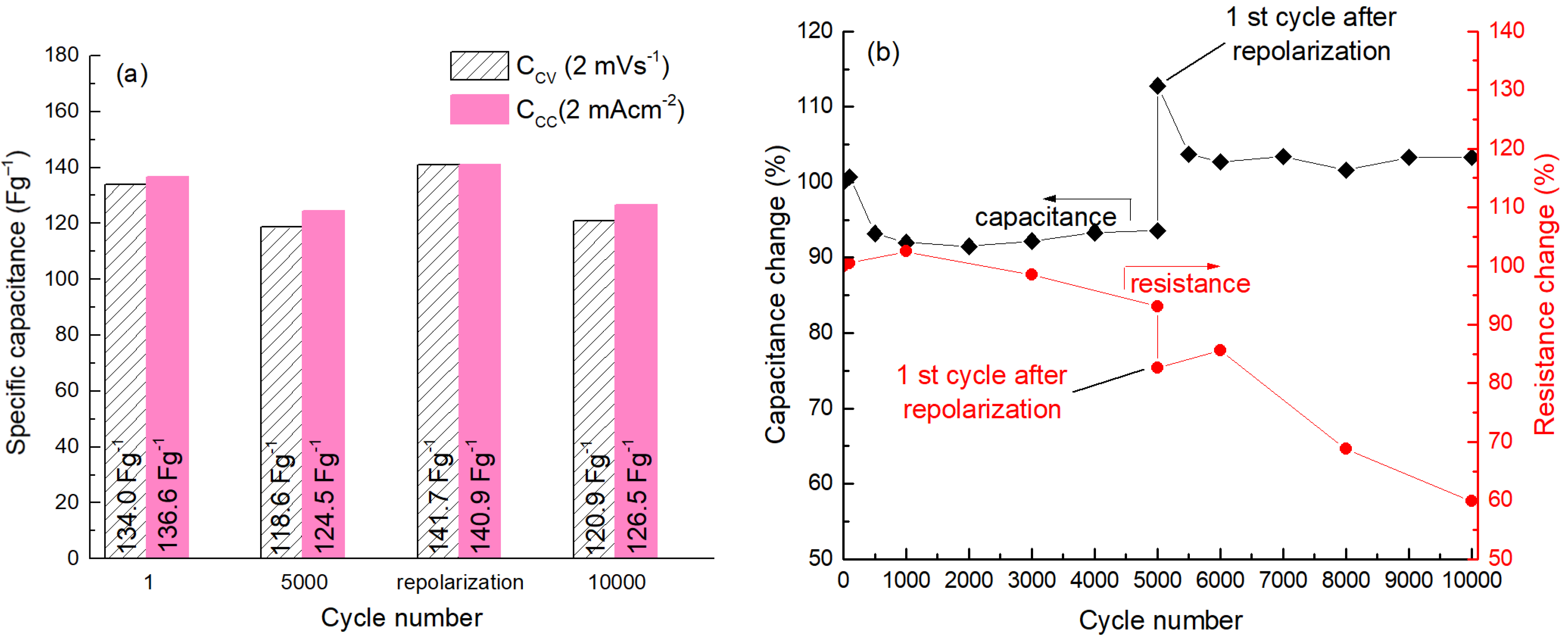
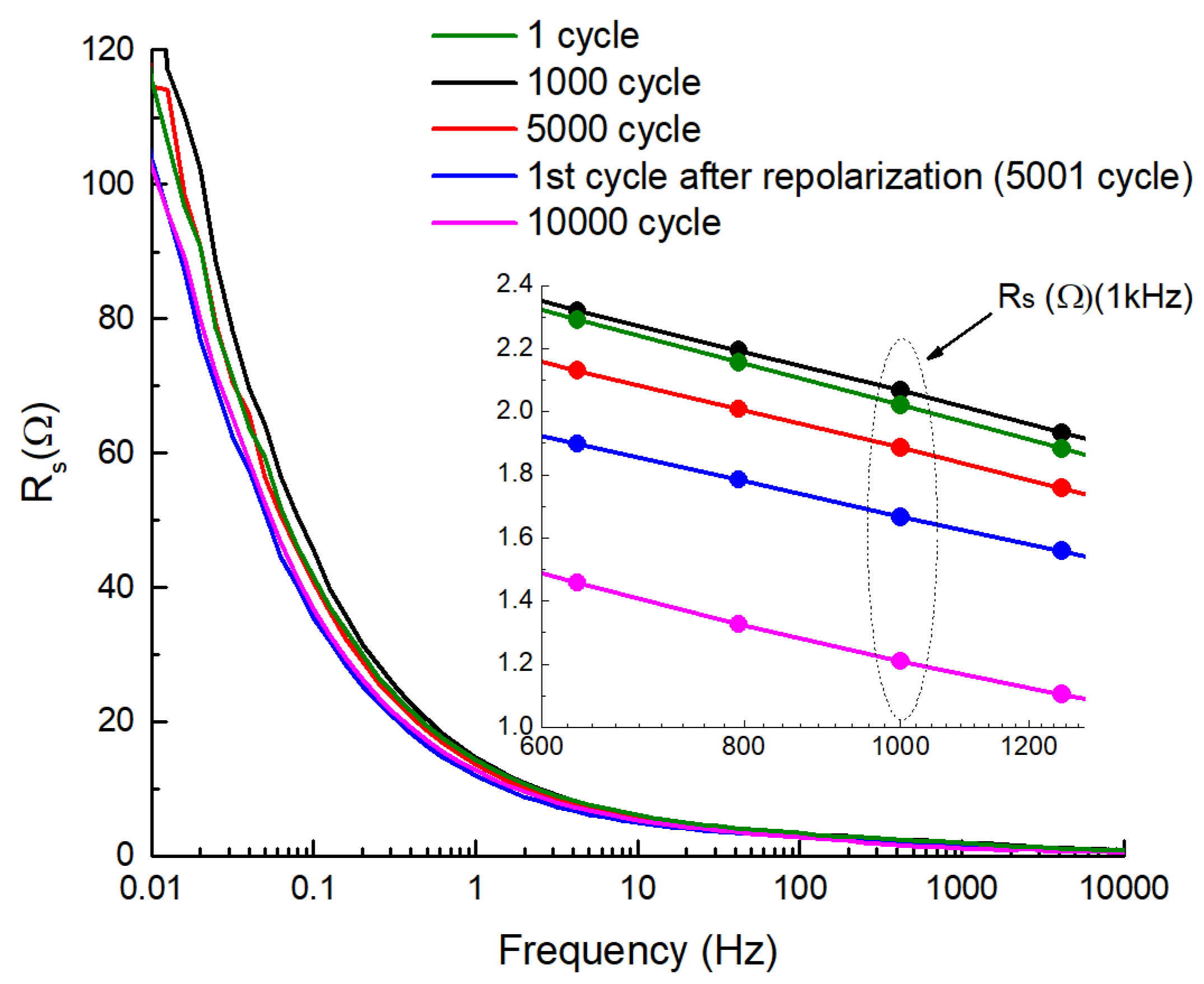
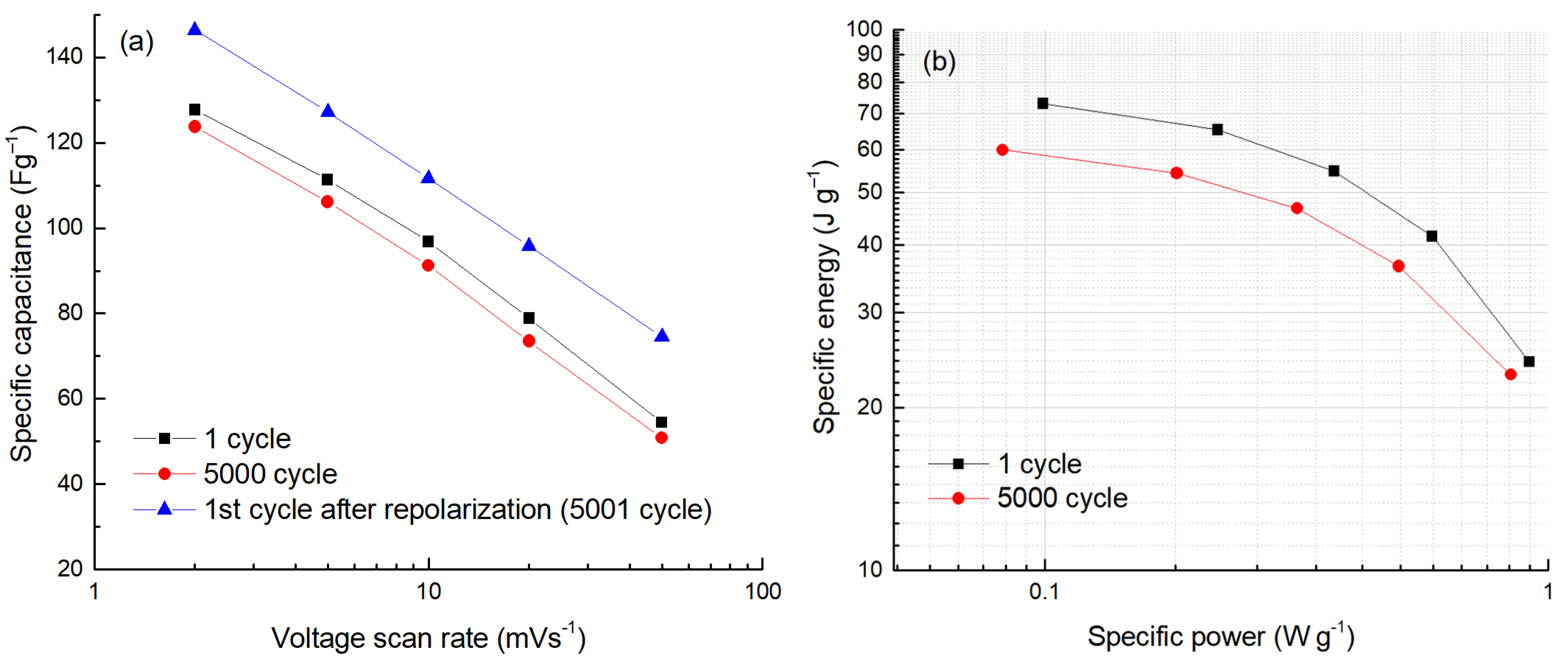
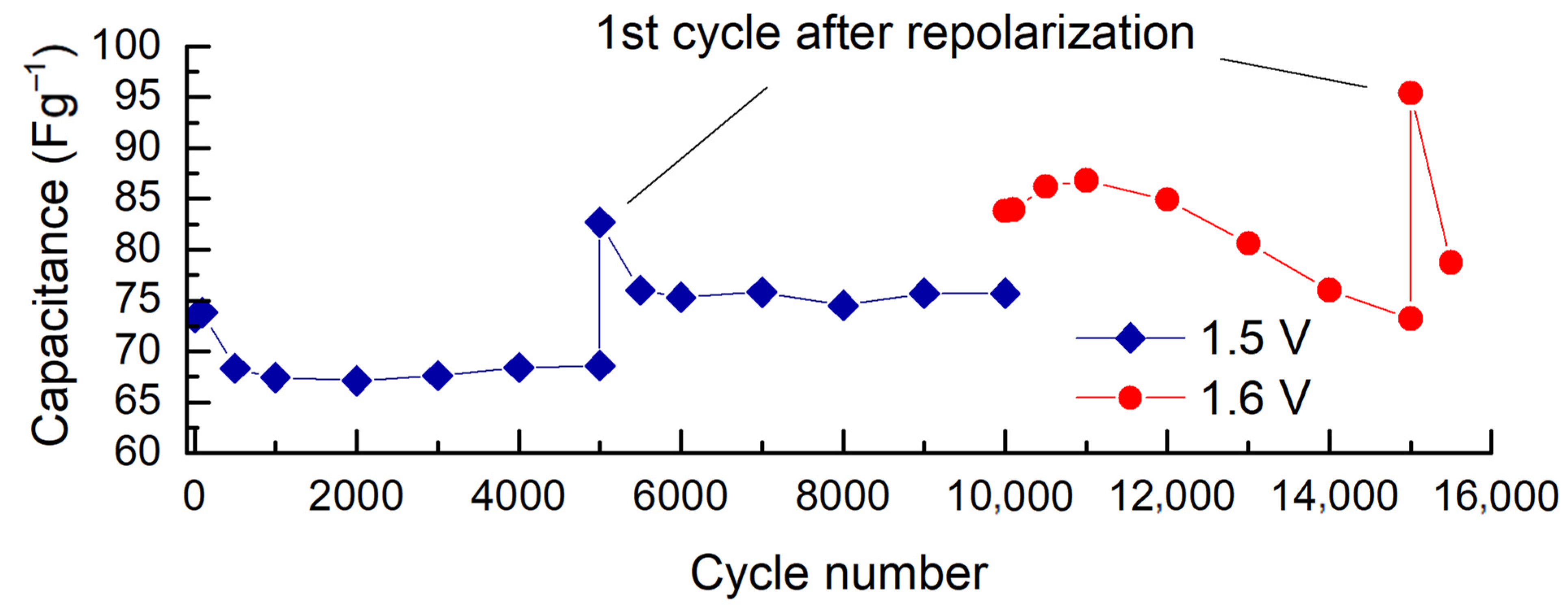
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis, Modification, and Characterizations of CDC Material
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-Derived Carbons—From Porous Networks to Nanotubes and Graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Dash, R.; Chmiola, J.; Yushin, G.; Gogotsi, Y.; Laudisio, G.; Singer, J.; Fischer, J.; Kucheyev, S. Titanium carbide derived nanoporous carbon for energy-related applications. Carbon 2006, 44, 2489–2497. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Dash, R.; Gogotsi, Y. Effect of pore size and surface area of carbide derived carbons on specific capacitance. J. Power Sources 2006, 158, 765–772. [Google Scholar] [CrossRef]
- Leis, J.; Arulepp, M.; Kuura, A.; Lätt, M.; Lust, E. Electrical double-layer characteristics of novel carbide-derived carbon materials. Carbon 2006, 44, 2122–2129. [Google Scholar] [CrossRef]
- Leis, J.; Arulepp, M.; Käärik, M.; Perkson, A. The effect of Mo2C derived carbon pore size on the electrical double-layer characteristics in propylene carbonate-based electrolyte. Carbon 2010, 48, 4001–4008. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Käärik, M.; Maran, U.; Leis, J. Characterization and prediction of double-layer capacitance of nanoporous carbon materials using the Quantitative nano-Structure-Property Relationship approach based on experimentally determined porosity descriptors. Carbon 2020, 158, 494–504. [Google Scholar] [CrossRef]
- Portet, C.; Lillo-Ródenas, M.Á.; Linares-Solano, A.; Gogotsi, Y. Capacitance of KOH activated carbide-derived carbons. Phys. Chem. Chem. Phys. 2009, 11, 4943. [Google Scholar] [CrossRef]
- Kierzek, K.; Frackowiak, E.; Lota, G.; Gryglewicz, G.; Machnikowski, J. Electrochemical capacitors based on highly porous carbons prepared by KOH activation. Electrochim. Acta 2004, 49, 515–523. [Google Scholar] [CrossRef]
- Osswald, S.; Portet, C.; Gogotsi, Y.; Laudisio, G.; Singer, J.P.; Fischer, J.E.; Sokolov, V.V.; Kukushkina, J.A.; Kravchik, A.E. Porosity control in nanoporous carbide-derived carbon by oxidation in air and carbon dioxide. J. Solid State Chem. 2009, 182, 1733–1741. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Kook, M.; Mäeorg, U.; Kozlova, J.; Sammelselg, V.; Perkson, A.; Leis, J. Characterisation of steam-treated nanoporous carbide-derived carbon of TiC origin: Structure and enhanced electrochemical performance. J. Porous Mater. 2017, 25, 1057–1070. [Google Scholar] [CrossRef]
- Tee, E.; Tallo, I.; Lust, E.; Jänes, A.; Thomberg, T. Electrical Double Layer Capacitors Based on Steam and CO2-Steam Co-Activated Carbon Electrodes and Ionic Liquid Electrolyte. J. Electrochem. Soc. 2019, 166, A1558–A1567. [Google Scholar] [CrossRef]
- Käärik, M.; Maran, U.; Arulepp, M.; Perkson, A.; Leis, J. Quantitative Nano-Structure–Property Relationships for the Nanoporous Carbon: Predicting the Performance of Energy Storage Materials. ACS Appl. Energy Mater. 2018, 1, 4016–4024. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Béguin, F.; Frąckowiak, E. (Eds.) Supercapacitors: Materials, Systems, and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science+Business Media: New York, NY, USA, 1999. [Google Scholar]
- Fic, K.; He, M.; Berg, E.J.; Novák, P.; Frackowiak, E. Comparative operando study of degradation mechanisms in carbon-based electrochemical capacitors with Li2SO4 and LiNO3 electrolytes. Carbon 2017, 120, 281–293. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Env. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- He, M.; Fic, K.; Fra, E.; Novák, P.; Berg, E.J. Ageing phenomena in high-voltage aqueous supercapacitors investigated by in situ gas analysis. Energy Env. Sci. 2016, 9, 623–633. [Google Scholar] [CrossRef]
- Piwek, J.; Platek, A.; Frackowiak, E.; Fic, K. Mechanisms of the performance fading of carbon-based electrochemical capacitors operating in a LiNO3 electrolyte. J. Power Sources 2019, 438, 227029. [Google Scholar] [CrossRef]
- Gao, Q.; Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte. Energy Environ. Sci. 2012, 5, 9611. [Google Scholar] [CrossRef]
- Fic, K.; Płatek, A.; Piwek, J.; Menzel, J.; Ślesiński, A.; Bujewska, P.; Galek, P.; Frąckowiak, E. Revisited insights into charge storage mechanisms in electrochemical capacitors with Li2SO4-based electrolyte. Energy Storage Mater. 2019, 22, 1–14. [Google Scholar] [CrossRef]
- Piwek, J.; Platek-Mielczarek, A.; Frackowiak, E.; Fic, K. Enhancing capacitor lifetime by alternate constant polarization. J. Power Sources 2021, 506, 230131. [Google Scholar] [CrossRef]
- Eskusson, J.; Jänes, A.; Kikas, A.; Matisen, L.; Lust, E. Physical and electrochemical characteristics of supercapacitors based on carbide derived carbon electrodes in aqueous electrolytes. J. Power Sources 2011, 196, 4109–4116. [Google Scholar] [CrossRef]
- Malmberg, S.; Arulepp, M.; Laanemets, K.; Käärik, M.; Laheäär, A.; Tarasova, E.; Vassiljeva, V.; Krasnou, I.; Krumme, A. The Performance of Fibrous CDC Electrodes in Aqueous and Non-Aqueous Electrolytes. J. Carbon Res. 2021, 7, 46. [Google Scholar] [CrossRef]
- Käärik, M.; Arulepp, M.; Kozlova, J.; Aruväli, J.; Mäeorg, U.; Kikas, A.; Kisand, V.; Tamm, A.; Leis, J. Effect of partial oxidation and repolarization of TiC-derived nanoporous carbon electrodes on supercapacitor performance using a pH-neutral aqueous electrolyte. J. Solid State Electrochem. 2022, 26, 2365–2378. [Google Scholar] [CrossRef]
- Qu, Q.T.; Wang, B.; Yang, L.C.; Shi, Y.; Tian, S.; Wu, Y.P. Study on electrochemical performance of activated carbon in aqueous Li2SO4, Na2SO4 and K2SO4 electrolytes. Electrochem. Commun. 2008, 10, 1652–1655. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Abbas, Q.; Ogwu, A.; Hall, P.; Goldin, M.; Mirzaeian, M.; Jirandehi, H.F. Electrode and electrolyte materials for electrochemical capacitors. Int. J. Hydrogen Energy 2017, 42, 25565–25587. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Liu, X.; Tong, Y.; Wu, Y.; Zheng, J.; Sun, Y.; Niu, L.; Li, H. Synergistically enhanced electrochemical performance using nitrogen, phosphorus and sulfur tri-doped hollow carbon for advanced potassium ion storage device. Chem. Eng. J. 2022, 431, 133986. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Liu, Z.; Yin, Y.; Sun, Y.; Li, H. Fabricating multi-porous carbon anode with remarkable initial coulombic efficiency and enhanced rate capability for sodium-ion batteries. Chin. Chem. Lett. 2023, 34, 107443. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Tong, Y.; Sun, Y.; Li, H. Dual-Carbon confinement strategy of antimony anode material enabling advanced potassium ion storage. J. Colloid Interface Sci. 2022, 622, 738–747. [Google Scholar] [CrossRef]
- Fischer, J.; Thümmler, K.; Fischer, S.; Martinez, I.G.G.; Oswald, S.; Mikhailova, D. Activated Carbon Derived from Cellulose and Cellulose Acetate Microspheres as Electrode Materials for Symmetric Supercapacitors in Aqueous Electrolytes. Energy Fuels 2021, 35, 12653–12665. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Huang, Z.; Zhang, H. Effects of oxygen-containing functional groups on the supercapacitor performance of incompletely reduced graphene oxides. Int. J. Hydrogen Energy 2017, 42, 7186–7194. [Google Scholar] [CrossRef]
- Oschatz, M.; Boukhalfa, S.; Nickel, W.; Hofmann, J.P.; Fischer, C.; Yushin, G.; Kaskel, S. Carbide-derived carbon aerogels with tunable pore structure as versatile electrode material in high power supercapacitors. Carbon 2017, 113, 283–291. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Leis, J.; Arulepp, M.; Lätt, M.; Kuura, H. A method of Making the Porous Carbon Material and Porous Carbon Materials Produced by the Method. U.S.patent US7,803,345, 28 September 2010. [Google Scholar]
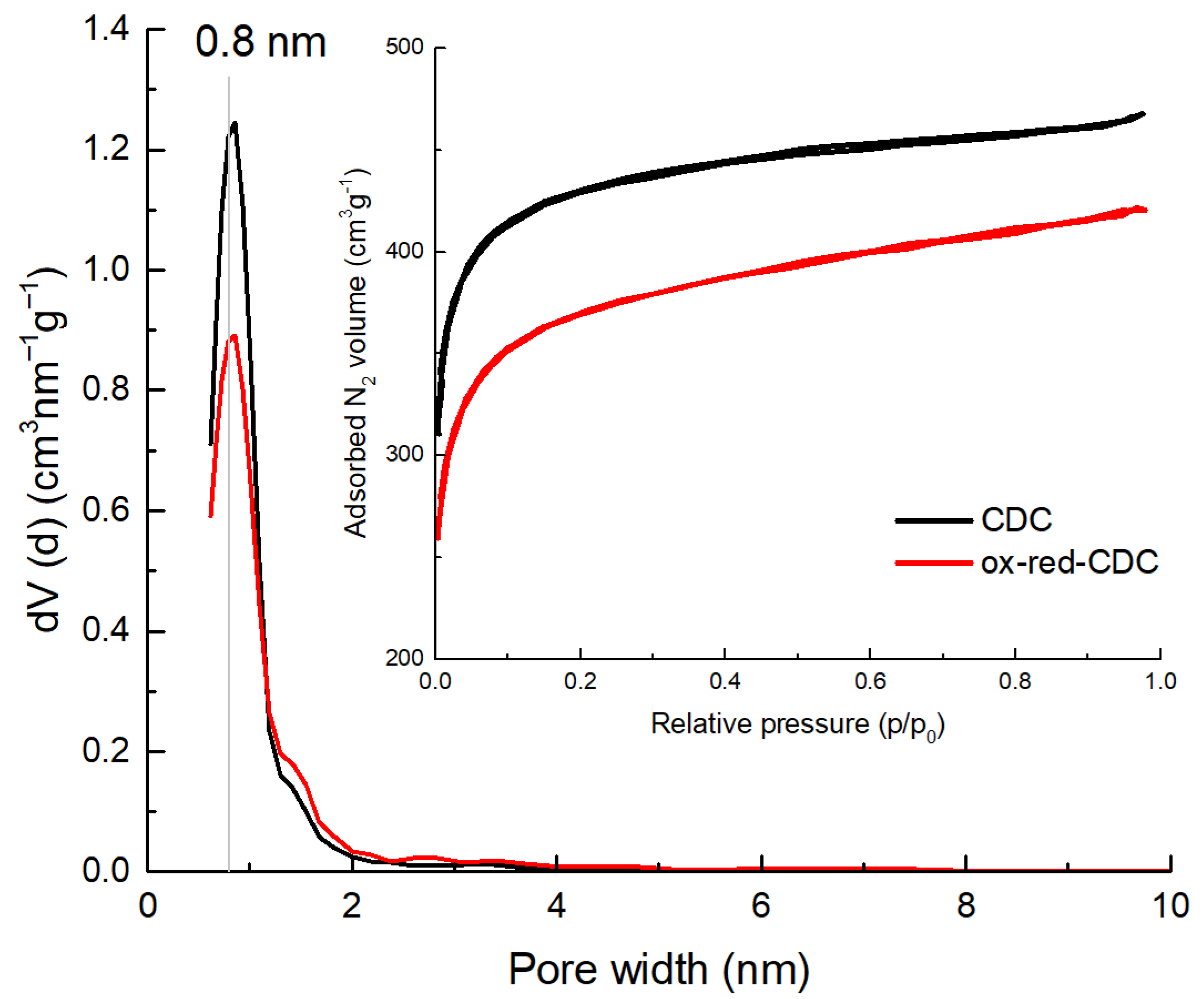


| Carbon | SBET | Sdft | Vt | Vµ |
|---|---|---|---|---|
| Sample | (m2 g−1) | (m2 g−1) | (cm3 g−1) | (cm3 g−1) |
| CDC | 1499 | 1511 | 0.73 | 0.63 |
| ox-red-CDC | 1300 | 1285 | 0.65 | 0.54 |
| Carbon Sample | (F cm−3) | (F cm−3) | (F cm−3) | (F cm−3) |
|---|---|---|---|---|
| CDC | 150 | 105 | 97 | 68 |
| Ox-red-CDC | 132 | 108 | 93 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Käärik, M.; Arulepp, M.; Leis, J. Partial Oxidation to Extend the Lifetime of Nanoporous Carbon in an Ultracapacitor with Li2SO4 Electrolyte. Molecules 2023, 28, 2944. https://doi.org/10.3390/molecules28072944
Käärik M, Arulepp M, Leis J. Partial Oxidation to Extend the Lifetime of Nanoporous Carbon in an Ultracapacitor with Li2SO4 Electrolyte. Molecules. 2023; 28(7):2944. https://doi.org/10.3390/molecules28072944
Chicago/Turabian StyleKäärik, Maike, Mati Arulepp, and Jaan Leis. 2023. "Partial Oxidation to Extend the Lifetime of Nanoporous Carbon in an Ultracapacitor with Li2SO4 Electrolyte" Molecules 28, no. 7: 2944. https://doi.org/10.3390/molecules28072944
APA StyleKäärik, M., Arulepp, M., & Leis, J. (2023). Partial Oxidation to Extend the Lifetime of Nanoporous Carbon in an Ultracapacitor with Li2SO4 Electrolyte. Molecules, 28(7), 2944. https://doi.org/10.3390/molecules28072944