Preclinical Evaluation of [155/161Tb]Tb-Crown-TATE—A Novel SPECT Imaging Theranostic Agent Targeting Neuroendocrine Tumours
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Crown-TATE
2.2. Radiochemistry
2.2.1. Radionuclide Production
2.2.2. Radiolabelling Studies
2.3. Preclinical Studies
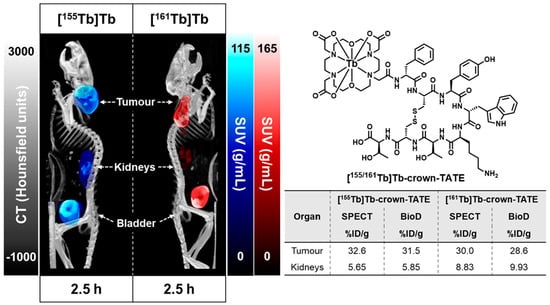
2.3.1. SPECT/CT Studies
2.3.2. Biodistribution Studies
3. Materials & Methods
3.1. General
3.2. Synthesis of Crown-TATE
3.3. Radionuclide Production
3.3.1. [155Tb]Tb3+
3.3.2. [161Tb]Tb3+
3.4. Radiolabelling Studies
3.5. Radio-HPLC
3.6. Human Serum Stability
3.7. Preclinical Studies
3.7.1. Tumour Inoculation
3.7.2. Radiotracer Preparation
3.7.3. SPECT/CT Studies
3.7.4. Biodistribution Studies
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| %ID/g | % injected dose per gram tissue |
| CT | Computed tomography |
| GEP | Gastroenteropancreatic |
| HPIC | High pressure ion chromatography |
| HPLC | High performance liquid chromatography |
| ISAC | Isotope separator and accelerator |
| ISOL | Isotope separation on-line |
| MAE | Meitner–Auger electron |
| NETs | Neuroendocrine tumours |
| OS | Overall survival |
| PET | Positron emission tomography |
| PFS | Progression free survival |
| RCP | Radiochemical purity |
| RCY | Radiochemical yield |
| PRRT | Peptide receptor radionuclide therapy |
| ROIs | Regions of interest |
| SPE | Solid-phase extraction |
| SPECT | Single photon emission computed tomography |
| SSTR2 | Somatostatin receptor-2 |
| SUV | Standardised uptake value |
| TAT | Targeted alpha therapy |
| UHS | Ultra-high sensitivity |
| VECTor | Versatile emission computed tomography |
References
- Ahmadi Bidakhvidi, N.; Goffin, K.; Dekervel, J.; Baete, K.; Nackaerts, K.; Clement, P.; van Cutsem, E.; Verslype, C.; Deroose, C.M. Peptide Receptor Radionuclide Therapy Targeting the Somatostatin Receptor: Basic Principles, Clinical Applications and Optimization Strategies. Cancers 2022, 14, 129. [Google Scholar] [CrossRef]
- Puliani, G.; Chiefari, A.; Mormando, M.; Bianchini, M.; Lauretta, R.; Appetecchia, M. New Insights in PRRT: Lessons From 2021. Front. Endocrinol. 2022, 13, 861434. [Google Scholar] [CrossRef] [PubMed]
- Camus, B.; Cottereau, A.-S.; Palmieri, L.-J.; Dermine, S.; Tenenbaum, F.; Brezault, C.; Coriat, R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J. Clin. Med. 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.E.; Zhernosekov, K. The Evolution of PRRT for the Treatment of Neuroendocrine Tumors; What Comes Next? Front. Endocrinol. 2022, 13, 941832. [Google Scholar] [CrossRef]
- Pencharz, D.; Walker, M.; Yalchin, M.; Quigley, A.M.; Caplin, M.; Toumpanakis, C.; Navalkissoor, S. Early Efficacy of and Toxicity from Lutetium-177-DOTATATE Treatment in Patients with Progressive Metastatic NET. Nucl. Med. Commun. 2017, 38, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Minczeles, N.S.; de Herder, W.W.; Feelders, R.A.; Verburg, F.A.; Hofland, J.; Brabander, T. Long-Term Outcomes of Submaximal Activities of Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients. J. Nucl. Med. 2023, 64, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.E.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. Final Overall Survival in the Phase 3 NETTER-1 Study of Lutetium-177-DOTATATE in Patients with Midgut Neuroendocrine Tumors. J. Clin. Oncol. 2021, 39 (Suppl. S15), 4112. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and Safety of 225Ac-DOTATATE Targeted Alpha Therapy in Metastatic Paragangliomas: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening Horizons with 225Ac-DOTATATE Targeted Alpha Therapy for Gastroenteropancreatic Neuroendocrine Tumour Patients Stable or Refractory to 177Lu-DOTATATE PRRT: First Clinical Experience on the Efficacy and Safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; van der Meulen, N.P. Scandium and Terbium Radionuclides for Radiotheranostics: Current State of Development towards Clinical Application. Br. J. Radiol. 2018, 91, 20180074. [Google Scholar] [CrossRef]
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; van der Walt, N.T.; Türler, A.; Schibli, R. A Unique Matched Quadruplet of Terbium Radioisotopes for PET and SPECT and for α- and β−-Radionuclide Therapy: An In Vivo Proof-of-Concept Study with a New Receptor-Targeted Folate Derivative. J. Nucl. Med. 2012, 53, 1951–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.; Singh, A.; Umbricht, C.A.; Kulkarni, H.R.; Johnston, K.; Benešová, M.; Senftleben, S.; Müller, D.; Vermeulen, C.; Schibli, R.; et al. Preclinical Investigations and First-in-Human Application of 152Tb-PSMA-617 for PET/CT Imaging of Prostate Cancer. EJNMMI Res. 2019, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Borgna, F.; Haller, S.; Rodriguez, J.M.M.; Ginj, M.; Grundler, P.V.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P.; Müller, C. Combination of Terbium-161 with Somatostatin Receptor Antagonists—A Potential Paradigm Shift for the Treatment of Neuroendocrine Neoplasms. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Grünberg, J.; Lindenblatt, D.; Dorrer, H.; Cohrs, S.; Zhernosekov, K.; Köster, U.; Türler, A.; Fischer, E.; Schibli, R. Anti-L1CAM Radioimmunotherapy Is More Effective with the Radiolanthanide Terbium-161 Compared to Lutetium-177 in an Ovarian Cancer Model. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, C.; Yuan, Z.; Rodriguez-Rodriguez, C.; Robertson, A.; Radchenko, V.; Perron, R.; Gendron, D.; Causey, P.; Gao, F.; et al. Synthesis and Evaluation of a Macrocyclic Actinium-225 Chelator, Quality Control and In Vivo Evaluation of 225Ac-crown-αMSH Peptide. Chem.—Eur. J. 2020, 26, 11435–11440. [Google Scholar] [CrossRef]
- Yang, H.; Gao, F.; Yuan, Z.; Rodríguez-Rodríguez, C.; Merkens, H.; Robertson, A.; Radchenko, V.; Causey, P.; Bénard, F.; Schaffer, P. A Novel Actinium Bifunctional Chelator Crown and Biodistribution of Ac-225-Crown-TATE. J. Nucl. Med. 2020, 61 (Suppl. S1), 1235. [Google Scholar]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC Receptor-Targeted Alpha-Radionuclide Therapy Induces Remission in Neuroendocrine Tumours Refractory to Beta Radiation: A First-in-Human Experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Baum, R.P.; Kluge, A.W.; Kulkarni, H.; Schorr-Neufing, U.; Niepsch, K.; Bitterlich, N.; van Echteld, C.J.A. [177Lu-DOTA]0-D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) for Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics 2016, 6, 501–510. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Kulkarni, H.R.; Bernhardt, P.; Rydén, T.; Schuchardt, C.; Gracheva, N.; Grundler, P.V.; Köster, U.; Müller, D.; et al. First-in-Human Application of Terbium-161: A Feasibility Study Using 161Tb-DOTATOC. J. Nucl. Med. 2021, 62, 1391–1397. [Google Scholar] [CrossRef]
- Laznicek, M.; Laznickova, A.; Maecke, H.R. Receptor Affinity and Preclinical Biodistribution of Radiolabeled Somatostatin Analogs. Anticancer Res. 2012, 32, 761–766. [Google Scholar]
- Petrou, C.; Magafa, V.; Nikolopoulou, A.; Pairas, G.; Nock, B.; Maina, T.; Cordopatis, P. Synthesis and Sst2 Binding Profiles of New (Tyr3)Octreotate Analogs. J. Pept. Sci. 2008, 14, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Schmitt, J.S.; Chen, J.; Waser, B.; Reubi, J.C.; de Jong, M.; Schulz, S.; Maecke, H.R. Design, Synthesis, and Biological Evaluation of Somatostatin-Based Radiopeptides. Chem. Biol. 2006, 13, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albericio, F.; Hammer, R.P.; García-Echeverría, C.; Molins, M.A.; Chang, J.L.; Munson, M.C.; Pons, M.; Giralt, E.; Barany, G. Cyclization of Disulfide-Containing Peptides in Solid-Phase Synthesis. Int. J. Pept. Protein Res. 2009, 37, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Otaka, A.; Funakoshi, S.; Bessho, K.; Watanabe, T.; Akaji, K.; Yajima, H. Studies on Peptides. CLI. Syntheses of Cystine-Peptides by Oxidation of s-Protected Cysteine-Peptides with Thallium(III) Trifluoroacetate. Chem. Pharm. Bull. 1987, 35, 2339–2347. [Google Scholar] [CrossRef] [Green Version]
- Fiaccabrino, D.E.; Kunz, P.; Radchenko, V. Potential for Production of Medical Radionuclides with On-Line Isotope Separation at the ISAC Facility at TRIUMF and Particular Discussion of the Examples of 165Er and 155Tb. Nucl. Med. Biol. 2021, 94–95, 81–91. [Google Scholar] [CrossRef]
- Cassells, I.; Ahenkorah, S.; Burgoyne, A.R.; van de Voorde, M.; Deroose, C.M.; Cardinaels, T.; Bormans, G.; Ooms, M.; Cleeren, F. Radiolabeling of Human Serum Albumin with Terbium-161 Using Mild Conditions and Evaluation of in Vivo Stability. Front. Med. 2021, 8, 675122. [Google Scholar] [CrossRef]
- McNeil, S.W.; van de Voorde, M.; Zhang, C.; Ooms, M.; Bénard, F.; Radchenko, V.; Yang, H. A Simple and Automated Method for 161Tb Purification and ICP-MS Analysis of 161Tb. EJNMMI Radiopharm. Chem. 2022, 7, 31. [Google Scholar] [CrossRef]
- Rylova, S.N.; Stoykow, C.; Del Pozzo, L.; Abiraj, K.; Tamma, M.L.; Kiefer, Y.; Fani, M.; Maecke, H.R. The Somatostatin Receptor 2 Antagonist 64Cu-NODAGA-JR11 Outperforms 64Cu-DOTA-TATE in a Mouse Xenograft Model. PLoS ONE 2018, 13, e0195802. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, E.; Lau, J.; Zhang, Z.; Uribe, C.F.; Kuo, H.-T.; Zhang, C.; Zeisler, J.; Colpo, N.; Lin, K.-S.; Bénard, F. Effects of Adding an Albumin Binder Chain on [177Lu]Lu-DOTATATE. Nucl. Med. Biol. 2018, 66, 10–17. [Google Scholar] [CrossRef]
- Loening, A.M.; Gambhir, S.S. AMIDE: A Free Software Tool for Multimodality Medical Image Analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef]
- Bricault, P.G.; Ames, F.; Dombsky, M.; Kunz, P.; Lassen, J. Rare Isotope Beams at ISAC—Target & Ion Source Systems. Hyperfine Interact. 2014, 225, 25–49. [Google Scholar] [CrossRef]
- Minor, G.; Kapalka, J.; Fisher, C.; Paley, W.; Chen, K.; Kinakin, M.; Earle, I.; Moss, B.; Bricault, P.; Gottberg, A. Remote Handling Systems for the ISAC and ARIEL High-Power Fission and Spallation ISOL Target Facilities at TRIUMF. Nucl. Eng. Technol. 2021, 53, 1378–1389. [Google Scholar] [CrossRef]
- Ivashchenko, O.; van der Have, F.; Goorden, M.C.; Ramakers, R.M.; Beekman, F.J. Ultra-High-Sensitivity Submillimeter Mouse SPECT. J. Nucl. Med. 2015, 56, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Goorden, M.C.; van der Have, F.; Kreuger, R.; Ramakers, R.M.; Vastenhouw, B.; Burbach, J.P.H.; Booij, J.; Molthoff, C.F.M.; Beekman, F.J. VECTor: A Preclinical Imaging System for Simultaneous Submillimeter SPECT and PET. J. Nucl. Med. 2013, 54, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, B.; Morris, T. Physiological Parameters in Laboratory Animals and Humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Foster, H.L.; Small, J.D.; Fox, J.G. The Mouse in Biomedical Research; Academic Press: New York, NY, USA, 1983. [Google Scholar]






| Time (h) | Tumour | Kidneys + Adrenals | ||||
|---|---|---|---|---|---|---|
| SPECT | BioD | SPECT | BioD | |||
| SUVmean | %ID/g | %ID/g | SUVmean | %ID/g | %ID/g | |
| [155Tb]Tb-crown-TATE | ||||||
| 0 | 0 | 0 | - | 0 | 0 | - |
| 0.17 | 5.19 | 18.9 | - | 3.69 | 13.4 | - |
| 0.37 | 6.63 | 24.1 | - | 2.1 | 7.64 | - |
| 0.55 | 7.68 | 27.9 | - | 2.05 | 7.45 | - |
| 0.75 | 7.77 | 28.3 | - | 2.02 | 7.34 | - |
| 0.93 | 8.15 | 29.6 | - | 1.98 | 7.2 | - |
| 1.12 | 8.32 | 30.3 | - | 1.98 | 7.19 | - |
| 2.5 | 8.95 | 32.6 | 31.6 | 1.64 | 5.96 | 5.85 |
| [161Tb]Tb-crown-TATE | ||||||
| 0 | 0 | 0 | - | 0 | 0 | - |
| 0.13 | 6.27 | 27.0 | - | 6.44 | 27.8 | - |
| 0.32 | 8.02 | 34.6 | - | 2.93 | 12.6 | - |
| 0.51 | 8.63 | 37.2 | - | 2.71 | 11.7 | - |
| 0.70 | 8.86 | 38.2 | - | 2.53 | 10.9 | - |
| 0.90 | 9.01 | 38.8 | - | 2.42 | 10.4 | - |
| 1.09 | 9.00 | 38.8 | - | 2.40 | 10.4 | - |
| 1.92 | 6.98 | 30.1 | 28.6 | 2.05 | 8.82 | 9.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wharton, L.; McNeil, S.W.; Merkens, H.; Yuan, Z.; Van de Voorde, M.; Engudar, G.; Ingham, A.; Koniar, H.; Rodríguez-Rodríguez, C.; Radchenko, V.; et al. Preclinical Evaluation of [155/161Tb]Tb-Crown-TATE—A Novel SPECT Imaging Theranostic Agent Targeting Neuroendocrine Tumours. Molecules 2023, 28, 3155. https://doi.org/10.3390/molecules28073155
Wharton L, McNeil SW, Merkens H, Yuan Z, Van de Voorde M, Engudar G, Ingham A, Koniar H, Rodríguez-Rodríguez C, Radchenko V, et al. Preclinical Evaluation of [155/161Tb]Tb-Crown-TATE—A Novel SPECT Imaging Theranostic Agent Targeting Neuroendocrine Tumours. Molecules. 2023; 28(7):3155. https://doi.org/10.3390/molecules28073155
Chicago/Turabian StyleWharton, Luke, Scott W. McNeil, Helen Merkens, Zheliang Yuan, Michiel Van de Voorde, Gokce Engudar, Aidan Ingham, Helena Koniar, Cristina Rodríguez-Rodríguez, Valery Radchenko, and et al. 2023. "Preclinical Evaluation of [155/161Tb]Tb-Crown-TATE—A Novel SPECT Imaging Theranostic Agent Targeting Neuroendocrine Tumours" Molecules 28, no. 7: 3155. https://doi.org/10.3390/molecules28073155
APA StyleWharton, L., McNeil, S. W., Merkens, H., Yuan, Z., Van de Voorde, M., Engudar, G., Ingham, A., Koniar, H., Rodríguez-Rodríguez, C., Radchenko, V., Ooms, M., Kunz, P., Bénard, F., Schaffer, P., & Yang, H. (2023). Preclinical Evaluation of [155/161Tb]Tb-Crown-TATE—A Novel SPECT Imaging Theranostic Agent Targeting Neuroendocrine Tumours. Molecules, 28(7), 3155. https://doi.org/10.3390/molecules28073155