Photoelectrocatalytic Dioxygen Reduction Based on a Novel Thiophene-Functionalized Tricarbonylchloro(1,10-phenanthroline)rhenium(I)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials
2.3. Fabrication of the Modified Electrode
2.4. Optical, Electrochemical and Photoelectrochemical (PEC) Experiments
2.5. Computational Details
3. Results and Discussions
3.1. Synthesis and Characterization
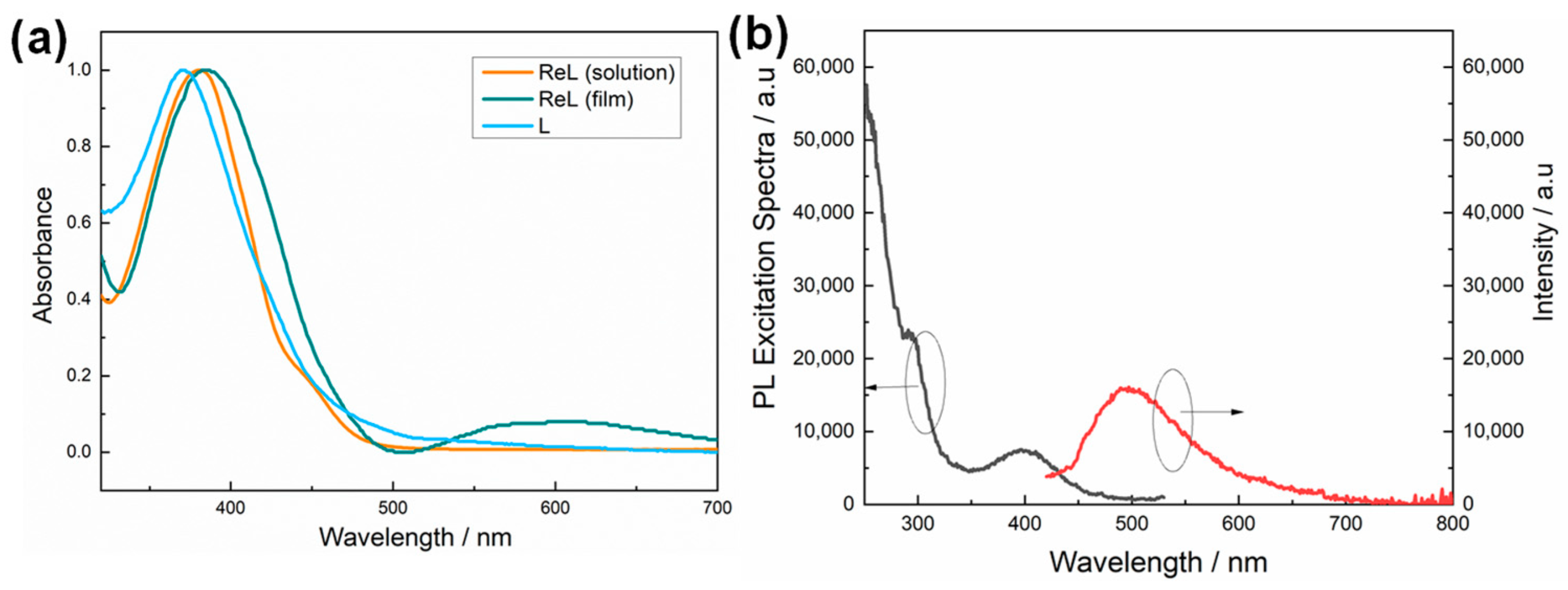
3.2. Optical Properties
3.3. DFT Calculation
3.4. Electrochemical Properties
3.5. PEC Properties
4. Conclusions
Supplementary Materials
 ,
,  and
and  , respectively); Figure S2: IR spectra of L (top line) and Re(CO)3ClL (bottom line) in KBr pellets; Figure S3: Optimized structures of Re(CO3)(o-Phen)Cl (a), Re(CO)3ClL-monomer (b) and Re(CO)3ClL- dimer (c) from DFT calculations. Cl green, Re cyan, O red, C gray, N blue, S yellow, H white; Figure S4: The distribution of MOs of Re(CO)3ClL- monomer. Cl green, Re cyan, O red, C gray, N blue, S yellow, H white; Figure S5: The distribution of MOs of Re(CO)3ClL- dimer. Cl green, Re cyan, O red, C gray, N blue, S yellow, H white; Figure S6: Partial molecular orbital energy levels of Re(CO)3ClL-monomer calculated by DFT; Figure S7: Partial molecular orbital energy levels of Re(CO)3ClL-dimer calculated by DFT; Figure S8: The UV-Vis absorption spectra of H2O2 at different concentrations, the top inset shows the dependence of absorbance at 350 nm on H2O2. (a) [H2O2] is 1–10 × 10−6 M, (b) [H2O2] is 1–10 × 10−5 M; Figure S9: Normalized UV-Vis absorption spectra of a CH2Cl2 solution (blue line), and drop-coated film of Re(CO)3ClL before (black line) and after (red line) photoelectrocatalysis for 5 h; Table S1: Computational energy levels of Re(CO)3ClL-monomer; Table S2: Comparison of computational selected bond lengths (Å), bond angles (°), and dihedral angels (°) of Re(CO)3ClLwith the atomic labelling scheme (left) and the molecular structure (right) shown below this table using the DFT-B3LYP at the LanL2DZ level with those of crystal structure of fac-[ReBr(CO)3(L3)], the Ref crystal, which was reported in text Ref. [57].
, respectively); Figure S2: IR spectra of L (top line) and Re(CO)3ClL (bottom line) in KBr pellets; Figure S3: Optimized structures of Re(CO3)(o-Phen)Cl (a), Re(CO)3ClL-monomer (b) and Re(CO)3ClL- dimer (c) from DFT calculations. Cl green, Re cyan, O red, C gray, N blue, S yellow, H white; Figure S4: The distribution of MOs of Re(CO)3ClL- monomer. Cl green, Re cyan, O red, C gray, N blue, S yellow, H white; Figure S5: The distribution of MOs of Re(CO)3ClL- dimer. Cl green, Re cyan, O red, C gray, N blue, S yellow, H white; Figure S6: Partial molecular orbital energy levels of Re(CO)3ClL-monomer calculated by DFT; Figure S7: Partial molecular orbital energy levels of Re(CO)3ClL-dimer calculated by DFT; Figure S8: The UV-Vis absorption spectra of H2O2 at different concentrations, the top inset shows the dependence of absorbance at 350 nm on H2O2. (a) [H2O2] is 1–10 × 10−6 M, (b) [H2O2] is 1–10 × 10−5 M; Figure S9: Normalized UV-Vis absorption spectra of a CH2Cl2 solution (blue line), and drop-coated film of Re(CO)3ClL before (black line) and after (red line) photoelectrocatalysis for 5 h; Table S1: Computational energy levels of Re(CO)3ClL-monomer; Table S2: Comparison of computational selected bond lengths (Å), bond angles (°), and dihedral angels (°) of Re(CO)3ClLwith the atomic labelling scheme (left) and the molecular structure (right) shown below this table using the DFT-B3LYP at the LanL2DZ level with those of crystal structure of fac-[ReBr(CO)3(L3)], the Ref crystal, which was reported in text Ref. [57].Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, C.; Dasgupta, N.P.; Yang, P. Semiconductor Nanowires for Artificial Photosynthesis. Chem. Mater. 2013, 26, 415–422. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-dimensional hybrid nanostructures for heterogeneous photocatalysis and photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Engelbert, P.; Stefanie, S.; Dogukan, A.; Kerstin, O.; Markus, H.; Daniel, A.M.E.; Helmut, N.; Günther, K.; Tsukasa, Y.; Niyazi Serdar, S. Using the Alkynyl-Substituted Rhenium(I) Complex (4,4′-Bisphenyl-Ethynyl-2,2′-Bipyridyl)Re(CO)3Cl as Catalyst for CO2 Reduction-Synthesis, Characterization, and Application. Electrocatalysis 2015, 6, 185–197. [Google Scholar]
- Fukuzumi, S. Production of Liquid Solar Fuels and Their Use in Fuel Cells. Joule 2017, 1, 689–738. [Google Scholar] [CrossRef] [Green Version]
- Sayama, K. Production of High-Value-Added Chemicals on Oxide Semiconductor Photoanodes under Visible Light for Solar Chemical-Conversion Processes. ACS Energy Lett. 2018, 3, 1093–1101. [Google Scholar] [CrossRef]
- Fuku, K.; Miyase, Y.; Miseki, Y.; Funaki, T.; Gunji, T.; Sayama, K. Photoelectrochemical Hydrogen Peroxide Production from Water on a WO3 /BiVO4 Photoanode and from O2 on an Au Cathode Without External Bias. Chem. Asian J. 2017, 12, 1111–1119. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Pan, Z.; Sayama, K. Electrochemical and Photoelectrochemical Water Oxidation for Hydrogen Peroxide Production. Angew. Chem. Int. Ed. Engl. 2021, 60, 10469–10480. [Google Scholar] [CrossRef]
- Orchanian, N.M.; Hong, L.E.; Skrainka, J.A.; Esterhuizen, J.A.; Popov, D.A.; Marinescu, S.C. Surface-Immobilized Conjugated Polymers Incorporating Rhenium Bipyridine Motifs for Electrocatalytic and Photocatalytic CO2 Reduction. ACS Energy Lett. 2018, 2, 110–123. [Google Scholar] [CrossRef]
- Nakada, A.; Ishitani, O. Selective Electrocatalysis of a Water-Soluble Rhenium(I) Complex for CO2 Reduction Using Water As an Electron Donor. ACS Catal. 2017, 8, 354–363. [Google Scholar] [CrossRef]
- Jürgens, S.; Herrmann, W.A.; Kühn, F.E. Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J. Organomet. Chem. 2014, 751, 83–89. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Tsang, K.H.-K. Bifunctional Luminescent Rhenium(I) Complexes Containing an Extended Planar Diimine Ligand and a Biotin Moiety. Organometallics 2004, 23, 3062–3070. [Google Scholar] [CrossRef]
- Lees, A.J. Organometallic complexes as luminescence probes in monitoring thermal and photochemical polymerizations. Coord. Chem. Rev. 1998, 177, 3–35. [Google Scholar] [CrossRef]
- Wang, K.; Huang, L.; Gao, L.; Jin, L.; Huang, C. Synthesis, crystal structure, and photoelectric properties of Re(CO)3ClL (L= 2-(1-ethylbenzimidazol-2-yl) pyridine). Inorg. Chem. 2002, 41, 3353. [Google Scholar] [CrossRef] [PubMed]
- Kuninobu, Y.; Takai, K. Organic Reactions Catalyzed by Rhenium Carbonyl Complexes. Chem. Rev. 2010, 111, 1938–1953. [Google Scholar] [CrossRef]
- Ju, C.-C.; Zhang, A.-G.; Sun, H.-L.; Wang, K.-Z.; Jiang, W.-L.; Bian, Z.-Q.; Huang, C.-H. Synthesis, Crystal Structure, and Optical and Photoelectrochemical Properties of a N∩O-Rhenium(I) Complex. Organometallics 2011, 30, 712–716. [Google Scholar] [CrossRef]
- Sun, C.; Prosperini, S.; Quagliotto, P.; Viscardi, G.; Yoon, S.S.; Gobetto, R.; Nervi, C. Electrocatalytic reduction of CO2 by thiophene-substituted rhenium(I) complexes and by their polymerized films. Dalton. Trans. 2016, 45, 14678–14688. [Google Scholar] [CrossRef]
- Popov, D.A.; Luna, J.M.; Orchanian, N.M.; Haiges, R.; Downes, C.A.; Marinescu, S.C. A 2,2′-bipyridine-containing covalent organic framework bearing rhenium(I) tricarbonyl moieties for CO2 reduction. Dalton. Trans. 2018, 47, 17450–17460. [Google Scholar] [CrossRef]
- Hideaki, T.; Akiko, S.; Yuji, O.; Kazuhide, K.; Hiroyuki, T.; Osamu, I. Control of Photochemical, Photophysical, Electrochemical, and Photocatalytic Properties of Rhenium(I) Complexes Using Intramolecular Weak Interactions between Ligands. J. Am. Chem. Soc. 2005, 127, 44. [Google Scholar]
- Kuramochi, Y.; Ishitani, O.; Ishida, H. Reaction mechanisms of catalytic photochemical CO2 reduction using Re(I) and Ru(II) complexes. Coord. Chem. Rev. 2018, 373, 333–356. [Google Scholar] [CrossRef]
- Nicholls, T.P.; Burt, L.K.; Simpson, P.V.; Massi, M.; Bissember, A.C. Tricarbonyl rhenium(I) tetrazolato and N-heterocyclic carbene complexes: Versatile visible-light-mediated photoredox catalysts. Dalton. Trans. 2019, 48, 12749–12754. [Google Scholar] [CrossRef]
- Oppelt, K.; Egbe, D.A.M.; Monkowius, U.; List, M.; Zabel, M.; Sariciftci, N.S.; Knör, G. Luminescence and spectroscopic studies of organometallic rhodium and rhenium multichromophore systems carrying polypyridyl acceptor sites and phenylethynyl antenna subunits. J. Organomet. Chem. 2011, 696, 2252–2258. [Google Scholar] [CrossRef]
- Portenkirchner, E.; Oppelt, K.; Ulbricht, C.; Egbe, D.A.M.; Neugebauer, H.; Knör, G.; Sariciftci, N.S. Electrocatalytic and photocatalytic reduction of carbon dioxide to carbon monoxide using the alkynyl-substituted rhenium(I) complex (5,5′-bisphenylethynyl-2,2′-bipyridyl)Re(CO)3Cl. J. Organomet. Chem. 2012, 716, 19–25. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.-X.; Li, Y.-J.; Fu, Y.-H.; Yin, Z.-H.; Gao, L.-H.; Wang, K.-Z. A 3D electropolymerized thin film based on a thiophene-functionalized Ru(II) complex: Electrochemical and photoelectrochemical insights. Inorg. Chem. Front. 2019, 6, 3518–3528. [Google Scholar] [CrossRef]
- Wolf, M.O. Transition-Metal-Polythiophene Hybrid Materials. Adv. Mater. 2001, 13, 545–553. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical−Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, T.; Yin, H.J.; Gao, L.H.; Wang, K.Z. Electrodeposited thiophene-containing organic small molecule-modified ITO electrode with highly efficient photoelectric conversion and photoelectrochemical oxygen reduction. Electrochim. Acta 2020, 362, 137150. [Google Scholar] [CrossRef]
- Wuttke, S.; Medina, D.D.; Rotter, J.M.; Begum, S.; Stassin, T.; Ameloot, R.; Oschatz, M.; Tsotsalas, M. Bringing Porous Organic and Carbon-Based Materials toward Thin-Film Applications. Adv. Funct. Mater. 2018, 28, 1801545. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Liang, Y.; Strohecker, D.; Lynch, V.; Holliday, B.J.; Jones, R.A. A Thiophene-Containing Conductive Metallopolymer Using an Fe(II) Bis(terpyridine) Core for Electrochromic Materials. ACS Appl. Mater. Interfaces 2016, 8, 34568–34580. [Google Scholar] [CrossRef]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Wang, J.-X.; Xia, H.-Y.; Liu, W.-Q.; Zhao, F.; Wang, Y.-B. Synthesis, spectroscopic, and DFT studies of rhenium(I) complexes with phenanthrolineimidazo ligands containing thienyl moieties. Inorg. Chim. Acta 2013, 394, 92–97. [Google Scholar] [CrossRef]
- Haga, M.-A.; Kobayashi, K.; Terada, K. Fabrication and functions of surface nanomaterials based on multilayered or nanoarrayed assembly of metal complexes. Coord. Chem. Rev. 2007, 251, 2688–2701. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Kumar, A.K.S.; Zhang, Y.; Li, D.; Compton, R.G. A mini-review: How reliable is the drop casting technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Wada, T.; Tsuge, K.; Tanaka, K. Electrochemical Oxidation of Water to Dioxygen Catalyzed by the Oxidized Form of the Bis(ruthenium–hydroxo) Complex in H2O. Angew. Chem. Int. Ed. 2000, 39, 1479–1482. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Drop-Casting to Make Efficient Perovskite Solar Cells under High Humidity. Angew. Chem. Int. Ed. 2021, 60, 11242–11246. [Google Scholar] [CrossRef]
- Xue, L.-X.; Duan, Z.-M.; Jia, J.; Wang, K.-Z.; Haga, M.-A. pH-induced photocurrent switching based on a highly stable drop-casting film of imidazole moiety-containing dinuclear Ru(II) Complex. Electrochim. Acta 2014, 146, 776–783. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The Measurement of Photoluminescence Quantum Yields. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of hydrogen peroxides and organic peroxides in aqueous suspensions of titanium dioxide, zinc oxide, and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. Int. J. Quantum Chem. 1988, 34, 245–255. [Google Scholar] [CrossRef]
- Whang, D.R.; Apaydin, D.H.; Park, S.Y.; Sariciftci, N.S. An electron-reservoir Re(I) complex for enhanced efficiency for reduction of CO2 to CO. J. Catal. 2018, 363, 191–196. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Löwdin, P.O. On the Non-Orthogonality Problem Connected with the Use of Atomic Wave Functions in the Theory of Molecules and Crystals. J. Chem. Phys. 1950, 18, 365–375. [Google Scholar] [CrossRef]
- Fujita, E.; Muckerman, J.T. Why Is Re-Re Bond Formation/Cleavage in [Re(bpy)(CO)3]2+ Different from That in [Re(CO)5]2+? Experimental and Theoretical Studies on the Dimers and Fragments. Inorg. Chem. 2004, 43, 7636–7647. [Google Scholar] [CrossRef]
- Jenks, T.C.; Bailey, M.D.; Corbin, B.A.; Kuda-Wedagedara, A.N.W.; Martin, P.D.; Schlegel, H.B.; Rabuffetti, F.A.; Allen, M.J. Photophysical characterization of a highly luminescent divalent-europium-containing azacryptate. Chem. Commun. 2018, 54, 4545–4548. [Google Scholar] [CrossRef]
- Ley, K.D.; Schanze, K.S. Photophysics of metal-organic π-conjugated polymers. Coord. Chem. Rev. 1998, 171, 287–307. [Google Scholar] [CrossRef]
- Fitzner, R.; Mena-Osteritz, E.; Walzer, K.; Pfeiffer, M.; Bäuerle, P. A-D-A-Type Oligothiophenes for Small Molecule Organic Solar Cells: Extending the π-System by Introduction of Ring-Locked Double Bonds. Adv. Funct. Mater. 2015, 25, 1845–1856. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Kapturkiewicz, A.; Lipkowski, J.; Nowacki, J. Re(I)(tricarbonyl)+ complexes with the 2-(2-pyridyl)-N-methyl-benzimidazole, 2-(2-pyridyl)benzoxazole and 2-(2-pyridyl)benzothiazole ligands-syntheses, structures, electrochemical and spectroscopic studies. Inorg. Chim. Acta 2005, 358, 2701–2710. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Kapturkiewicz, A.; Anulewicz-Ostrowska, R.; Nowacki, J. ReI(CO)3+ complexes with N∩O-bidentate ligands. J. Chem. Soc. Dalton Trans. 2002, 3434–3441. [Google Scholar] [CrossRef]
- Lan, Z.A.; Zhang, G.; Chen, X.; Zhang, Y.; Zhang, K.A.I.; Wang, X. Reducing the Exciton Binding Energy of Donor-Acceptor-Based Conjugated Polymers to Promote Charge-Induced Reactions. Angew. Chem. Int. Ed. Engl. 2019, 58, 10236–10240. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, Z.; Yang, Z.; Zhang, S.; Gong, X.; Hua, J. Bandgap engineering of novel peryleno[1,12-bcd]thiophene sulfone-based conjugated co-polymers for significantly enhanced hydrogen evolution without co-catalyst. J. Mater. Chem. A 2020, 8, 20062–20071. [Google Scholar] [CrossRef]
- Bonello, R.O.; Morgan, I.R.; Yeo, B.R.; Jones, L.E.J.; Kariuki, B.M.; Fallis, I.A.; Pope, S.J.A. Luminescent rhenium(I) complexes of substituted imidazole[4,5-f]-1,10-phenanthroline derivatives. J. Organomet. Chem. 2014, 749, 150–156. [Google Scholar] [CrossRef]
- Dai, C.; Xu, S.; Liu, W.; Gong, X.; Panahandeh-Fard, M.; Liu, Z.; Zhang, D.; Xue, C.; Loh, K.P.; Liu, B. Dibenzothiophene-S,S-Dioxide-Based Conjugated Polymers: Highly Efficient Photocatalyts for Hydrogen Production from Water under Visible Light. Small 2018, 14, 1801839. [Google Scholar] [CrossRef]
- Yang, L.; Ren, A.-M.; Feng, J.-K.; Liu, X.-D.; Ma, Y.-G.; Zhang, H.-X. Theoretical Studies of Ground and Excited Electronic States in a Series of Rhenium(I) Bipyridine Complexes Containing Diarylethynyl-Based Structure. Inorg. Chem. 2004, 43, 5961–5972. [Google Scholar] [CrossRef]
- Kaeffer, N.; Massin, J.; Lebrun, C.; Renault, O.; Chavarot-Kerlidou, M.; Artero, V. Covalent Design for Dye-Sensitized H2-Evolving Photocathodes Based on a Cobalt Diimine-Dioxime Catalyst. J. Am. Chem. Soc. 2016, 138, 12308–12311. [Google Scholar] [CrossRef] [Green Version]
- Savéant, J.-M. Electron hopping between localized sites: Coupling with electroinactive counterion transport. Phys. Rev. A 1988, 92, 1011–1013. [Google Scholar] [CrossRef]
- Denisevich, P.; Abruna, H.; Leidner, C.; Meyer, T.; Murray, R.W. Electropolymerization of vinylpyridine and vinylbipyridine complexes of iron and ruthenium: Homopolymers, copolymers, reactive polymers. Inorg. Chem. 1982, 21, 2153–2161. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kita, S.; Brunschwig, B.S.; Fujita, E. Involvement of a Binuclear Species with the Re-C(O)O-Re Moiety in CO2 Reduction Catalyzed by Tricarbonyl Rhenium (I) Complexes with Diimine Ligands: Strikingly Slow Formation of the Re-Re and Re-C(O)O-Re Species from Re(dmb)(CO)3S (dmb = 4,4′-Dimethyl-2,2′-bipyridine, S = Solvent). J. Am. Chem. Soc. 2003, 125, 11976–11987. [Google Scholar] [PubMed] [Green Version]
- Kalyanasundaram, K. Luminescence and redox reactions of the metal-to-ligand charge-transfer excited state of tricarbonylchloro-(polypyridyl) rhenium(I) complexes. J. Chem. Soc. Faraday Trans. 1986, 2, 2401–2415. [Google Scholar] [CrossRef]
- Luong, B.C.; Nadjo, L.; Wrighton, M.S. Ground and excited state electron transfer processes involving fac-tricarbonylchloro (1,10-phenanthroline) rhenium (I). Electrogenerated chemiluminescence and electron transfer quenching of the lowest excited state. J. Am. Chem. Soc. 1987, 100, 5790–5795. [Google Scholar] [CrossRef]
- Ng, C.-O.; Lo, L.T.-L.; Ng, S.-M.; Ko, C.-C.; Zhu, N. A New Class of Isocyanide-Containing Rhenium(I) Bipyridyl Luminophore with Readily Tunable and Highly Environmentally Sensitive Excited-State Properties. Inorg. Chem. 2008, 47, 7447–7449. [Google Scholar] [CrossRef]
- Groenendaala, L.; Zottib, G.; Jonas, F. Optical, conductive and magnetic properties of electrochemically prepared alkylated poly(3,4-ethylenedioxythiophene)s. Synth. Met. 2001, 118, 105–109. [Google Scholar] [CrossRef]
- Liu, T.; Han, L.-L.; Du, C.-M.; Yu, Z.-Y. Redox potentials of dopamine and its supramolecular complex with aspartic acid. Russ. J. Phys. Chem. A 2014, 88, 1085–1090. [Google Scholar] [CrossRef]
- Lattach, Y.; Fortage, J.; Deronzier, A.; Moutet, J.C. Polypyrrole-Ru(2,2′-bipyridine)32+/MoSx structured composite film as a photocathode for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 4476–4480. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.-T.; Zhang, Y.-Y.; Zhang, C.-C.; Cheng, Q.-R.; Hou, S.-M.; Liao, J.-H.; Wang, K.-Z. Three-dimensional high-rate electropolymerized thin film with exceptionally high photocurrent based on a triphenylamine-containing ruthenium complex. Electrochim. Acta 2019, 298, 265–278. [Google Scholar] [CrossRef]
- Ng, C.H.; Winther-Jensen, O.; Ohlin, C.A.; Winther-Jensen, B. Exploration and optimisation of poly(2,2′-bithiophene) as a stable photo-electrocatalyst for hydrogen production. J. Mater. Chem. A 2015, 3, 11358–11366. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Hou, Y.; Zuo, F.; Beran, G.J.; Feng, P. Boron carbides as efficient, metal-free, visible-light-responsive photocatalysts. Angew. Chem. Int. Ed. Engl. 2013, 52, 3241–3245. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.T.; Xue, L.X.; Wang, H.; Wang, K.Z.; Haga, M. pH controllable photocurrent switching and molecular half-subtractor calculations based on a monolayer composite film of a dinuclear RuII complex and graphene oxide. J. Mater. Chem. C 2017, 5, 3390–3396. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Z.-B.; Meng, T.-T.; Wang, K.-Z. Synergistically enhanced photoelectrochemical properties of a layer-by-layer hybrid film based on graphene oxide and a free terpyridyl-grafted ruthenium complex. J. Mater. Chem. A 2015, 3, 3441–3449. [Google Scholar] [CrossRef]
- Meng, T.T.; Zheng, Z.B.; Wang, K.Z. Layer-by-layer assembly of graphene oxide and a Ru(II) complex and significant photocurrent generation properties. Langmuir 2013, 29, 14314–14320. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-C.; Yang, W.; Chen, X.; Gao, L.-H.; Wang, K.-Z. Redox- and photovoltaic-active nanocomposite thin films of graphene oxide and a ruthenium terpyridyl complex. Electrochim. Acta 2014, 134, 319–326. [Google Scholar] [CrossRef]
- Xue, L.-X.; Meng, T.-T.; Zhao, Y.; Gao, L.-H.; Wang, K.-Z. Graphene oxide supported mononuclear aquaruthenium complex ultrathin films with enhanced photoelectric conversion and electrocatalytic water oxidation. Electrochim. Acta 2015, 172, 77–87. [Google Scholar] [CrossRef]
- Huang, Q.-Y.; Kang, S.-Y.; Lin, H.; Wang, K.-Z. Electrochemical and Photoelectrochemical Investigation of New Self-Assembled Films Based on Prussian Blue and a Terpyridyl RuII Complex. Aust. J. Chem. 2015, 68, 426. [Google Scholar] [CrossRef]
- Walsh, J.J.; Mallon, C.T.; Bond, A.M.; Keyes, T.E.; Forster, R.J. Enhanced photocurrent production from thin films of Ru(II) metallopolymer/Dawson polyoxotungstate adducts under visible irradiation. Chem. Commun. 2012, 48, 3593–3595. [Google Scholar] [CrossRef]
- Walsh, J.J.; Long, D.L.; Cronin, L.; Bond, A.M.; Forster, R.J.; Keyes, T.E. Electronic and photophysical properties of adducts of [Ru(bpy)3]2+ and Dawson-type sulfite polyoxomolybdates alpha/beta-[Mo18O54(SO3)2]4−. Dalton. Trans. 2011, 40, 2038–2045. [Google Scholar] [CrossRef]
- Walsh, J.J.; Zhu, J.; Bond, A.M.; Forster, R.J.; Keyes, T.E. Visible light sensitized photocurrent generation from electrostatically assembled thin films of [Ru(bpy)3]2+ and the polyoxometalate γ*-[W18O54(SO4)2]4−: Optimizing performance in a low electrolyte medium. J. Electroanal. Chem. 2013, 706, 93–101. [Google Scholar] [CrossRef]
- Zhu, J.; Walsh, J.J.; Bond, A.M.; Keyes, T.E.; Forster, R.J. Ruthenium metallopolymer: Dawson polyoxomolybdate alpha-[Mo18O54(SO4)2]4- adduct films: Sensitization for visible photoelectrocatalysis. Langmuir 2012, 28, 13536–13541. [Google Scholar] [CrossRef]
- Li, L.Y.; Yu, H.Y.; Lin, N.; Chen, X.; Wei, R.; Wang, K.Z. Preparation, characterization, and photoelectric properties of an electrostatically self-assembled film based on tungstophosphoric acid and a binuclear Ru(II) complex. J. Nanosci. Nanotechnol. 2011, 11, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.R.; Dupart, E.; Mingotaud, C.; Ravaine, S. Electrochemical and Photoelectrochemical Properties of New Hybrid Langmuir-Blodgett Films Containing Prussian Blue and a Tris(Bipyridine) Ruthenium Derivative. J. Phys. Chem. B 2000, 104, 9487–9490. [Google Scholar] [CrossRef]
- Aoki, A.; Abe, Y.; Miyashita, T. Effective Photoinduced Electron Transfer in Hetero-Deposited Redox Polymer LB Films. Langmuir 1999, 15, 1463–1469. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wang, C.; Xia, Y. Graphene oxide assisted solvothermal synthesis of LiMnPO 4 naonplates cathode materials for lithium ion batteries. Electrochim. Acta 2014, 146, 8–14. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Fei, X.; Zhang, Y.; Tong, J.; Song, X.-M. Significant photoelectric conversion properties of multilayer films formed by a cationic zinc phthalocyanine complex and graphene oxide. RSC Adv. 2016, 6, 67017–67024. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, H.; Zhang, Y.; Sun, Y.; Lin, S.; Liu, J.; Yang, Q.; Song, X.-M. Enhanced photovoltaic effect of ruthenium complex-modified graphene oxide with P-type conductivity. Mater. Chem. Phys. 2014, 147, 1140–1145. [Google Scholar] [CrossRef]
- Tong, B.; Yang, H.; Xiong, W.; Xie, F.; Shi, J.; Zhi, J.; Chan, W.K.; Dong, Y. Controlled fabrication and optoelectrical properties of metallosupramolecular films based on ruthenium(II) phthalocyanines and 4,4′-bipyridine covalently anchored on inorganic substrates. J. Phys. Chem. B 2013, 117, 5338–5344. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Lee, S.-H.; Chang, J.; Yi, W.; Han, S.-H. Improved Photocurrent in Ru(2,2′-bipyridine-4,4′-dicarboxylic acid)2(NCS)2/ Di(3-aminopropyl)viologen/Single-Walled Carbon Nanotubes/Indium Tin Oxide System: Suppression of Recombination Reaction by Use of Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 9110–9115. [Google Scholar] [CrossRef]
- Lockhart, P.; Little, B.K.; Slaten, B.L.; Mills, G. Photogeneration of H2O2 in Water-Swollen SPEEK/PVA Polymer Films. J. Phys. Chem. A 2016, 120, 3867–3877. [Google Scholar] [CrossRef]
- Jakešová, M.; Apaydin, D.H.; Sytnyk, M.; Oppelt, K.; Heiss, W.; Sariciftci, N.S.; Głowacki, E.D. Hydrogen-Bonded Organic Semiconductors as Stable Photoelectrocatalysts for Efficient Hydrogen Peroxide Photosynthesis. Adv. Funct. Mater. 2016, 26, 5248–5254. [Google Scholar] [CrossRef]
- Suppes, G.M.; Fortin, P.J.; Holdcrof, S. Photoelectrochemical Hydrogen Evolution: Single-Layer, Conjugated Polymer Films Bearing Surface-Deposited Pt Nanoparticles. J. Electrochem. Soc. 2015, 162, H551–H556. [Google Scholar] [CrossRef]
- Yin, H.J.; Zhang, C.; Yang, T.; Yan, D.; Wang, K.-Z. Oxidative electropolymerization films of a styrene-appending ruthenium complex with highly performed electrochemical, solar photoelectric conversion and photoelectrochemical oxygen reduction properties. Electrochim. Acta 2022, 403, 139672. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zhang, Y.; Zhao, G.; Wang, Y.; Wang, H.; Huan Wang, H.; Xu, R.; Wei, Q. Signal-enhanced electrochemiluminescence strategy using iron-based metal-organic frameworks modified with carboxylated Ru(II) complexes for neuron-specific enolase detection. Biosens. Bioelectron. 2022, 215, 114605. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-Q.; Wang, K.-Z. Photoelectrocatalytic Dioxygen Reduction Based on a Novel Thiophene-Functionalized Tricarbonylchloro(1,10-phenanthroline)rhenium(I). Molecules 2023, 28, 3229. https://doi.org/10.3390/molecules28073229
Li Y-Q, Wang K-Z. Photoelectrocatalytic Dioxygen Reduction Based on a Novel Thiophene-Functionalized Tricarbonylchloro(1,10-phenanthroline)rhenium(I). Molecules. 2023; 28(7):3229. https://doi.org/10.3390/molecules28073229
Chicago/Turabian StyleLi, Yu-Qin, and Ke-Zhi Wang. 2023. "Photoelectrocatalytic Dioxygen Reduction Based on a Novel Thiophene-Functionalized Tricarbonylchloro(1,10-phenanthroline)rhenium(I)" Molecules 28, no. 7: 3229. https://doi.org/10.3390/molecules28073229



