Metalorganic Chemical Vapor Deposition Approach to the Synthesis of Perovskite BaCeO3 and BaCe0.8Y0.2O3 Thin Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Characterization of the Precursors
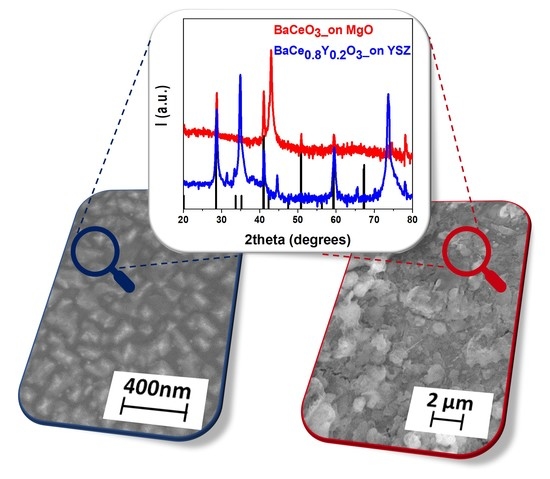
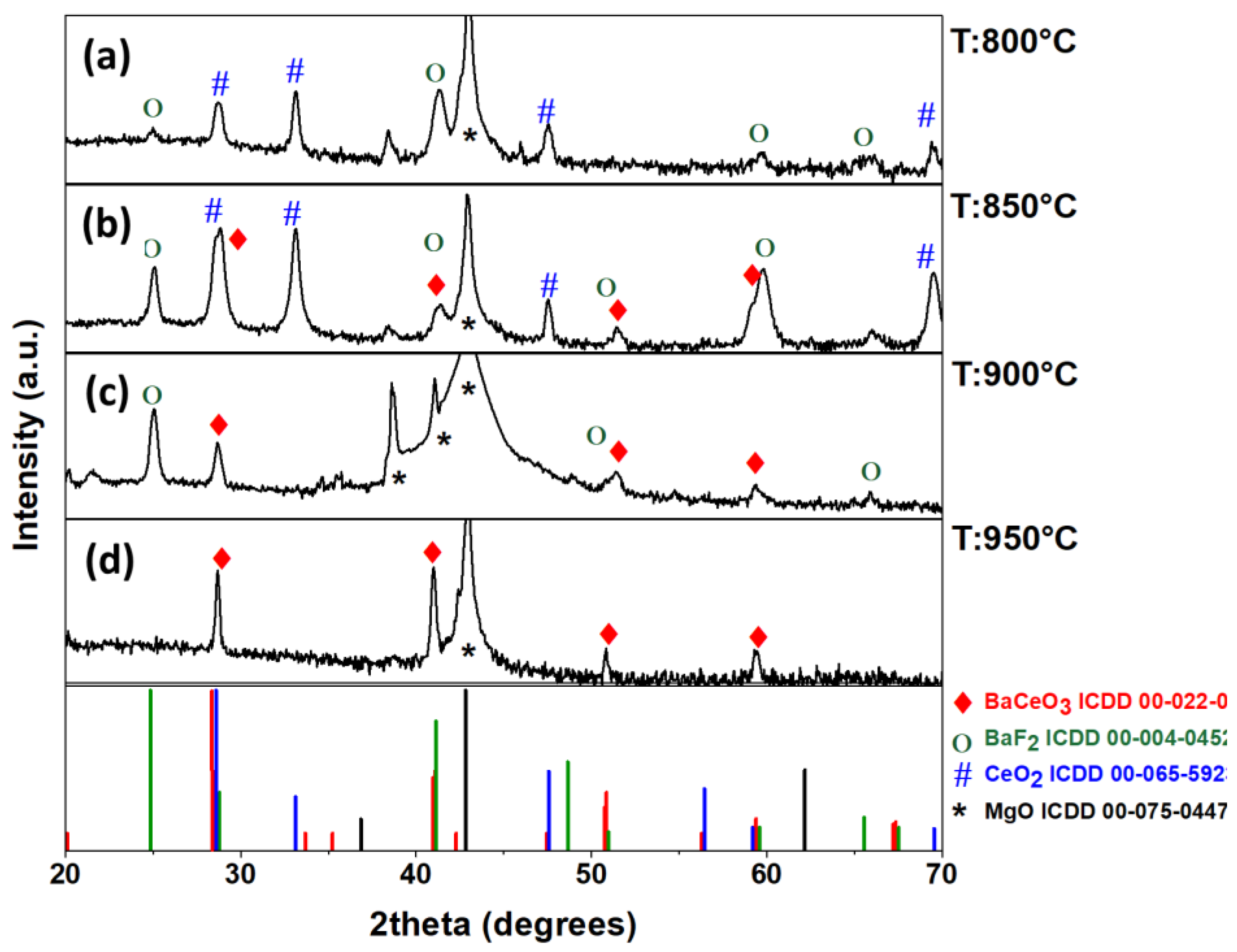
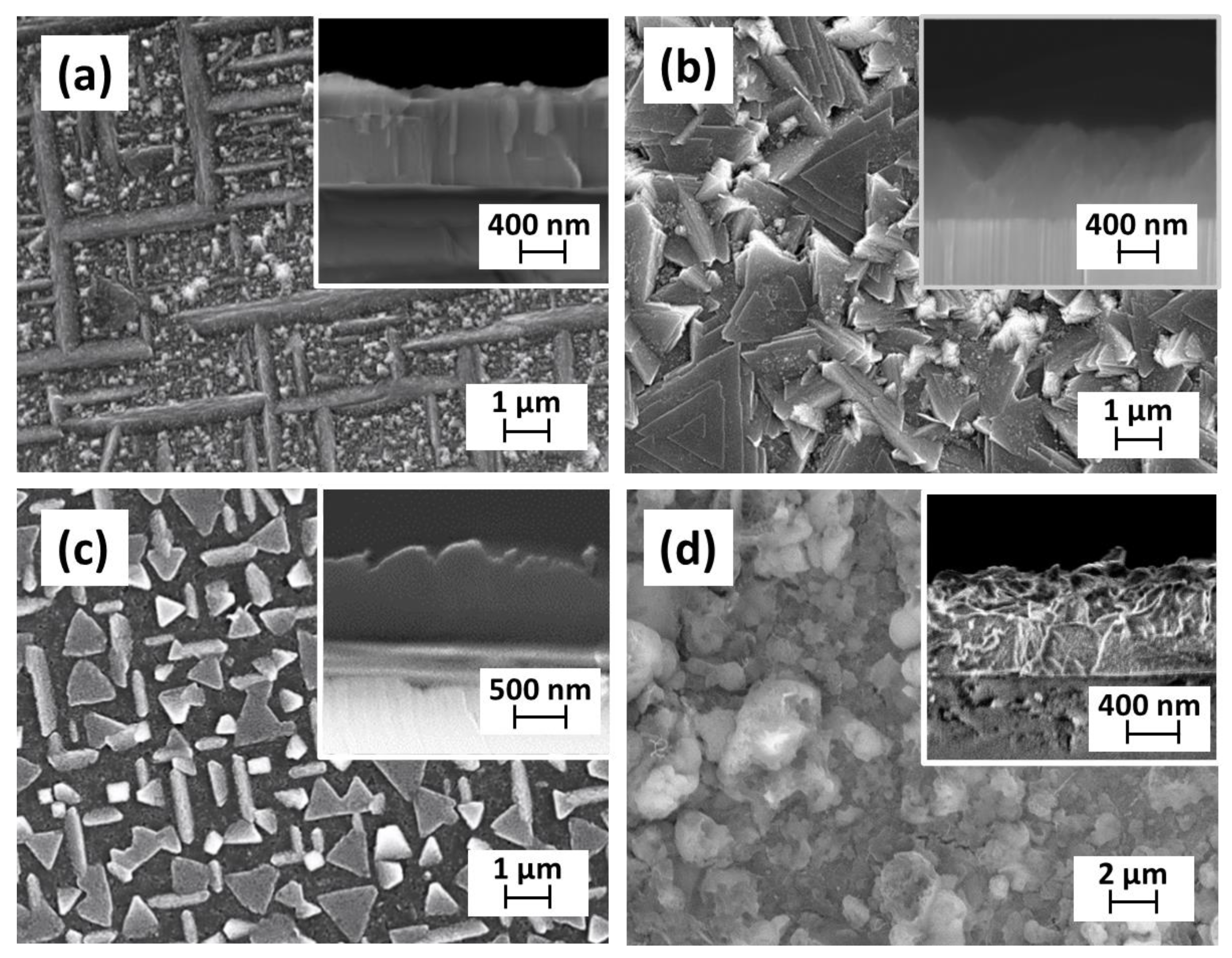
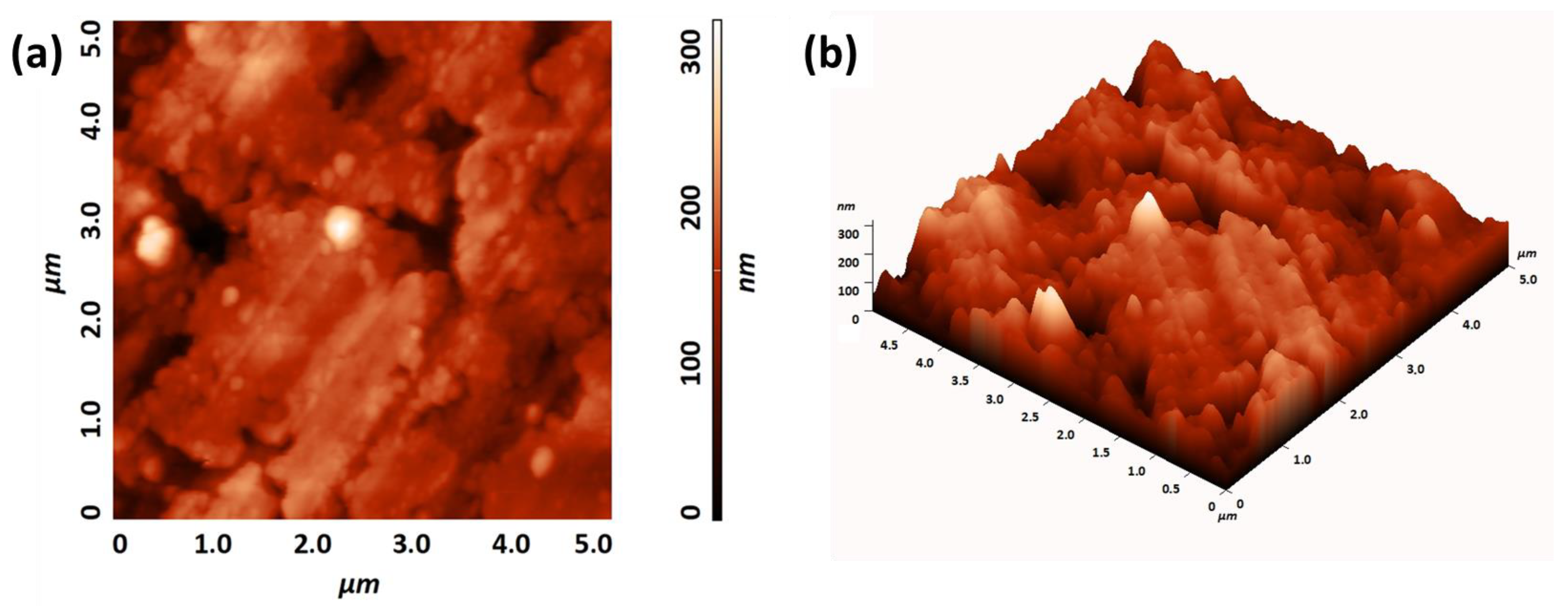
2.2. Deposition of BaCeO3 Films
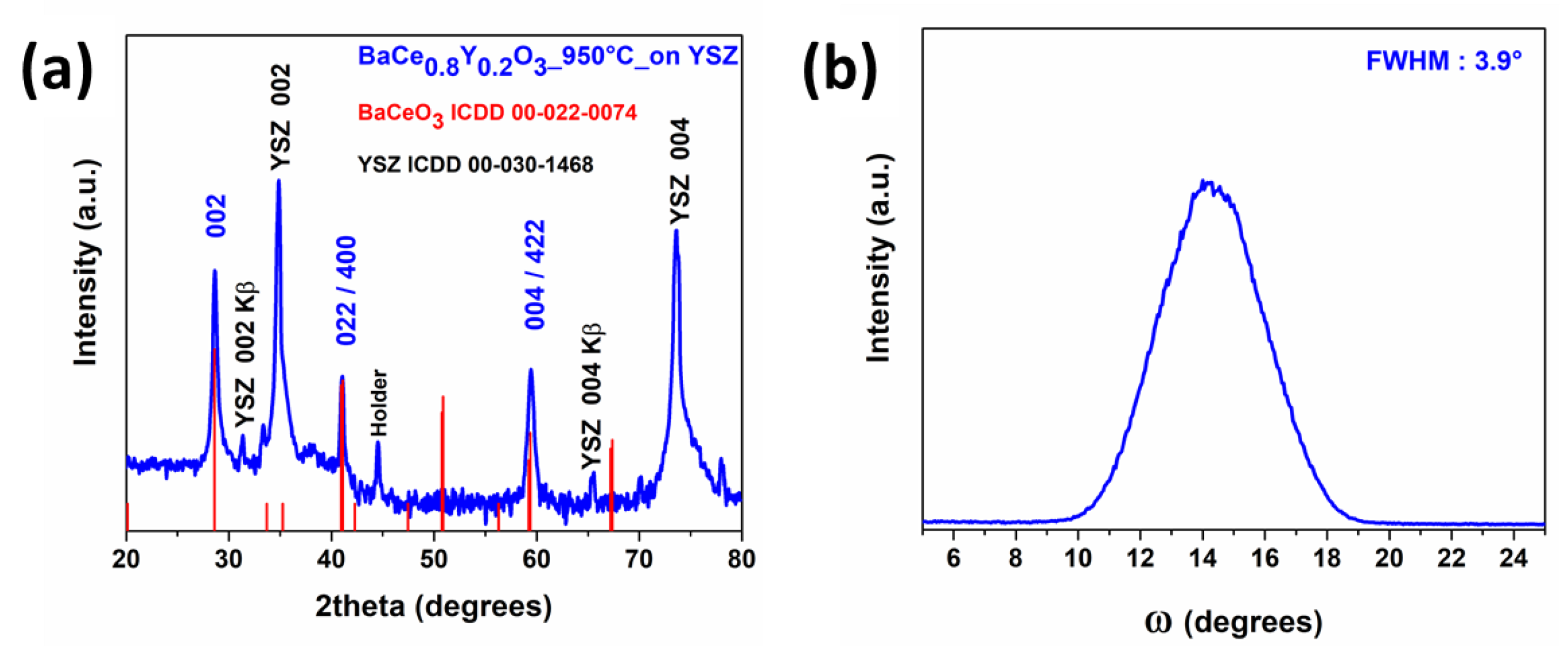
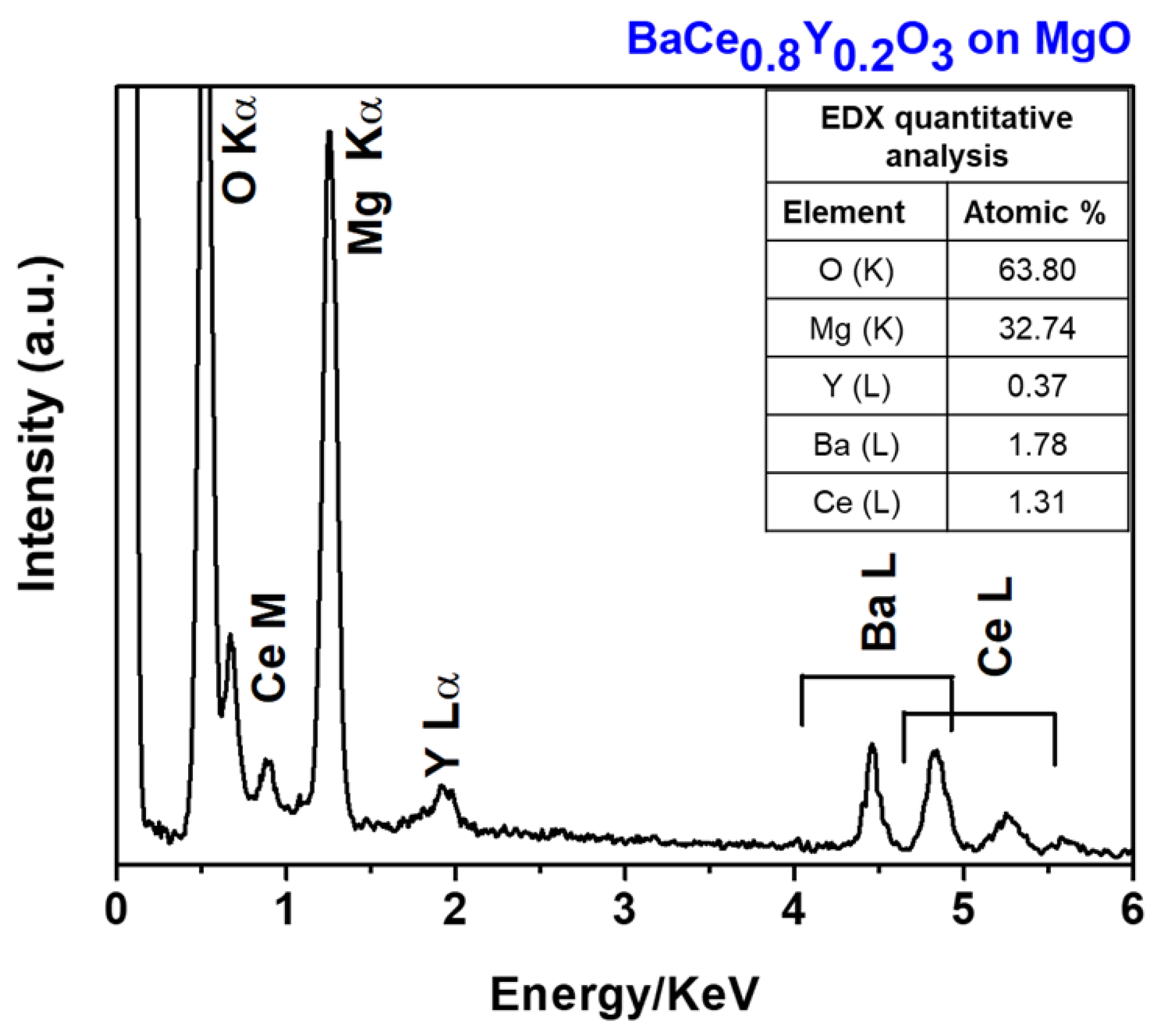
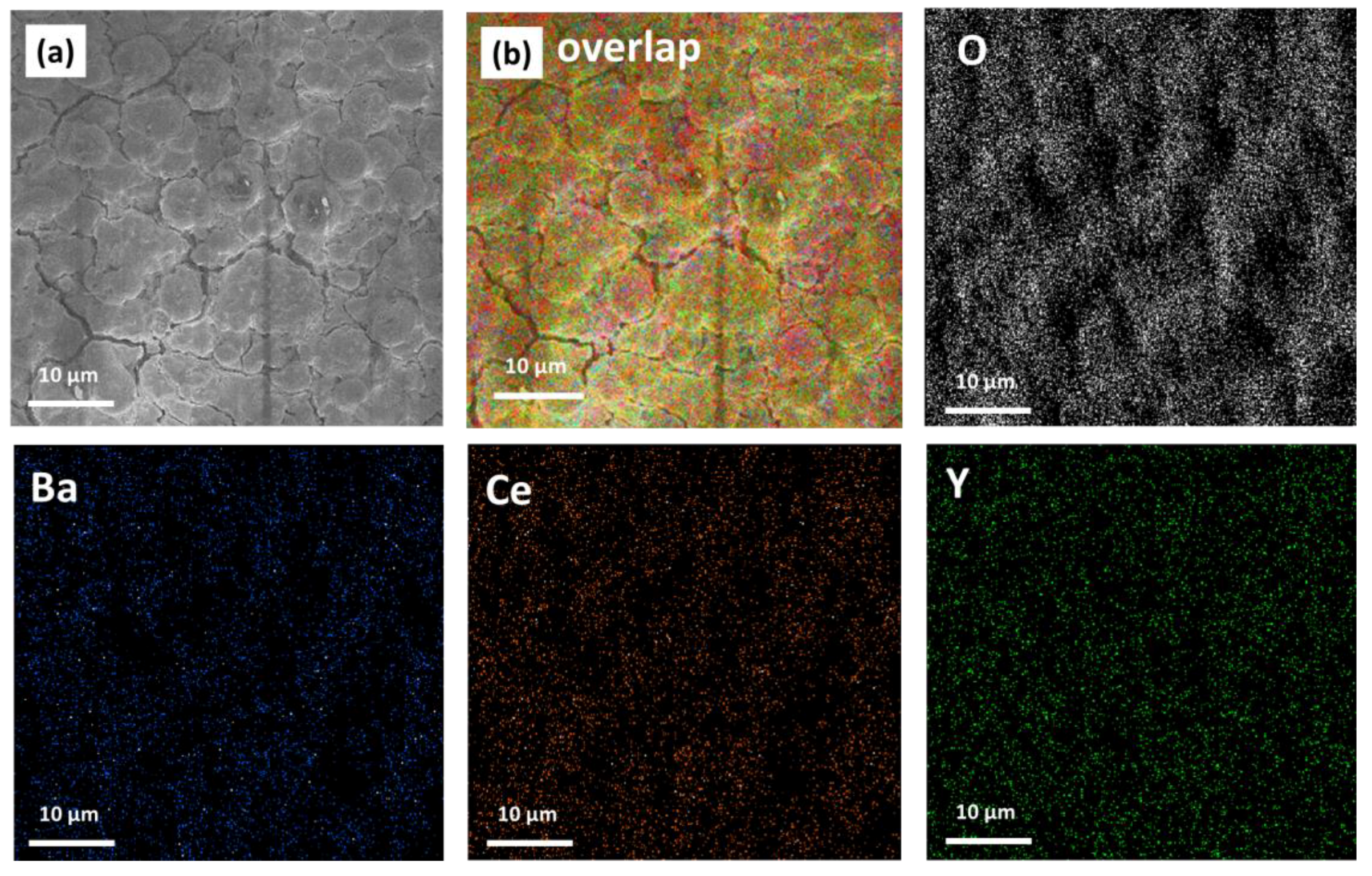
2.3. MOCVD Deposition of Y-Doped BaCeO3 Films
3. Materials and Methods
3.1. Reagents and Precursor Syntheses
3.1.1. Ba(hfa)2tetraglyme
3.1.2. Ce(hfa)3diglyme
3.1.3. Y(hfa)3diglyme
3.2. MOCVD Depositions
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Raiford, J.A.; Oyakhire, S.T.; Bent, S.F. Applications of atomic layer deposition and chemical vapor deposition for perovskite solar cells. Energy Environ. Sci. 2020, 13, 1997–2023. [Google Scholar] [CrossRef]
- Pellegrino, A.L.; Malandrino, G. Surfactant-Free Synthesis of the Full Inorganic Perovskite CsPbBr3: Evolution and Phase Stability of CsPbBr3 vs. CsPb2Br5 and Their Photocatalytic Properties. ACS Appl. Energy Mater. 2021, 4, 9431–9439. [Google Scholar] [CrossRef]
- Tilley, R.J.D. Perovskites: Structure-Property Relationships; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Raknual, D.; Suttiyarak, P.; Tubtimtae, A.; Vailikhit, V. Effect of indium doping in Nb2O5 thin films for electron transport layers: Investigation of structural, optical, and electrical properties. Mater. Lett. 2020, 259, 126828. [Google Scholar] [CrossRef]
- Suwannakham, N.; Tubtimtae, A.; Wongrat, E. Structural, linear/non-linear optical, optoelectrical, and electrical properties of novel crystalline antimony-doped tin oxide thin films synthesized by the chemical deposition method. Phys. B 2023, 649, 414440. [Google Scholar] [CrossRef]
- Panturotai, K.; Krataithong, C.; Pluengphon, P.; Wongrat, E.; Tubtimtae, A.; Inceesungvorn, B. Structural and optical properties of undoped and Sb-doped lead oxide thin films synthesized via the chemical bath deposition method. Opt. Mater. 2022, 126, 112179. [Google Scholar] [CrossRef]
- Sun, H.; Song, S.; Xu, X.; Dai, J.; Yu, J.; Zhou, W.; Shao, Z.; Jung, W.-C. Recent Progress on Structurally Ordered Materials for Electrocatalysis. Adv. Energy Mater. 2021, 11, 2101937. [Google Scholar] [CrossRef]
- Sun, H.; Kim, H.; Xu, X.; Fei, L.; Jung, W.-C.; Shao, Z. Thin Films Fabricated by Pulsed Laser Deposition for Electrocatalysis. Renewables 2023, 1, 21–38. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Jin, N.; Yang, X.; Sun, L.; Wang, Y.; Xie, L.; Chen, X.; Lei, L.; Rozier, P.; et al. Boosting the lithium-ion storage performance of perovskite SrxVO3 via Sr cation and O anion deficient engineering. Sci. Bull. 2022, 67, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Hao, S.; Janolin, P.-E. Enhancing properties of lead-free ferroelectric BaTiO3 through doping. J. Eur. Ceram. Soc. 2022, 42, 4693–4701. [Google Scholar] [CrossRef]
- Shukla, A.; Parey, V.; Thakur, R.; Srivastava, A.; Gaur, N.K. Thermal properties of perovskite RCeO3 (R = Ba, Sr). Thermochim. Acta 2015, 614, 213–217. [Google Scholar] [CrossRef]
- Wu, J.; Davies, R.A.; Islam, M.S.; Haile, S.M. Atomistic Study of Doped BaCeO3: Dopant Site-Selectivity and Cation Nonstoichiometry. Chem. Mater. 2005, 17, 846–851. [Google Scholar] [CrossRef]
- Gu, Y.; Luo, G.; Chen, Z.; Huo, Y.; Wu, F.Z. Enhanced chemical stability and electrochemical performance of BaCe0.8Y0.1Ni0.04Sm0.06O3-δ perovskite electrolytes as proton conductors. Ceram. Int. 2022, 48, 10650–10658. [Google Scholar] [CrossRef]
- He, L.; Xuan, Y.; Zhang, F.; Wang, X.; Pan, H.; Ren, J.; Chen, M. A new perspective of co-doping and Nd segregation effect on proton stability and transportation in Y and Nd co-doped BaCeO3. Int. J. Hydrogen Energy 2021, 46, 1096–1105. [Google Scholar] [CrossRef]
- Matskevich, N.I.; Semerikova, A.N.; Gelfond, N.V.; Matskevich, M.Y.; Anyfrieva, O.I.; Tkachev, E.N. The effect of substituting of rare earth elements (R = Ho, Yb) on energetic characteristics of doped barium cerates. Thermochim. Acta 2021, 705, 179048. [Google Scholar] [CrossRef]
- Hu, W.; Chen, W.; Wang, H. Electrochemical Properties of Lutetium and Zirconium codoped SrCeO3 Composite Electrolyte for Intermediate Temperature for Solid Oxide Fuel Cell. Int. J. Electrochem. Sci. 2020, 15, 3157–3163. [Google Scholar] [CrossRef]
- Ouzaouit, K.; Aboulaich, A. Nd-Doped Barium Cerate Nano-Sized Catalyst Converts CH4 into CO2 at Lower Temperature Compared to Noble Metal-Based Pd/Al2O3. Catalyst. J. Environ. Nanotechnol. 2021, 10, 1–8. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Ran, R.; Cao, J.; Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 2000, 30, 764–774. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef]
- Tomita, A.; Hibino, T.; Suzuki, M.; Sano, M. Proton conduction at the surface of Y-doped BaCeO3 and its application to an air/fuel sensor. J. Mater. Sci. 2004, 39, 2493–2497. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Suzuki, M.; Sano, M. A Solid Oxide Fuel Cell Using Y-Doped BaCeO3 with Pd-Loaded FeO Anode and Ba0.5Pr0.5CoO3 Cathode at Low Temperatures. J. Electrochem. Soc. 2002, 149, 1503–1508. [Google Scholar] [CrossRef]
- Song, S.; Wachsman, E.; Dorris, S.; Balachandran, U. Defect chemistry modeling of high-temperature proton-conducting cerates. Solid State Ion. 2002, 149, 1–10. [Google Scholar] [CrossRef]
- Hui, Z.; Michele, P. Preparation, chemical stability, and electrical properties of Ba(Ce1 − xBix)O3 (x = 0.0–0.5). J. Mater. Chem. 2002, 12, 3787–3791. [Google Scholar] [CrossRef]
- Coors, W.; Readey, D.J. Proton Conductivity Measurements in Yttrium Barium Cerate by Impedance Spectroscopy. J. Am. Ceram. Soc. 2002, 85, 2637–2640. [Google Scholar] [CrossRef]
- Song, J.; Birdja, Y.Y.; Pant, D.; Chen, Z.; Vaes, J. Recent progress in the structure optimization and development of proton-conducting electrolyte materials for low-temperature solid oxide cells. Int. J. Miner. Metal. Mater. 2022, 29, 848–869. [Google Scholar] [CrossRef]
- Medvedev, D.; Murashkina, A.; Pikalova, E.; Demin, A.; Podias, A.; Tsiakaras, P. BaCeO3: Materials development, properties and application. Prog. Mater. Sci. 2014, 60, 72–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Liang, L.; Sai, Q.; Ni, J.; Au, C.; Lin, X.; Wang, X.; Zheng, Y.; Zheng, L.; et al. Unraveling the size-dependent effect of Ru-based catalysts on Ammonia synthesis at mild conditions. J. Catal. 2021, 404, 501–511. [Google Scholar] [CrossRef]
- Owaku, T.; Iida, Y.; Fukawa, H.; Higuchi, T. Structural and electrical properties of BaCe0.90Y0.10O3-δ thin film on Al2O3 substrate. Trans. Mater. Res. Soc. Jpn. 2012, 37, 81–84. [Google Scholar] [CrossRef]
- Li, Y.; Su, P.-C.; Wong, L.M.; Wang, S. Chemical stability study of nanoscale thin film yttria-doped barium cerate electrolyte for micro solid oxide fuel cells. J. Power Sources 2014, 268, 804–809. [Google Scholar] [CrossRef]
- Dubala, S.U.; Bhosalea, C.H.; Jadhav, L.D. Performance of spray deposited Gd-doped barium cerate thin films for proton conducting SOFCs. Ceram. Int. 2015, 41, 5607–5613. [Google Scholar] [CrossRef]
- Lo Presti, F.; Pellegrino, A.L.; Malandrino, G. Journey of a molecule from the solid to the gas phase and vice versa: Direct estimation of vapor pressure of alkaline-earth metalorganic precursors for atmospheric pressure vapor phase deposition of fluoride films. Dalton Trans. 2022, 51, 7352–7362. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, G.; Lo Nigro, R.; Benelli, C.; Castelli, F.; Fragalà, I.L. Volatile CeIII Hexafluoroacetylacetonate Glyme Adducts as Promising Precursors for the MOCVD of CeO2 Thin Films. Chem. Vap. Depos. 2000, 6, 233–238. [Google Scholar] [CrossRef]
- Malandrino, G.; Lo Nigro, R.; Fragalà, I.L.; Benelli, C. Yttrium β-Diketonate Glyme MOCVD Precursors: Effects of the Polyether Length on Stabilities, Mass Transport Properties and Coordination Spheres. Eur. J. Inorg. Chem. 2004, 2004, 500–511. [Google Scholar] [CrossRef]
- Lo Nigro, R.; Fisichella, G.; Battiato, S.; Greco, G.; Fiorenza, P.; Roccaforte, F.; Malandrino, G. An insight into the epitaxial nanostructures of NiO and CeO2 thin film dielectrics for AlGaN/GaN heterostructures. Mater. Chem. Phys. 2015, 162, 461–468. [Google Scholar] [CrossRef]
- Micard, Q.; Pellegrino, A.L.; Lo Nigro, R.; Bartasyte, A.; Condorelli, G.G.; Malandrino, G. Piezoelectric Ba and Ti co-doped BiFeO3 textured films: Selective growth of solid solutions or nanocomposites. J. Mater. Chem. C 2020, 8, 16168–16179. [Google Scholar] [CrossRef]
- Malandrino, G.; Grigoli, G.; Fragalà, I.L. Heteroepitaxial YAlO3 Films on (100) SrTiO3 Substrates: The Use of Pole Figures as a Non-invasive Tool to Assess the Direction of Growth. Chem. Vap. Depos. 2008, 14, 46–50. [Google Scholar] [CrossRef]
- Pellegrino, A.L.; Lucchini, G.; Speghini, A.; Malandrino, G. Energy conversion systems: Molecular architecture engineering of metal precursors and their applications to vapor phase and solution routes. J. Mater. Res. 2020, 35, 2950–2966. [Google Scholar] [CrossRef]
- Melekh, B.-T.; Egorov, V.M.; Baikov, Y.M.; Kartenko, N.F.; Filin, Y.N.; Kompan, M.E.; Novak, I.I.; Venus, G.B.; Kulik, V.B. Structure, phase transitions and optical properties of pure and rare earth doped BaCeO3, SrCeO3 prepared by inductive melting. Solid State Ion. 1997, 97, 465–470. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lo Presti, F.; Pellegrino, A.L.; Malandrino, G. Metal-Organic Chemical Vapor Deposition of Oxide Perovskite Films: A Facile Route to Complex Functional Systems. Adv. Mater. Interfaces 2022, 9, 2102501. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Y.; Zheng, W.; Li, H.; Wu, X.; Hu, X.; Zhang, X.; Zhu, X.; Zhang, Q.; Wang, X.; et al. Controllable Growth and Formation Mechanisms of Dislocated WS2 Spirals. Nano Lett. 2018, 18, 3885–3892. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, A.L.; Lo Presti, F.; Malandrino, G. Metalorganic Chemical Vapor Deposition Approach to the Synthesis of Perovskite BaCeO3 and BaCe0.8Y0.2O3 Thin Films. Molecules 2023, 28, 3303. https://doi.org/10.3390/molecules28083303
Pellegrino AL, Lo Presti F, Malandrino G. Metalorganic Chemical Vapor Deposition Approach to the Synthesis of Perovskite BaCeO3 and BaCe0.8Y0.2O3 Thin Films. Molecules. 2023; 28(8):3303. https://doi.org/10.3390/molecules28083303
Chicago/Turabian StylePellegrino, Anna L., Francesca Lo Presti, and Graziella Malandrino. 2023. "Metalorganic Chemical Vapor Deposition Approach to the Synthesis of Perovskite BaCeO3 and BaCe0.8Y0.2O3 Thin Films" Molecules 28, no. 8: 3303. https://doi.org/10.3390/molecules28083303
APA StylePellegrino, A. L., Lo Presti, F., & Malandrino, G. (2023). Metalorganic Chemical Vapor Deposition Approach to the Synthesis of Perovskite BaCeO3 and BaCe0.8Y0.2O3 Thin Films. Molecules, 28(8), 3303. https://doi.org/10.3390/molecules28083303