One Pot Synthesis, Biological Efficacy of AuNPs and Au-Amoxicillin Conjugates Functionalized with Crude Flavonoids Extract of Micromeria biflora
Abstract
:1. Introduction
2. Results
2.1. Synthesis of AuNPs and Au-Amoxicillin Conjugate
 |
| A schematic representation of AuNP and Au-amoxi synthesis. |
UV–Visible Spectroscopy (Uv–Vis)
2.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3. Scanning Electron Microscopy (SEM)
2.4. XRD, EDX, and TGA Analyses
2.5. Zeta Potential
2.6. Effect of pH and NaCl on AuNPs Stability
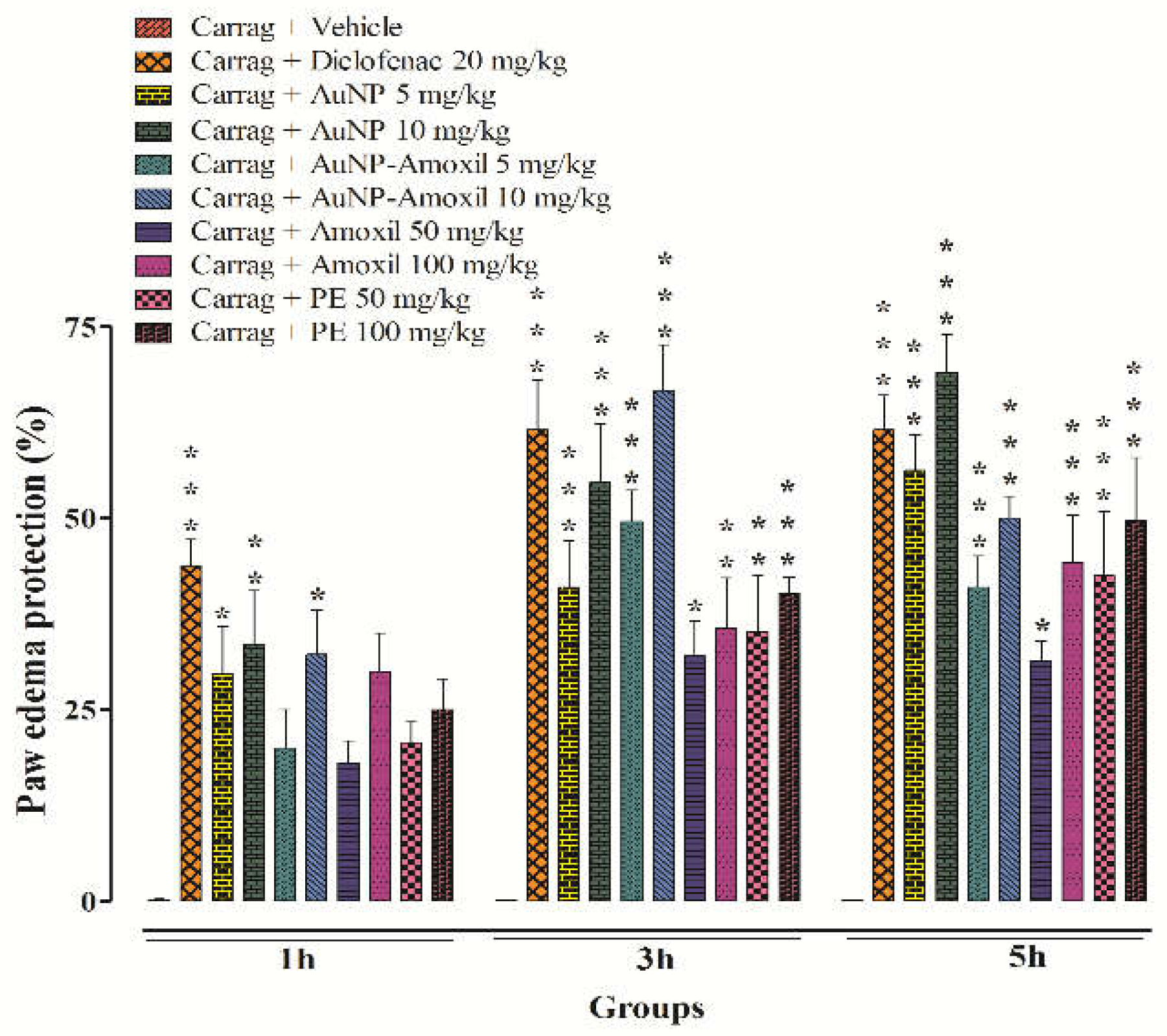
2.7. Biological Efficacy
2.7.1. Inflammatory and Antinociceptive Activities
2.7.2. Writhing Test
2.7.3. Hot Plate Test
3. Discussion
4. Experimental Section
4.1. Materials and Methods
4.2. Preparation of Crude Flavonoids Micromeria Extract
4.3. Preparation of Amoxicillin Solution
4.4. Synthesis of AuNPs Using Crude Flavonoids Extract of Micromeria biflora
Synthesis of Gold Nanoparticles-Amoxicillin Conjugate (AuNPs-amoxi)
4.5. Characterization Techniques
4.6. Bioactivities Tests
4.6.1. In Vivo Studies: Anti-Inflammatory and Antinociceptive Activities
In Vivo Carrageenan-Induced Hind Paw Edema Model
4.6.2. Antinociceptive Activities
Acetic-Acid-Induced Writhing Test
Hot Plate Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Ejobi, F.; Tweyongyere, R.; Lubwama, M.; Kirabira, J.B.; Wampande, E.M. Nanotechnological solutions for controlling transmission and emergence of antimicrobial-resistant bacteria, future prospects, and challenges: A systematic review. J. Nanopart. Res. 2020, 22, 117. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.; Bari, A.; Ullah, R.; Mathanmohun, M.; Veeraraghavan, V.P.; Sun, Z. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J. Photochem. Photobiol. B Biol. 2019, 201, 111643. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khan, R.S.; Zahoor, M.; Islam, N.U.; Khan, T.; Muhammad, Z.; Ullah, R.; Bari, A. Alnus nitida and urea-doped Alnus nitida-based silver nanoparticles synthesis, characterization, their effects on the biomass and elicitation of secondary metabolites in wheat seeds under in vitro conditions. Heliyon 2023, 9, e14579. [Google Scholar] [CrossRef]
- Khuda, F.; Jamil, M.; Khalil, A.A.K.; Ullah, R.; Ullah, N.; Naureen, F.; Abbas, M.; Khan, M.S.; Ali, S.; Farooqi, H.M.U.; et al. Assessment of antioxidant and cytotoxic potential of silver nanoparticles synthesized from root extract of Reynoutria japonica Houtt. Arab. J. Chem. 2022, 15, 104327. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. Int. 2020, 27, 19151–19168. [Google Scholar] [CrossRef]
- Ocsoy, I.; Yusufbeyoglu, S.; Yılmaz, V.; McLamore, E.S.; Ildız, N.; Ülgen, A. DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-Resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2017, 159, 16–22. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Bonder, M.J.; Balakrishnan, S.; Wang, X.; Mao, H.; Hadjipanayis, G.C. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small 2008, 4, 1925–1929. [Google Scholar] [CrossRef]
- Hutter, E.; Maysinger, D. Gold-nanoparticle-based biosensors for detection of enzyme activity. Trends Pharmacol. Sci. 2013, 34, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Parida, U.K.; Bindhani, B.K.; Nayak, P. Green Synthesis and Characterization of Gold Nanoparticles Using Onion (Allium cepa) Extract. World J. Nano Sci. Eng. 2011, 1, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Singh, A.K.; Talat, M.; Singh, D.P.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Ling, L.; Jiang, Y.; Liu, Y.; Li, H.; Bari, A.; Ullah, R.; Xue, J. Role of gold nanoparticle from Cinnamomum verum against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) induced mice model. J. Photochem. Photobiol. B: Biol. 2019, 201, 111657. [Google Scholar] [CrossRef]
- Gatadi, S.; Madhavi, Y.V.; Nanduri, S. Nanoparticle drug conjugates treating microbial and viral infections: A review. J. Mol. Struct. 2021, 1228, 129750. [Google Scholar] [CrossRef]
- Masri, A.; Anwar, A.; Khan, N.A.; Siddiqui, R. The Use of Nanomedicine for Targeted Therapy against Bacterial Infections. Antibiotics 2019, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Hagbani, T.A.; Yadav, H.; Moin, A.; Lila, A.S.A.; Mehmood, K.; Alshammari, F.; Khan, S.; Khafagy, E.S.; Hussain, T.; Rizvi, S.M.D.; et al. Enhancement of Vancomycin Potential against Pathogenic Bacterial Strains via Gold Nano-Formulations: A Nano-Antibiotic Approach. Materials 2022, 15, 1108. [Google Scholar] [CrossRef]
- Muenraya, P.; Sawatdee, S.; Srichana, T.; Atipairin, A. Silver Nanoparticles Conjugated with Colistin Enhanced the Antimicrobial Activity against Gram-Negative Bacteria. Molecules 2022, 27, 5780. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Hosny, S. Green Synthesis of Ag and Au Nanoparticles from Micro and Macro Algae—Review. Int. J. Atmos. Ocean. Sci. 2018, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef]
- Uchiyama, M.K.; Hebeda, C.B.; Sandri, S.; Paula-Silva, M.D.; Romano, M.; Cardoso, R.M.; Toma, S.H.; Araki, K.; Farsky, S.H. In vivo evaluation of toxicity and anti-inflammatory activity of iron oxide nanoparticles conjugated with ibuprofen. Nanomedicine 2021, 16, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ahn, S.; Kang, J.P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [PubMed] [Green Version]
- Gul, A.; Shaheen, A.; Ahmad, I.; Khattak, B.; Ahmad, M.; Ullah, R.; Bari, A.; Ali, S.S.; Alobaid, A.; Asmari, M.M.; et al. Green Synthesis, Characterization, Enzyme Inhibition, Antimicrobial Potential, and Cytotoxic Activity of Plant Mediated Silver Nanoparticle Using Ricinus communis Leaf and Root Extracts. Biomolecules 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Hilton, S.; Liechty, K.; Zgheib, C.; Dewberry, L.C.; Hodges, M.; Hu, J.; Xu, J.; Seal, S.; Nozik-Grayck, E. Cerium Oxide Nanoparticle Conjugated with MicroRNA-146a Decreases Lung Inflammation and Fibrosis in Bleomycin Murine Model. Pediatrics 2019, 144, 359. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, Z.Y.; Sun, C.S.; Wang, C.Y.; Jiang, T.Y.; Wang, S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials 2010, 31, 908–915. [Google Scholar] [CrossRef]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Hussain, T.; Alshammari, T.M.; Ahmad, W.; Tabrez, S.; Al-Qahtani, M.H.; Abuzenadah, A.M. Synthesis and Characterization of Cefotaxime Conjugated Gold Nanoparticles and Their Use to Target Drug-Resistant CTX-M-Producing Bacterial Pathogens. J. Cell. Biochem. 2017, 118, 2802–2808. [Google Scholar] [CrossRef]
- Qi, R.; Wang, Y.; Bruno, P.M.; Xiao, H.; Yu, Y.; Li, T.; Lauffer, S.; Wei, W.; Chen, Q.; Kang, X.; et al. Nanoparticle conjugates of a highly potent toxin enhance safety and circumvent platinum resistance in ovarian cancer. Nat. Commun. 2017, 8, 2166. [Google Scholar] [CrossRef] [Green Version]
- Park, Y. A New Paradigam Shift for Green Synthesis of the Antibacterial Agnps Utilizing Plant Extracts. Toxicol. Res. 2014, 30, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Yahyaei, B.; Pourali, P. One step conjugation of some chemotherapeutic drugs to the biologically produced gold nanoparticles and assessment of their anticancer effects. Sci. Rep. 2019, 9, 10242. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xia, R.; Hu, H.; Peng, T. Biosynthesis, characterization and cytotoxicity of gold nanoparticles and their loading with N-acetylcarnosine for cataract treatment. J. Photochem. Photobiol. B 2018, 187, 180–183. [Google Scholar] [CrossRef]
- Ramadan, A.K.M. Manipulation of Nanoparticles to Control Multidrug Resistant Bacteria—Manipulation-of-nanoparticles-to-control-multidrug-resistant-bacteria. J. Pharmacol. Rev. Rep. 2017, 1, 2. [Google Scholar]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Melhus, Å. Effects of amoxicillin on the expression of cytokines during experimental acute otitis media caused by non-typeable Haemophilus influenzae. J. Antimicrob. Chemother. 2001, 48, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Picoli, S.U.; Mazzoleni, L.E.; Fernández, H.; De Bona, L.R.; Neuhauss, E.; Longo, L.; Prolla, J.C. Resistance to amoxicillin, clarithromycin and ciprofloxacin of Helicobacter pylori isolated from Southern Brazil patients. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 197–200. [Google Scholar] [CrossRef]
- Wyckoff, R.W. Cubic closest packed, ccp, structure. Cryst. Struct. 1963, 1, 7–83. [Google Scholar]
- Sun, D. Effect of Zeta Potential and Particle Size on the Stability of SiO2 Nanospheres as Carrier for Ultrasound Imaging Contrast Agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Haider, M.J.; Mehdi, M.S. Study of morphology and Zeta Potential analyzer for the Silver Nanoparticles. Int. J. Sci. Eng. Res. 2014, 5, 381–387. [Google Scholar]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2019, 8, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Akkam, Y.; Al Zoubi, M.S.; Al-Batayneh, K.M.; Al-Trad, B.; Abo Alrob, O.; Alkilany, A.M.; Benamara, M.; Evans, D.J. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus and their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef] [Green Version]
- Titus, D.; Samuel, E.J.J.; Roopan, S.M. Chapter 12—Nanoparticle characterization techniques. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–319. [Google Scholar]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Ilahi, I.; Khuda, F.; Sahibzada, M.U.K.; Alghamdi, S.; Rahim, U.; Dablool, A.S.; Alam, M.; Khan, A.; Khalil, A.A.K. Synthesis of silver nanoparticles using root extract of Duchesnea indica and assessment of its biological activities. Arab. J. Chem. 2021, 14, 103110. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis. Biotechnol. Adv. 2018, 36, 1709–1723. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Bukhari, I.A.; Gilani, A.H.; Meo, S.A.; Saeed, A. Analgesic, anti-inflammatory and anti-platelet activities of Buddleja crispa. BMC Complement. Altern. Med. 2016, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Lingadurai, S.; Nath, L.K.; Kar, P.K.; Besra, S.E.; Joseph, R.V. Anti-inflammatory and anti-nociceptive activities of methanolic extract of the leaves of Fraxinus floribunda Wallich. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Munir, A.; Khushal, A.; Saeed, K.; Sadiq, A.; Rahim, U.; Ali, G.; Ashraf, Z.; Mughal, E.U.; Jan, M.S.; Rashid, U.; et al. Synthesis, in-vitro, in-vivo anti-inflammatory activities and molecular docking studies of acyl and salicylic acid hydrazide derivatives. Bioorg. Chem. 2020, 104, 104168. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil, K.; Ahmad, S.; Islam, N.; Ullah, R.; Jalil, Q.; Sulaiman, S.; Sajjad, A.; Ullah, R.; Alqahtani, A.S.; Bari, A.; et al. One Pot Synthesis, Biological Efficacy of AuNPs and Au-Amoxicillin Conjugates Functionalized with Crude Flavonoids Extract of Micromeria biflora. Molecules 2023, 28, 3320. https://doi.org/10.3390/molecules28083320
Jalil K, Ahmad S, Islam N, Ullah R, Jalil Q, Sulaiman S, Sajjad A, Ullah R, Alqahtani AS, Bari A, et al. One Pot Synthesis, Biological Efficacy of AuNPs and Au-Amoxicillin Conjugates Functionalized with Crude Flavonoids Extract of Micromeria biflora. Molecules. 2023; 28(8):3320. https://doi.org/10.3390/molecules28083320
Chicago/Turabian StyleJalil, Kamran, Shabir Ahmad, Nazrul Islam, Rahim Ullah, Qudsia Jalil, Sulaiman Sulaiman, Anoosha Sajjad, Riaz Ullah, Ali S. Alqahtani, Ahmed Bari, and et al. 2023. "One Pot Synthesis, Biological Efficacy of AuNPs and Au-Amoxicillin Conjugates Functionalized with Crude Flavonoids Extract of Micromeria biflora" Molecules 28, no. 8: 3320. https://doi.org/10.3390/molecules28083320
APA StyleJalil, K., Ahmad, S., Islam, N., Ullah, R., Jalil, Q., Sulaiman, S., Sajjad, A., Ullah, R., Alqahtani, A. S., Bari, A., Hussain, H., & Ali, E. A. (2023). One Pot Synthesis, Biological Efficacy of AuNPs and Au-Amoxicillin Conjugates Functionalized with Crude Flavonoids Extract of Micromeria biflora. Molecules, 28(8), 3320. https://doi.org/10.3390/molecules28083320






