Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants
Abstract
:1. Introduction
2. Results and Discussion
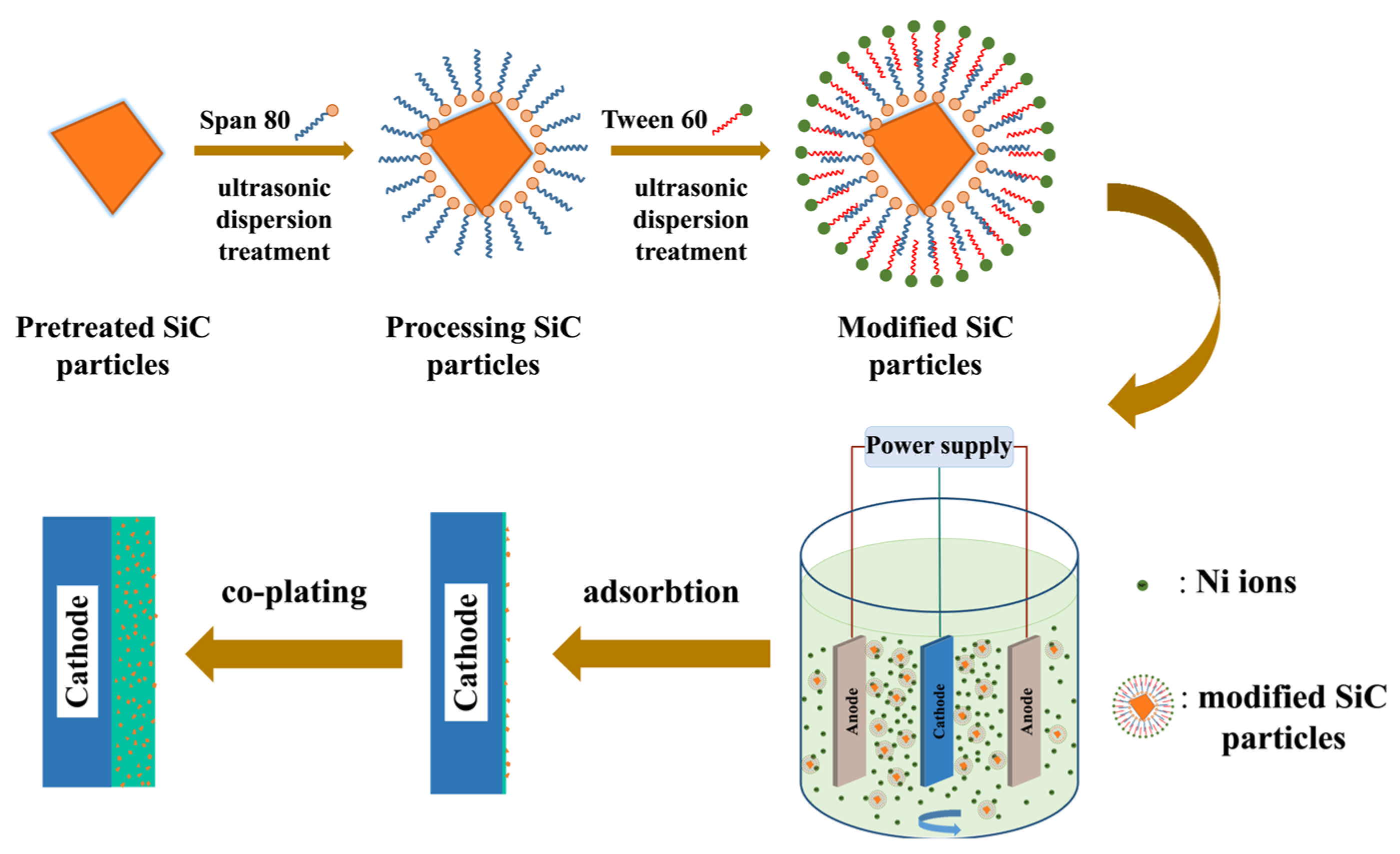
2.1. Modification and Dispersion of SiC Particles
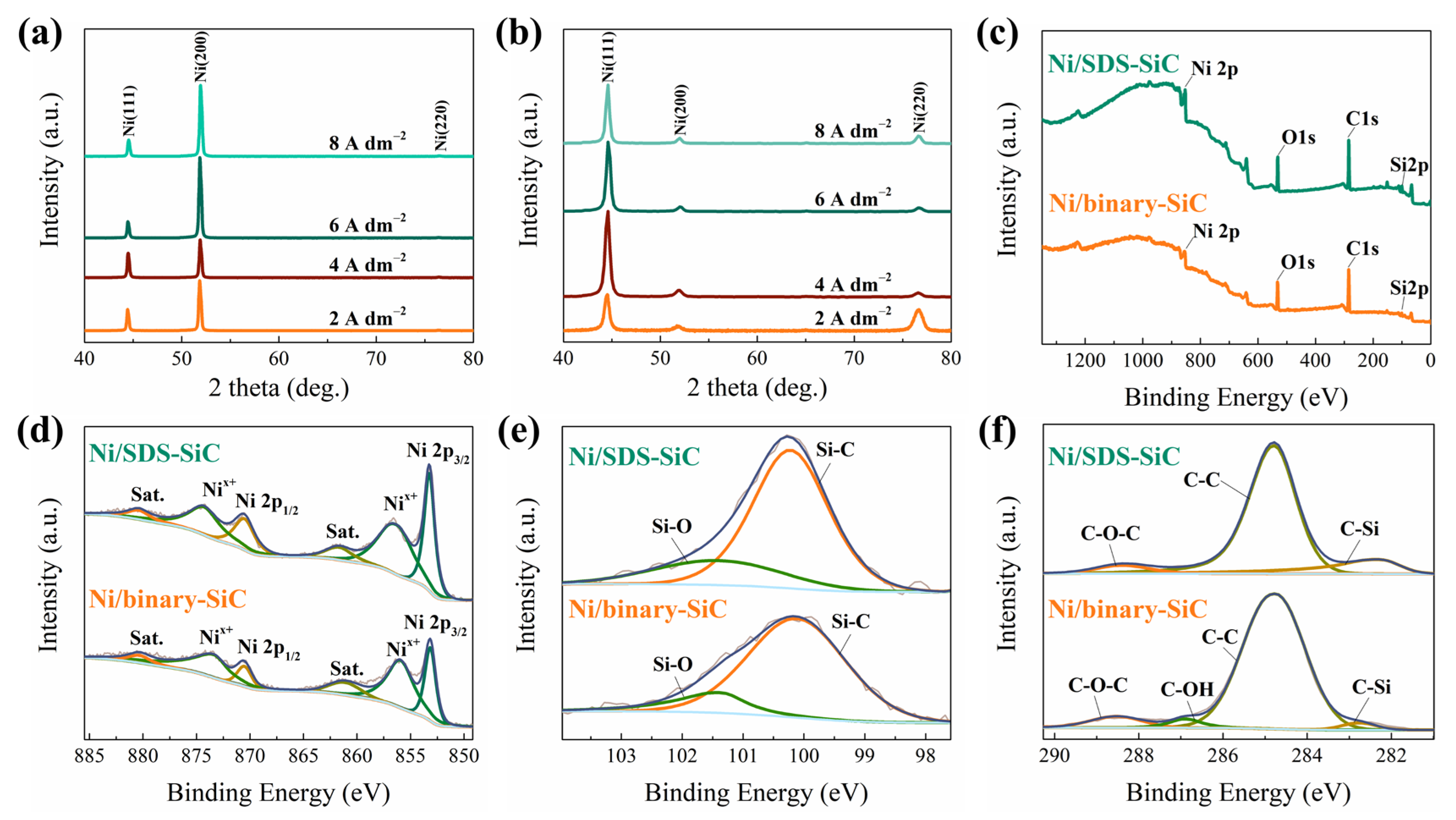
2.2. Phase Structure and Grain Size
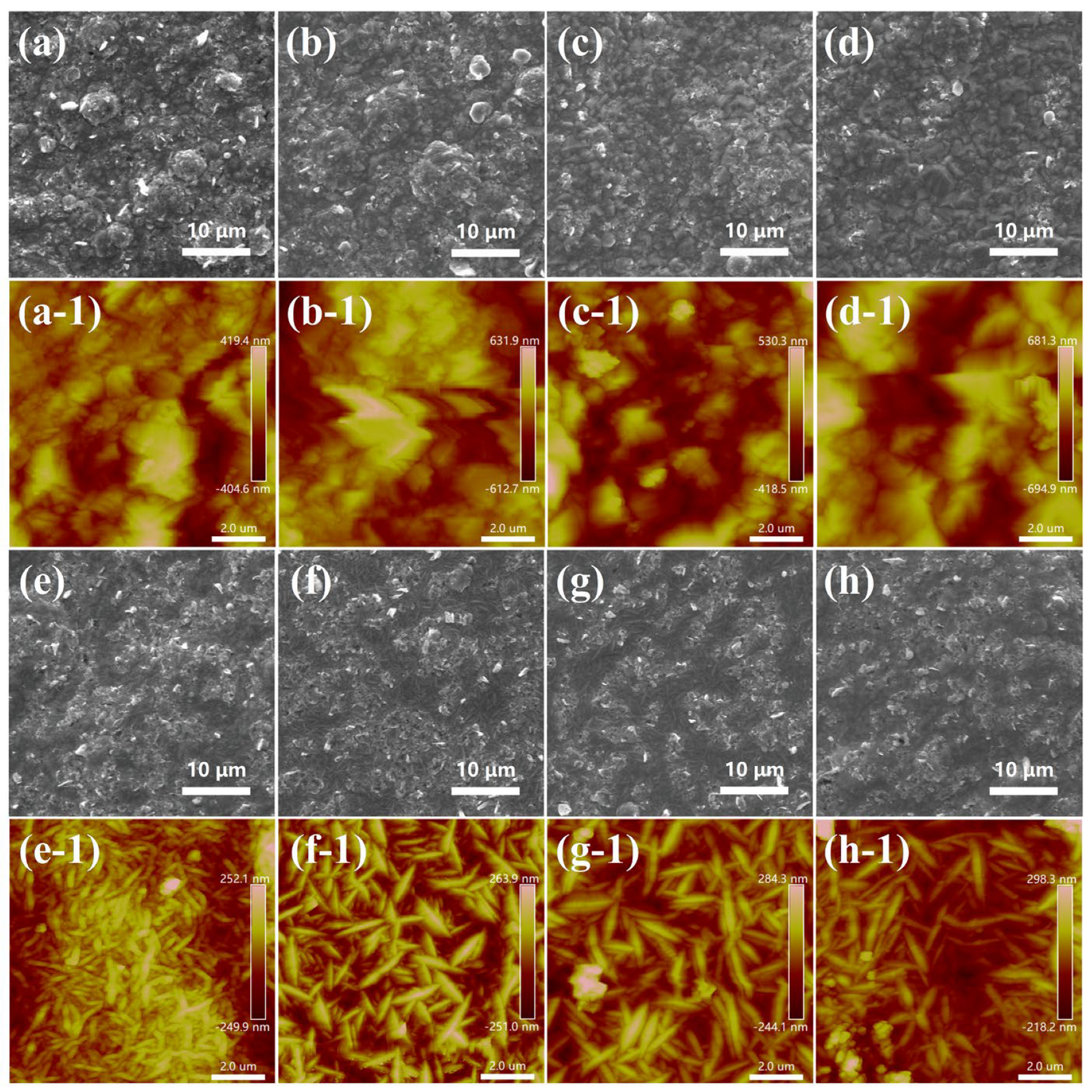
2.3. Morphology of Composite Coatings
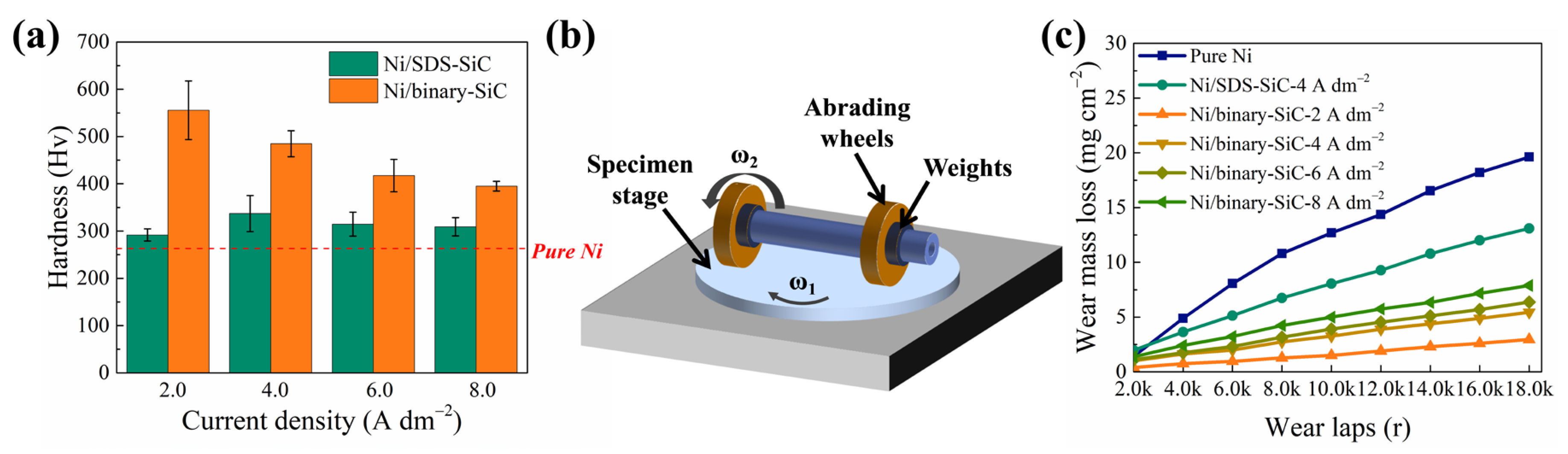
2.4. Hardness and Wear-Resisting Properties
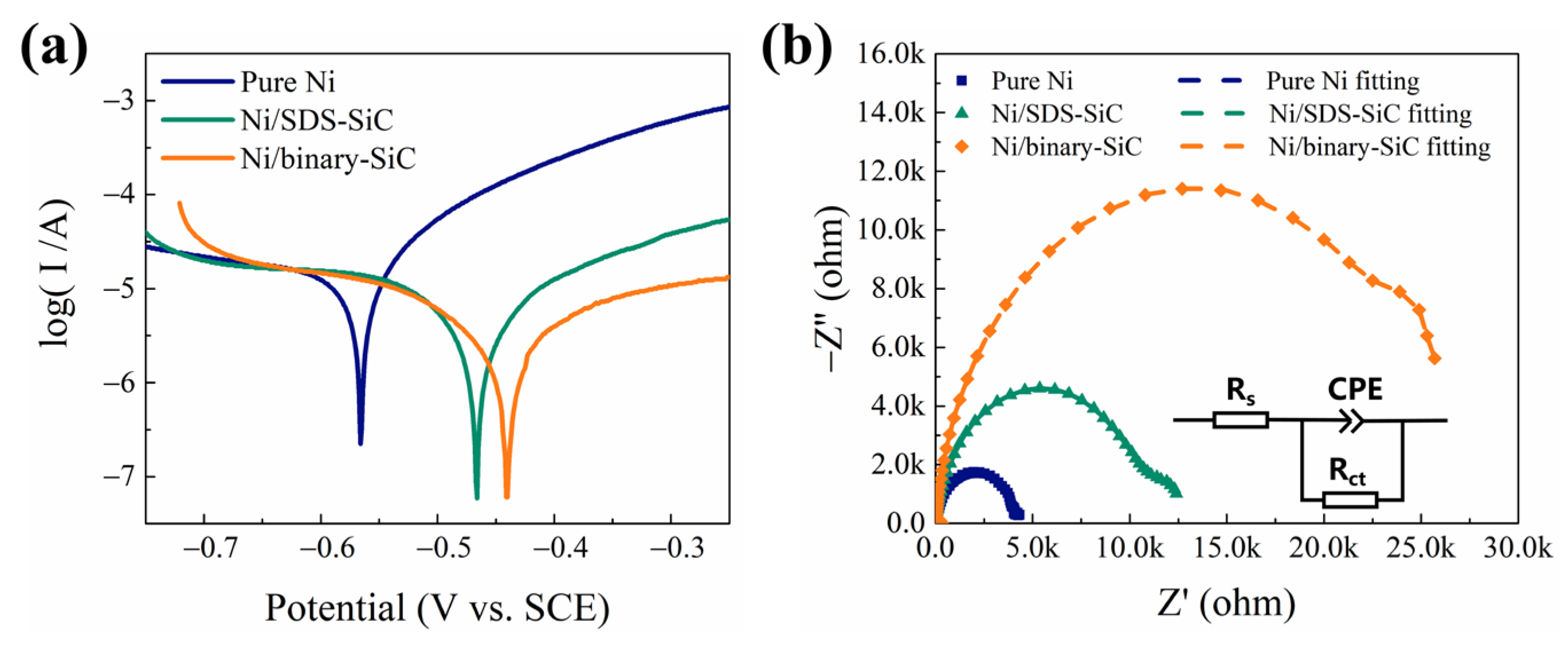
2.5. Corrosion Resistance
3. Materials and Methods
3.1. Materials
3.2. Electrodeposition
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huczko, A.; Dąbrowska, A.; Savchyn, V.; Popov, A.I.; Karbovnyk, I. Silicon carbide nanowires: Synthesis and cathodoluminescence. Phys. Status Solidi B 2009, 246, 2806–2808. [Google Scholar] [CrossRef]
- Cao, G.P.; Konishi, H.; Li, X.C. Mechanical properties and microstructure of Mg/SiC nanocomposites fabricated by ultrasonic cavitation based nanomanufacturing. J. Manuf. Sci. Eng. Trans. Asme 2008, 130, 031105. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, L.; Zhong, W.; Wang, S.; Liu, J.; Zhang, C. Damage and annealing behavior in neutron-irradiated SiC used as a post-irradiation temperature monitor. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2022, 512, 91–95. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, J.D.; Chen, S.X.; Wang, H.; He, Y.; Ma, C.Y. Ni-SiC composite coatings with improved wear and corrosion resistance synthesized via ultrasonic electrodeposition. Ceram. Int. 2021, 47, 9437–9446. [Google Scholar] [CrossRef]
- Garcia, I.; Fransaer, J.; Celis, J.P. Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni-SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Zhao, K.N.; Wei, G.Y.; Ge, H.L.; Yu, Y.D. Study on Performance of Co-W/SiC Composite Coatings Electrodeposited on Copper. Int. J. Electrochem. Sci. 2021, 16, 210241. [Google Scholar] [CrossRef]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, M.; Liggieri, L. Effect of nanoparticles on the interfacial properties of liquid/liquid and liquid/air surface layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef]
- Yan, Y.T.; Lu, L.F.; Huo, Y.Q. Dry Sliding Tribological Behaviors of Electrodeposited Ni-GO/SiC Composite Coating on the 2218 Aluminum Alloy. Materials 2022, 15, 2834. [Google Scholar] [CrossRef]
- Ger, M.D. Electrochemical deposition of nickel/SiC composites in the presence of surfactants. Mater. Chem. Phys. 2004, 87, 67–74. [Google Scholar] [CrossRef]
- Kan, H.M.; Meng, Y.Y.; Reddy, R.G. Influence of particle size and surfactants on uniformity and quantity of silicon carbide particles in electrodeposited nickel-silicon carbide coatings. J. Cent. South Univ. 2021, 28, 1627–1636. [Google Scholar] [CrossRef]
- Jiang, S.W.; Yang, L.; Pang, J.N.; Lin, H.; Wang, Z.Q. Electrodeposition of Ni-Al2O3 composite coatings with combined addition of SDS and HPB surfactants. Surf. Coat. Technol. 2016, 286, 197–205. [Google Scholar] [CrossRef]
- Afroukhteh, S.; Dehghanian, C.; Emamy, M. Corrosion behavior of Ni-P/nano-TiC composite coating prepared in electroless baths containing different types of surfactant. Prog. Nat. Sci. Mater. Int. 2012, 22, 480–487. [Google Scholar] [CrossRef]
- Sabri, M.; Sarabi, A.A.; Kondelo, S.M.N. The effect of sodium dodecyl sulfate surfactant on the electrodeposition of Ni-alumina composite coatings. Mater. Chem. Phys. 2012, 136, 566–569. [Google Scholar] [CrossRef]
- Qin, L.J.; Huang, Y.C. Improvement in Cr Nanoparticle Content in Ni-Cr Film by Co-deposition with Combined Surfactant HPB and CTAB. Acta Metall. Sin. Engl. Lett. 2017, 30, 999–1007. [Google Scholar] [CrossRef]
- Parida, G.; Chaira, D.; Chopkar, M.; Basu, A. Synthesis and characterization of Ni-TiO2 composite coatings by electro-co-deposition. Surf. Coat. Technol. 2011, 205, 4871–4879. [Google Scholar] [CrossRef]
- Hou, K.H.; Ger, M.D.; Wang, L.M.; Ke, S.T. The wear behaviour of electro-codeposited Ni-SiC composites. Wear 2002, 253, 994–1003. [Google Scholar] [CrossRef]
- Masalova, I.; Kharatyan, E. Effect of silica particles on stability of highly concentrated water-in-oil emulsions with non-ionic surfactant. Colloid J. 2013, 75, 95–102. [Google Scholar] [CrossRef]
- Czagany, M.; Baumli, P. Effect of surfactants on the behavior of the Ni-P bath and on the formation of electroless Ni-P-TiC composite coatings. Surf. Coat. Technol. 2019, 361, 42–49. [Google Scholar] [CrossRef]
- Djaoued, Y.; Priya, S.; Balaji, S. Low temperature synthesis of nanocrystalline WO3 films by sol-gel process. J. Non-Cryst. Solids 2008, 354, 673–679. [Google Scholar] [CrossRef]
- Luo, Z.B.; Su, Y.C.; Yue, S.X.; Yu, Q.S.; Tursun, R.; Zhang, J. Effect of Fluorocarbon Surfactant on Electroforming of Copper Nano-Powders. Int. J. Electrochem. Sci. 2021, 16, 210232. [Google Scholar] [CrossRef]
- Kazimierczak, H.; Szymkiewicz, K.; Gileadi, E.; Eliaz, N. The Effect of Direct and Pulsed Current in the Presence of Surfactants on the Electrodeposition of Zn-SiC Nanocomposite Coatings. Coatings 2019, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Wang, J.C.; Chen, T.C. Analysis of suspension and heat transfer characteristics of Al2O3 nanofluids prepared through ultrasonic vibration. Appl. Energy 2011, 88, 4527–4533. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; McClements, D.J. Modification of interfacial characteristics of monodisperse droplets produced using membrane emulsification by surfactant displacement and/or polyelectrolyte electrostatic deposition. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Martinsson, E.; Shahjamali, M.M.; Large, N.; Zaraee, N.; Zhou, Y.; Schatz, G.C.; Mirkin, C.A.; Aili, D. Influence of surfactant bilayers on the refractive index sensitivity and catalytic properties of anisotropic gold nanoparticles. Small 2016, 12, 330–342. [Google Scholar] [CrossRef]
- Sharma, K.P.; Aswal, V.K.; Kumaraswamy, G. Adsorption of nonionic surfactant on silica nanoparticles: Structure and resultant interparticle interactions. J. Phys. Chem. B 2010, 114, 10986–10994. [Google Scholar] [CrossRef]
- Boakye-Ansah, S.; Khan, M.A.; Haase, M.F. Controlling surfactant adsorption on highly charged nanoparticles to stabilize bijels. J. Phys. Chem. C 2020, 124, 12417–12423. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Shin, S.H.R.; Abezgauz, L.L.; Lewis, S.A.; Chirsan, A.M.; Danino, D.D.; Bishop, K.J.M. Integration of gold nanoparticles into bilayer structures via adaptive surface chemistry. J. Am. Chem. Soc. 2013, 135, 5950–5953. [Google Scholar] [CrossRef]
- Jiménez-Ángeles, F.; Khoshnood, A.; Firoozabadi, A. Molecular dynamics simulation of the adsorption and aggregation of ionic surfactants at liquid–solid interfaces. J. Phys. Chem. C 2017, 121, 25908–25920. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009. [Google Scholar] [CrossRef]
- Karbovnyk, I.; Savchyn, P.; Huczko, A.; Cestelli Guidi, M.; Mirri, C.; Popov, A. FTIR studies of silicon carbide 1D-nanostructures. Mater. Sci. Forum 2015, 821–823, 261–264. [Google Scholar] [CrossRef]
- Vahid, N.F.; Marvi, M.R.; Naimi-Jamal, M.R.; Naghib, S.M.; Ghaffarinejad, A. Effect of surfactant type on buckypaper electrochemical performance. Micro Nano Lett. 2018, 13, 927–930. [Google Scholar] [CrossRef]
- Yi, J.; He, X.; Sun, Y.; Li, Y. Optical properties of SIC/SIO2 composite thin film. Microw. Opt. Technol. Lett. 2007, 49, 1551–1553. [Google Scholar] [CrossRef]
- Zhang, J.; Jianga, D.; Tana, S.; Gui, L.; Ruan, M.L. Aqueous processing of SiC green sheets I: Dispersant. J. Mater. Res. 2002, 17, 2012–2018. [Google Scholar] [CrossRef]
- Somasundaran, P.; Snell, E.; Fu, E.; Xu, Q. Effect of adsorption of non-ionic surfactant and non-ionic—Anionic surfactant mixtures on silica—Liquid interfacial properties. Colloids Surf. 1992, 63, 49–54. [Google Scholar] [CrossRef]
- White, B.; Banerjee, S.; O’Brien, S.; Turro, N.J.; Herman, I.P. Zeta-potential measurements of surfactant-wrapped individual single-walled carbon nanotubes. J. Phys. Chem. C 2007, 111, 13684–13690. [Google Scholar] [CrossRef]
- Prosser, A.J.; Franses, E.I. Adsorption and surface tension of ionic surfactants at the air-water interface: Review and evaluation of equilibrium models. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 1–40. [Google Scholar] [CrossRef]
- Benrraou, M.; Bales, B.L.; Zana, R. Effect of the nature of the counterion on the properties of anionic surfactants: 1. Cmc, ionization degree at the cmc and aggregation number of micelles of sodium, cesium, tetramethylammonium, tetraethylammonium, tetrapropylammonium, and tetrabutylammonium dodecyl sulfates. J. Phys. Chem. B 2003, 107, 13432–13440. [Google Scholar]
- Senac, C.; Urbach, W.; Kurtisovski, E.; Hünenberger, P.H.; Horta, B.A.; Taulier, N.; Fuchs, P.F. Simulating bilayers of nonionic surfactants with the GROMOS-compatible 2016H66 force field. Langmuir 2017, 33, 10225–10238. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Haridoss, P.; Seshadri, S.K.; Sundararajan, G. Influence of mode of electrodeposition, current density and saccharin on the microstructure and hardness of electrodeposited nanocrystalline nickel coatings. Surf. Coat. Technol. 2016, 291, 130–140. [Google Scholar] [CrossRef]
- Baghal, S.M.L.; Amadeh, A.; Sohi, M.H.; Hadavi, S.M.M. The effect of SDS surfactant on tensile properties of electrodeposited Ni-Co/SiC nanocomposites. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2013, 559, 583–590. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Liu, H.C.; Zhu, H.Y.; Jiang, X.Y.; Zhu, L.Q.; Li, W.P.; Chen, H.N. Amorphous/amorphous Ni-P/Ni(OH)2 heterostructure nanotubes for an efficient alkaline hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 10169–10179. [Google Scholar] [CrossRef]
- Dalla Torre, F.; Van Swygenhoven, H.; Victoria, M. Nanocrystalline electrodeposited Ni: Microstructure and tensile properties. Acta Mater. 2002, 50, 3957–3970. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Gong, M.; Huang, W. Microstructure and mechanical properties of Ni-P-Si3N4 nanowire electroless composite coatings. Appl. Surf. Sci. 2015, 357, 328–332. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Pavlatou, E.A.; Stroumbouli, M.; Gyftou, P.; Spyrellis, N. Hardening effect induced by incorporation of SiC particles in nickel electrodeposits. J. Appl. Electrochem. 2006, 36, 385–394. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A.; Nasirpouri, F.; Hosseini, M.G. Corrosion resistance of Ni-Co alloy and Ni-Co/SiC nanocomposite coatings electrodeposited by sediment codeposition technique. Appl. Surf. Sci. 2014, 307, 351–359. [Google Scholar] [CrossRef]
- Özkan, S.; Hapçı, G.; Orhan, G.; Kazmanlı, K. Electrodeposited Ni/SiC nanocomposite coatings and evaluation of wear and corrosion properties. Surf. Coat. Technol. 2013, 232, 734–741. [Google Scholar] [CrossRef]
- García, I.; Conde, A.; Langelaan, G.; Fransaer, J.; Celis, J.-P. Improved corrosion resistance through microstructural modifications induced by codepositing SiC-particles with electrolytic nickel. Corros. Sci. 2003, 45, 1173–1189. [Google Scholar] [CrossRef]


| Current Density (A dm−2) | Ni/SDS-SiC | Ni/Binary-SiC | ||||||
|---|---|---|---|---|---|---|---|---|
| Texture Coefficient (%) | Grain size (nm) | Texture Coefficient (%) | Grain Size (nm) | |||||
| (111) | (200) | (220) | (111) | (200) | (220) | |||
| 2.0 | 14.6 | 80.2 | 5.2 | 32 | 18.1 | 10.2 | 71.7 | 14 |
| 4.0 | 20.1 | 72.9 | 7.0 | 32 | 51.8 | 16.0 | 32.2 | 15 |
| 6.0 | 7.9 | 88.6 | 3.5 | 32 | 50.2 | 15.2 | 34.6 | 16 |
| 8.0 | 8.8 | 87.5 | 3.7 | 31 | 38.2 | 15.0 | 46.8 | 19 |
| Coatings | βa (mV dec−1) | βc (mV dec−1) | Ecorr (V) | Icorr (μA cm−2) | Rp (kΩ cm2) |
|---|---|---|---|---|---|
| Pure nickel | 111 | 622 | −0.57 | 21.99 | 1.86 |
| Ni/SDS-SiC | 228 | 345 | −0.47 | 8.78 | 6.79 |
| Ni/binary-SiC | 261 | 221 | −0.44 | 4.74 | 10.41 |
| Coatings | Rs (Ω) | CPE | Rct (kΩ cm2) | |
|---|---|---|---|---|
| Q (Ω−1 cm−2 s−n) | n | |||
| Pure nickel | 0.51 | 1.34 × 10−4 | 0.83 | 4.29 |
| Ni/SDS-SiC | 1.21 | 3.77× 10−5 | 0.93 | 11.02 |
| Ni/binary-SiC | 0.97 | 4.08 × 10−5 | 0.93 | 26.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, H.; Li, W.; Zhao, F.; Song, Y.; Liu, H.; Zhu, L.; Chen, H. Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants. Molecules 2023, 28, 3344. https://doi.org/10.3390/molecules28083344
Rao H, Li W, Zhao F, Song Y, Liu H, Zhu L, Chen H. Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants. Molecules. 2023; 28(8):3344. https://doi.org/10.3390/molecules28083344
Chicago/Turabian StyleRao, Han, Weiping Li, Fuzhen Zhao, Yongfa Song, Huicong Liu, Liqun Zhu, and Haining Chen. 2023. "Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants" Molecules 28, no. 8: 3344. https://doi.org/10.3390/molecules28083344
APA StyleRao, H., Li, W., Zhao, F., Song, Y., Liu, H., Zhu, L., & Chen, H. (2023). Electrodeposition of High-Quality Ni/SiC Composite Coatings by Using Binary Non-Ionic Surfactants. Molecules, 28(8), 3344. https://doi.org/10.3390/molecules28083344





