A Study on the Gaseous Benzene Removal Based on Adsorption onto the Cost-Effective and Environmentally Friendly Adsorbent
Abstract
:1. Introduction
2. Results
2.1. Characterization Results
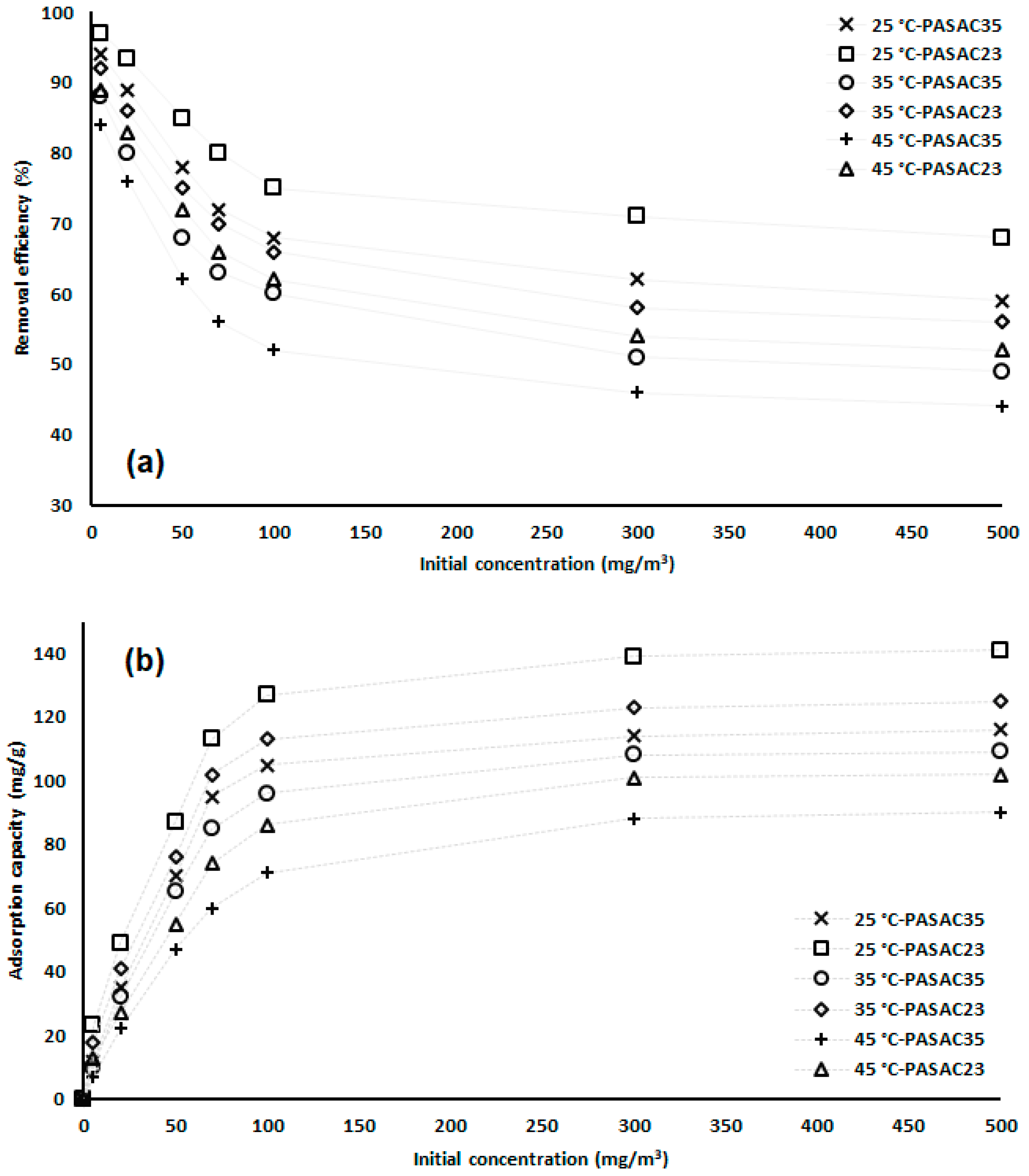
2.2. Benzene Adsorption Capacities of the PASACs
2.3. Effect of Initial Concentration
2.4. Effect of Contact Time
2.5. Regeneration Process
3. Discussion
4. Materials and Methods
4.1. Materials and PASAC Yield
4.2. Environmentally Adsorbent Preparation
4.3. Structural Characterization
4.4. Batch Equilibrium Details
4.5. Quality Control and Quality Assurance
4.6. Recyclability and Reusability of Spent PASAC
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lyu, X.; Guo, H.; Wang, Y.; Zhang, F.; Nie, K.; Dang, J.; Liang, Z.; Dong, S.; Zeren, Y.; Zhou, B.; et al. Hazardous volatile organic compounds in ambient air of China. Chemosphere 2020, 246, 125731. [Google Scholar] [CrossRef]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of Biogenic Volatile Organic Compounds (BVOC) emitted by urban trees on ozone concentration in cities: A review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Garzón, J.P.; Huertas, J.I.; Magaña, M.; Huertas, M.E.; Cárdenas, B.; Watanabe, T.; Maeda, T.; Wakamatsu, S.; Blanco, S. Volatile organic compounds in the atmosphere of Mexico City. Atmos. Environ. 2015, 119, 415–429. [Google Scholar] [CrossRef] [Green Version]
- WHO. Benzene Air Quality Guidelines for Europe, 2nd ed.; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000.
- Zhou, J.; You, Y.; Bai, Z.; Hu, Y.; Zhang, J.; Zhang, N. Health risk assessment of personal inhalation exposure to volatile organic compounds in Tianjin, China. Sci. Total Environ. 2011, 409, 452–459. [Google Scholar] [CrossRef]
- IARC. Summaries & Evaluations: Benzene (Group 1); IARC Monographs on the Carcinogenicity of Chemicals to Humans, Supplement 7; International Agency for Research on Cancer: Lyon, France, 1987; p. 120.
- Gong, Y.; Wei, Y.; Cheng, J.; Jiang, T.; Chen, L.; Xu, B. Health risk assessment and personal exposure to Volatile Organic Compounds (VOCs) in metro carriages—A case study in Shanghai, China. Sci. Total Environ. 2017, 574, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, D.A.; Williams, A.E.; Onasch, T.B.; Wood, E.; Herndon, S.C.; Kolb, C.E.; Knighton, W.B.; Zavala, M.; Molina, L.T.; Marr, L.C. Application of positive matrix factorization to on-road measurements for source apportionment of diesel-and gasoline-powered vehicle emissions in Mexico City. Atmos. Chem. Phys. 2010, 10, 3629–3644. [Google Scholar] [CrossRef] [Green Version]
- Shuai, J.; Kim, S.; Ryu, H.; Park, J.; Lee, C.K.; Kim, G.-B.; Ultra, V.U., Jr.; Yang, W. Health risk assessment of volatile organic compounds exposure near Daegu dyeing industrial complex in South Korea. BMC Public Health 2018, 18, 528. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Yin, Y.; Liu, J.; Dai, X. An eight-city study of volatile organic compounds in Chinese residences: Compounds, concentrations, and characteristics. Sci. Total Environ. 2020, 698, 134137. [Google Scholar] [CrossRef]
- Song, M.; Kim, K.; Cho, C.; Kim, D. Reduction of volatile organic compounds (Vocs) emissions from laundry dry-cleaning by an integrated treatment process of condensation and adsorption. Processes 2021, 9, 1658. [Google Scholar] [CrossRef]
- Mercier, F.; Gilles, E.; Saramito, G.; Glorennec, P.; Le Bot, B. A multi-residue method for the simultaneous analysis in indoor dust of several classes of semi-volatile organic compounds by pressurized liquid extraction and gas chromatography/tandem mass spectrometry. J. Chromatogr. A 2014, 1336, 101–111. [Google Scholar] [CrossRef]
- Marco, E.; Grimalt, J.O. A rapid method for the chromatographic analysis of volatile organic compounds in exhaled breath of tobacco cigarette and electronic cigarette smokers. J. Chromatogr. A 2015, 1410, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Che, X.; Gao, S.; Chen, X.; Pan, M.; Jiang, M.; Zhang, S.; Jia, H.; Duan, Y. Volatile organic compounds emission inventory of organic chemical raw material industry. Atmos. Pollut. Res. 2022, 13, 101276. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and human health impacts of volatile organic compounds: A perspective review. Chemosphere 2022, 313, 137489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Zhang, M. Personal exposure and source characteristics of carbonyl compounds and BTEXs within homes in Beijing, China. Build. Environ. 2013, 61, 210–216. [Google Scholar] [CrossRef]
- Vikrant, K.; Park, C.M.; Kim, K.H.; Kumar, S.; Jeon, E.C. Recent advancements in photocatalyst-based platforms for the destruction of gaseous benzene: Performance evaluation of different modes of photocatalytic operations and against adsorption techniques. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100316. [Google Scholar] [CrossRef]
- Isinkaralar, K. High-efficiency removal of benzene vapor using activated carbon from Althaea officinalis L. biomass as a lignocellulosic precursor. Environ. Sci. Pollut. Res. 2022, 29, 66728–66740. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Rashidi, R.; Khavanin, A. The efficacy of GAC/MgO composite for destructive adsorption of benzene from waste air stream. Chem. Eng. J. 2013, 228, 741–747. [Google Scholar] [CrossRef]
- Alshahrani, F.; Tawabini, B.; Saleh, T.; Alrayaan, M.; Alaama, S.; Nasser, R.; Soupios, P.; Kirmizakis, P.; Mahmoud, M.; Oyehan, T.; et al. Removal of benzene, MTBE and toluene from contaminated waters using biochar-based liquid activated carbon. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Ha, S.H.; Younis, S.A.; Vikrant, K.; Szulejko, J.E.; Kim, K.H. Evidence of the dominant role of particle size in controlling the dynamic adsorption breakthrough behavior of gaseous benzene in a microporous carbon bed system. Chem. Eng. J. 2022, 427, 130977. [Google Scholar] [CrossRef]
- Liu, B.; Younis, S.A.; Kim, K.H. The dynamic competition in adsorption between gaseous benzene and moisture on metal-organic frameworks across their varying concentration levels. Chem. Eng. J. 2021, 421, 127813. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Gullu, G.; Turkyilmaz, A. Experimental study of formaldehyde and BTEX adsorption onto activated carbon from lignocellulosic biomass. Biomass Convers. Biorefinery 2022, 13, 4279–4289. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Gullu, G.; Turkyilmaz, A.; Dogan, M.; Turhan, O. Activated carbon production from horse chestnut shells for hydrogen storage. Int. J. Glob. Warm. 2022, 26, 361–373. [Google Scholar] [CrossRef]
- Shi, G.; He, S.; Chen, G.; Ruan, C.; Ma, Y.; Chen, Q.; Jin, X.; Liu, X.; He, C.; Du, C.; et al. Crayfish shell-based micro-mesoporous activated carbon: Insight into preparation and gaseous benzene adsorption mechanism. Chem. Eng. J. 2022, 428, 131148. [Google Scholar] [CrossRef]
- Osuchowski, Ł.; Szczęśniak, B.; Choma, J.; Jaroniec, M. High benzene adsorption capacity of micro-mesoporous carbon spheres prepared from XAD-4 resin beads with pores protected effectively by silica. J. Mater. Sci. 2019, 54, 13892–13900. [Google Scholar] [CrossRef]
- Tehrani, N.H.M.H.; Alivand, M.S.; Rashidi, A.; Shamskar, K.R.; Samipoorgiri, M.; Esrafili, M.D.; Maklavany, D.M.; Shafiei-Alavijeh, M. Preparation and characterization of a new waste-derived mesoporous carbon structure for ultrahigh adsorption of benzene and toluene at ambient conditions. J. Hazard. Mater. 2020, 384, 121317. [Google Scholar] [CrossRef]
- Gil, E.R.; Ruiz, B.; Lozano, M.S.; Martín, M.J.; Fuente, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Li, S.; Song, K.; Zhao, D.; Rugarabamu, J.R.; Diao, R.; Gu, Y. Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Microporous Mesoporous Mater. 2020, 302, 110220. [Google Scholar] [CrossRef]
- Isinkaralar, K. Theoretical removal study of gas BTEX onto activated carbon produced from Digitalis purpurea L. biomass. Biomass Convers. Biorefinery 2022, 12, 4171–4181. [Google Scholar] [CrossRef]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Raman, A.A.A.; Buthiyappan, A.; Chan, A.A.; Khor, Y.Y. Oxidant-assisted adsorption using lignocellulosic biomass-based activated carbon. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Turkyilmaz, A. Simultaneous adsorption of selected VOCs in the gas environment by low-cost adsorbent from Ricinus communis. Carbon Lett. 2022, 32, 1781–1789. [Google Scholar] [CrossRef]
- Faried, M.; Shameli, K.; Miyake, M.; Hajalilou, A.; Zamanian, A.; Zakaria, Z.; Abouzari-Lotf, E.; Hara, H.; Khairudin, N.B.B.A.; Nordin, M.F. A green approach for the synthesis of silver nanoparticles using ultrasonic radiation’s times in sodium alginate media: Characterization and antibacterial evaluation. J. Nanomater. 2016, 2016, 4941231. [Google Scholar] [CrossRef] [Green Version]
- Hackbarth, F.V.; Girardi, F.; de Souza, S.M.A.G.U.; de Souza, A.A.U.; Boaventura, R.A.; Vilar, V.J. Marine macroalgae Pelvetia canaliculata (Phaeophyceae) as a natural cation exchanger for cadmium and lead ions separation in aqueous solutions. Chem. Eng. J. 2014, 242, 294–305. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.; Ferreira, C.; Botelho, C.M.; Boaventura, R.A. Optimization of nickel biosorption by chemically modified brown macroalgae (Pelvetia canaliculata). Chem. Eng. J. 2012, 193, 256–266. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.S. Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef]
- Salomón, Y.L.D.O.; Georgin, J.; Franco, D.S.; Netto, M.S.; Grassi, P.; Piccilli, D.G.; Oliveira, M.L.; Dotto, G.L. Powdered biosorbent from pecan pericarp (Carya illinoensis) as an efficient material to uptake methyl violet 2B from effluents in batch and column operations. Adv. Powder Technol. 2020, 31, 2843–2852. [Google Scholar] [CrossRef]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M. Low-cost activated carbon preparation from Corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: Study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind. Crop. Prod. 2022, 178, 114546. [Google Scholar] [CrossRef]
- Kim, B.C.; Park, S.W. Fracture toughness of the nano-particle reinforced epoxy composite. Compos. Struct. 2008, 86, 69–77. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef]
- Smith, M.T. Advances in understanding benzene health effects and susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, A.J.; Faridi, S.; Momeniha, F. Temporal variations of atmospheric benzene and its health effects in Tehran megacity (2010–2013). Environ. Sci. Pollut. Res. 2019, 26, 17214–17223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ho, S.S.H.; Ho, K.F.; Lee, S.C.; Yu, J.Z.; Louie, P.K. Characteristics and health impacts of VOCs and carbonyls associated with residential cooking activities in Hong Kong. J. Hazard. Mater. 2011, 186, 344–351. [Google Scholar] [CrossRef]
- Chaiklieng, S.; Suggaravetsiri, P.; Autrup, H. Risk assessment on benzene exposure among gasoline station workers. Int. J. Environ. Res. Public Health 2019, 16, 2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhinjer, H.S.; Singh, A.; Bhattacharya, S.; Jassal, M.; Agrawal, A.K. Metal-organic frameworks functionalized smart textiles for adsorptive removal of hazardous aromatic pollutants from ambient air. J. Hazard. Mater. 2021, 411, 125056. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhu, J. Straw-based activated carbon: Optimization of the preparation procedure and performance of volatile organic compounds adsorption. Materials 2021, 14, 3284. [Google Scholar] [CrossRef]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Cohn, S.; Fisk, W.J. Energy efficient indoor VOC air cleaning with activated carbon fiber (ACF) filters. Build. Environ. 2012, 47, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Baur, G.B.; Yuranov, I.; Renken, A.; Kiwi-Minsker, L. Activated carbon fibers for efficient VOC removal from diluted streams: The role of surface morphology. Adsorption 2015, 21, 479–488. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Isinkaralar, K. Experimental evaluation of benzene adsorption in the gas phase using activated carbon from waste biomass. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Y.J. Evaluation of activated carbon for remediating benzene contamination: Adsorption and oxidative regeneration. J. Hazard. Mater. 2010, 182, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Szulejko, J.E.; Samaddar, P.; Kim, K.H.; Eom, W.; Ambade, S.B.; Han, T.H. The effect of diverse metal oxides in graphene composites on the adsorption isotherm of gaseous benzene. Environ. Res. 2019, 172, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Anand, B.; Kim, K.H.; Younis, S.A. Insights into the performance of the two contrasting dynamic adsorption platforms in the removal of gaseous benzene on microporous carbon materials. J. Clean. Prod. 2022, 364, 132520. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Ha, S.H.; Kim, K.H.; Younis, S.A.; Dou, X. The interactive roles of space velocity and particle size in a microporous carbon bed system in controlling adsorptive removal of gaseous benzene under ambient conditions. Chem. Eng. J. 2020, 401, 126010. [Google Scholar] [CrossRef]
- Shen, X.; Ou, R.; Lu, Y.; Yuan, A.; Liu, J.; Gu, J.; Hu, X.; Yang, Z.; Yang, F. Record-high capture of volatile benzene and toluene enabled by activator implant-optimized banana peel-derived engineering carbonaceous adsorbents. Environ. Int. 2020, 143, 105774. [Google Scholar] [CrossRef]
- ASTM Standard E872-85; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 2006.
- ASTM Standard E871-82; Standard Test Method for MOISTURE analysis of Particulate Wood Fuels. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 2006.
- ASTM Standard E1755-01; Standard Test Method for Ash in Biomass. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 2007.
- US EPA. Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes, Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition Compendium Method TO-17; U.S. Environmental Protection Agency (US EPA): Washington, DC, USA, 1999.
- Lv, Y.; Sun, J.; Yu, G.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. Hydrophobic design of adsorbent for VOC removal in humid environment and quick regeneration by microwave. Microporous Mesoporous Mater. 2020, 294, 109869. [Google Scholar] [CrossRef]
- Charola, S.; Patel, H.; Chandna, S.; Maiti, S. Optimization to prepare porous carbon from mustard husk using response surface methodology adopted with central composite design. J. Clean. Prod. 2019, 223, 969–979. [Google Scholar] [CrossRef]
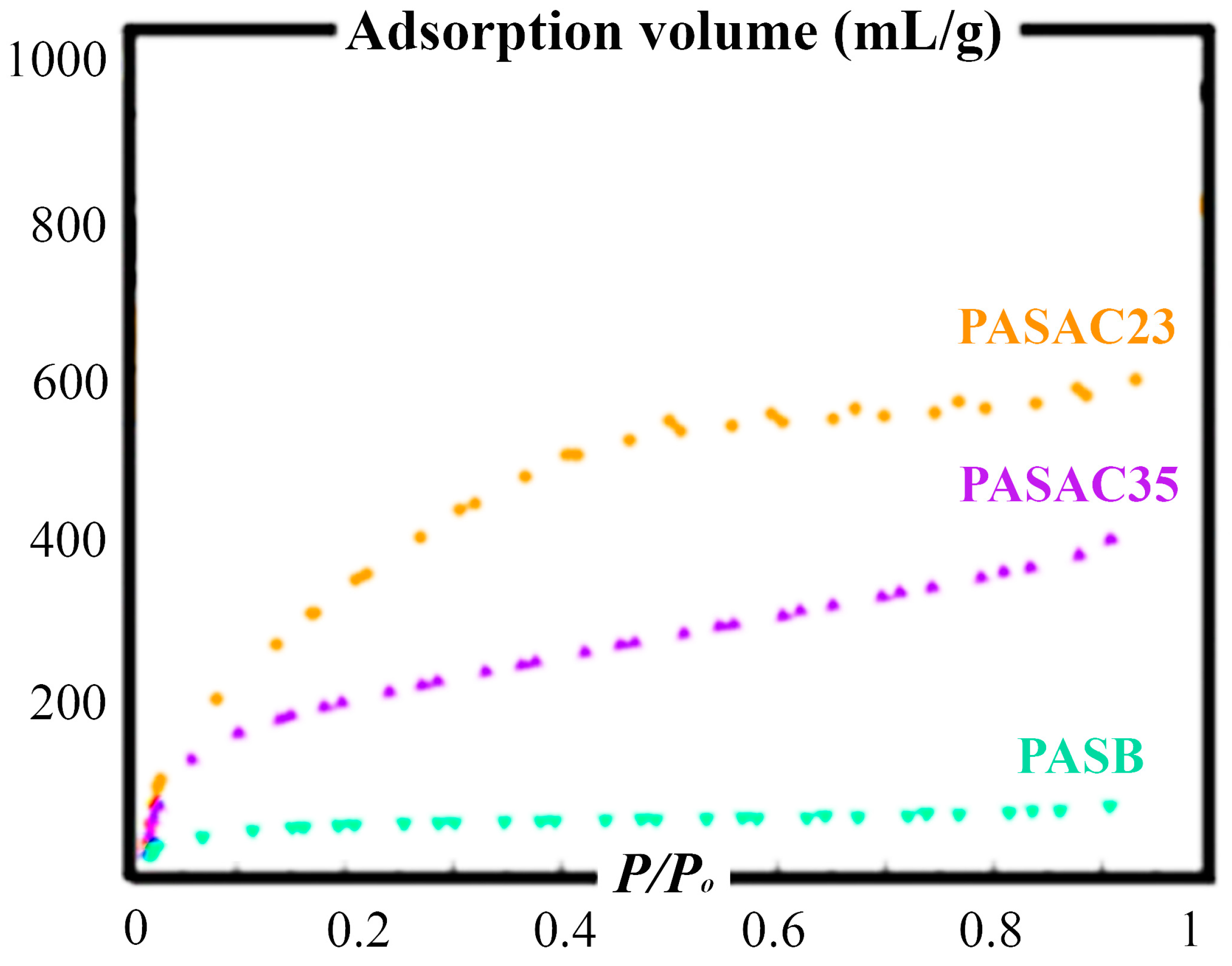


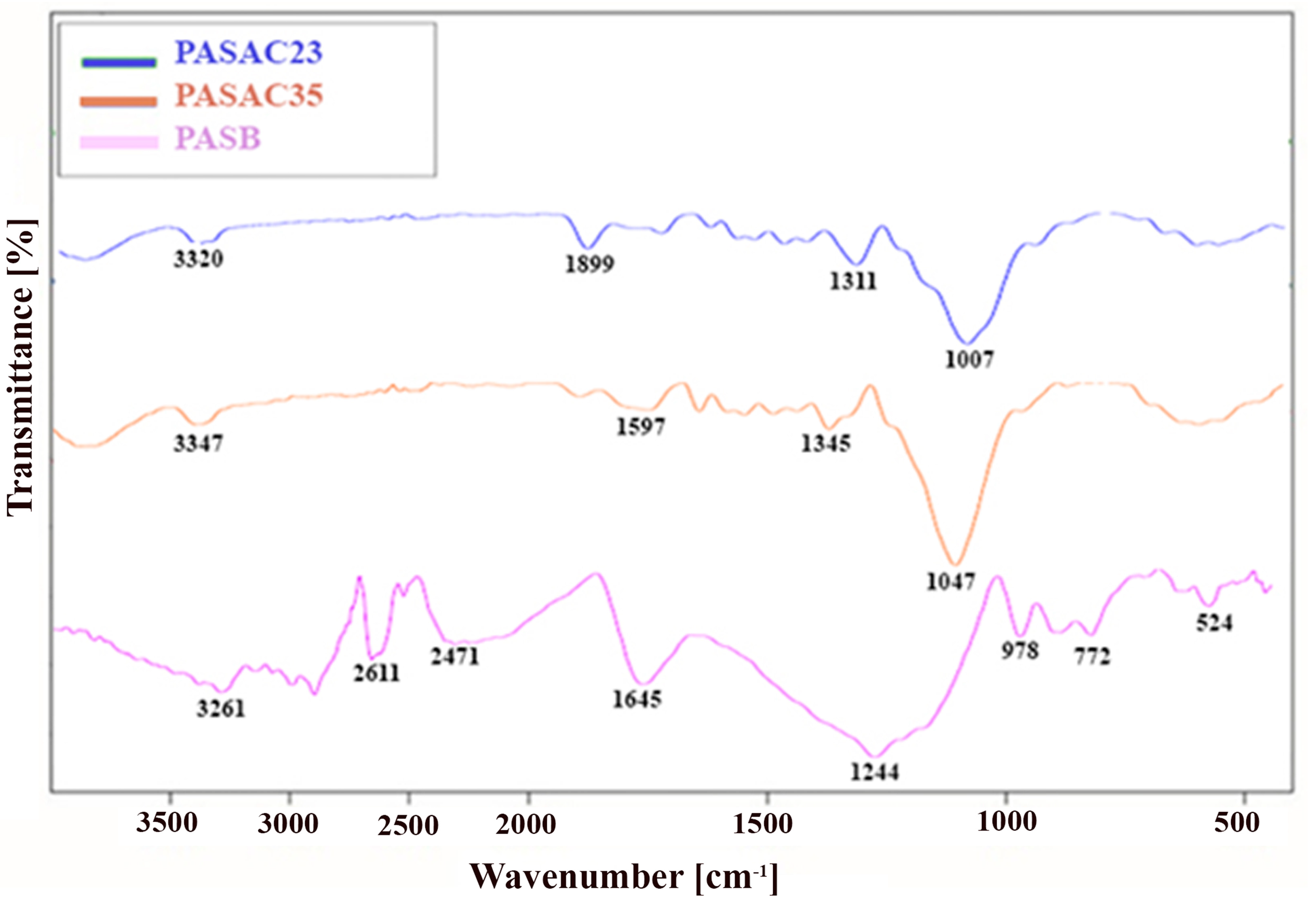
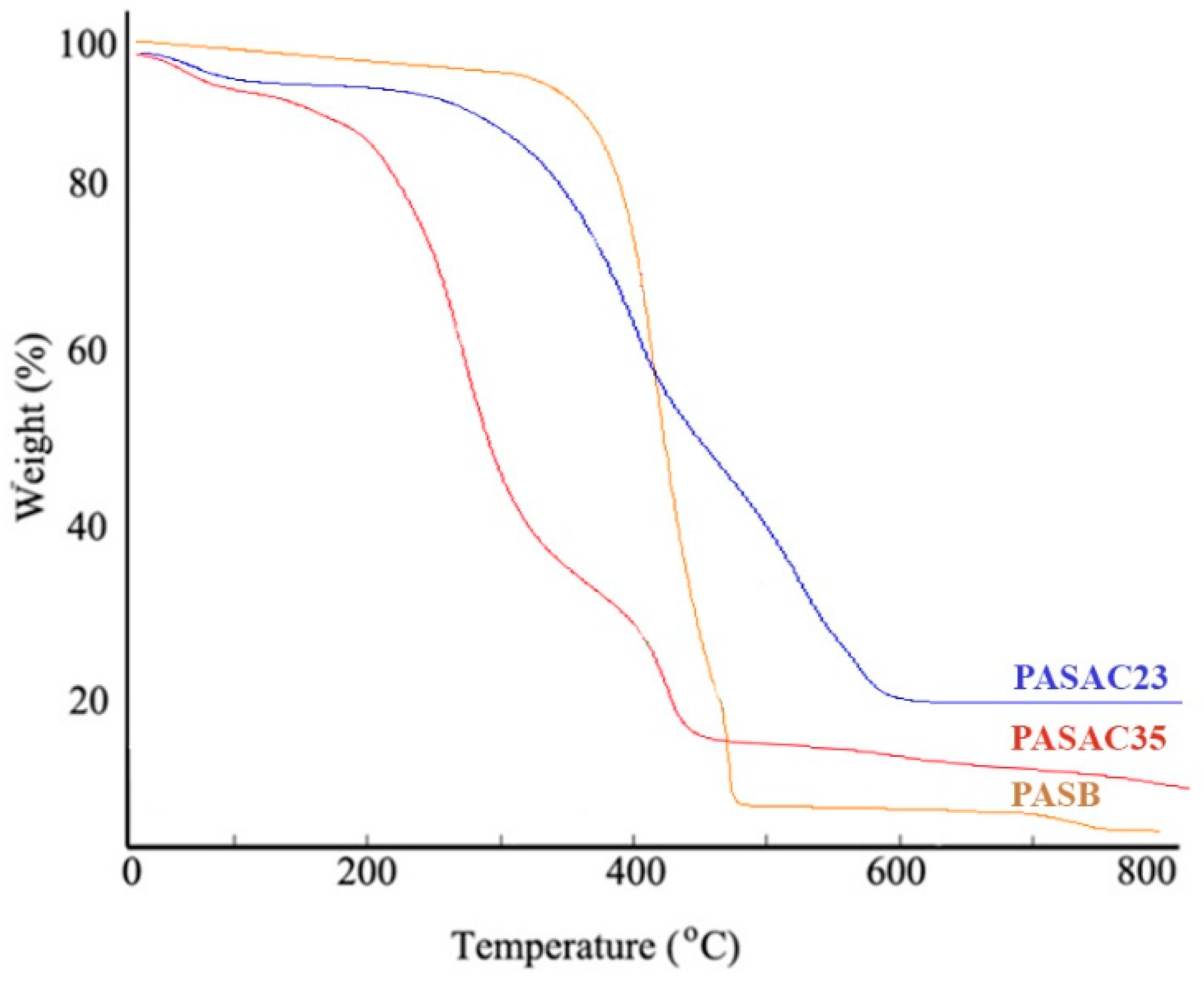



| Element (wt%) | PASB | PASAC23 | PASAC35 |
|---|---|---|---|
| C | 42.56 | 70.83 | 67.94 |
| H | 4.71 | 3.67 | 2.87 |
| O | 50.25 | 22.17 | 19.64 |
| N | 2.56 | 3.33 | 9.45 |
| Proximate (wt%) | PASB | PASAC23 | PASAC35 |
|---|---|---|---|
| Moisture | 10.66 | 2.58 | 5.53 |
| Volatile substant | 73.27 | 17.27 | 21.61 |
| Fixed carbon | 15.42 | 74.38 | 68.24 |
| Ash | 0.65 | 5.77 | 4.62 |
| Materials | PASB | PASAC23 | PASAC35 |
|---|---|---|---|
| SBET (m2/g) | 3 | 657 | 581 |
| Vtotal (cm3/g) | 0.01 | 0.36 | 0.32 |
| Vmicro (cm3/g) | - | 0.21 | 0.19 |
| Vmeso (cm3/g) | - | 0.15 | 0.13 |
| Yield (%) | - | 38.6 | 41.5 |
| dp (nm) | - | 1.54 | 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isinkaralar, K. A Study on the Gaseous Benzene Removal Based on Adsorption onto the Cost-Effective and Environmentally Friendly Adsorbent. Molecules 2023, 28, 3453. https://doi.org/10.3390/molecules28083453
Isinkaralar K. A Study on the Gaseous Benzene Removal Based on Adsorption onto the Cost-Effective and Environmentally Friendly Adsorbent. Molecules. 2023; 28(8):3453. https://doi.org/10.3390/molecules28083453
Chicago/Turabian StyleIsinkaralar, Kaan. 2023. "A Study on the Gaseous Benzene Removal Based on Adsorption onto the Cost-Effective and Environmentally Friendly Adsorbent" Molecules 28, no. 8: 3453. https://doi.org/10.3390/molecules28083453





