Panchromatic Light-Absorbing [70]Fullerene-Perylene-BODIPY Triad with Cascade of Energy Transfer as an Efficient Singlet Oxygen Sensitizer
Abstract
1. Introduction
2. Results
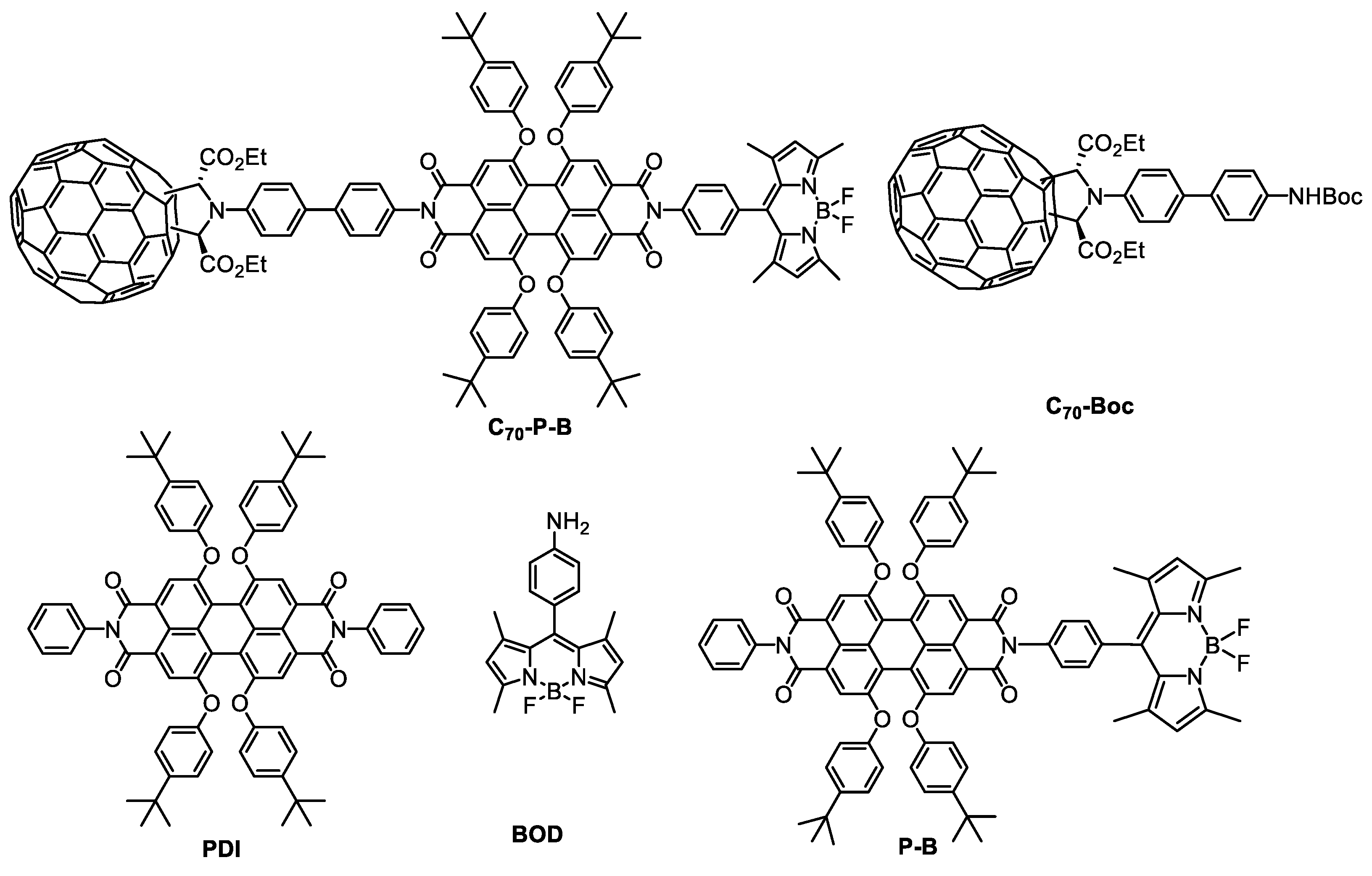
2.1. Synthesis
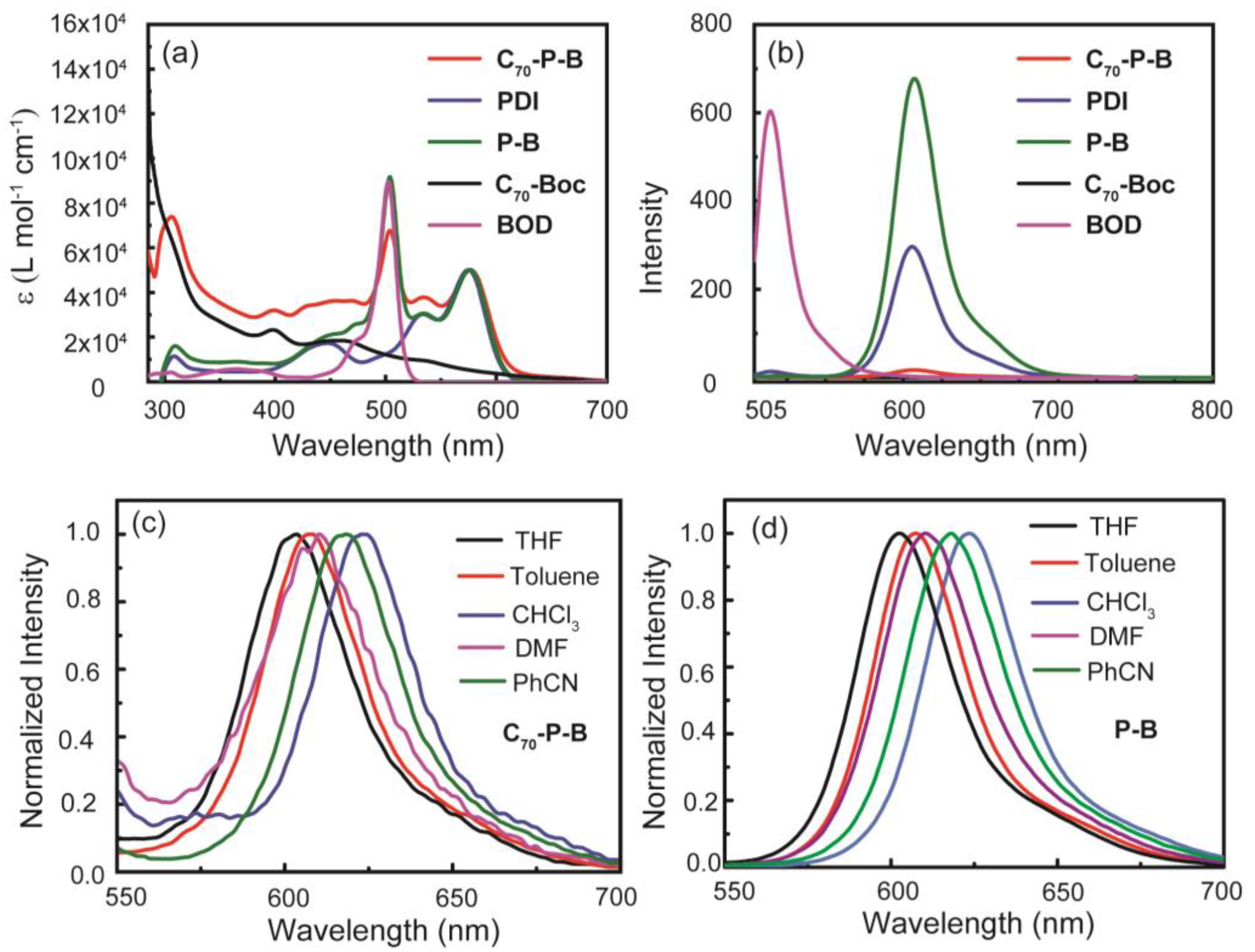
2.2. UV-Vis Absorption and Steady-State Fluorescence
2.3. Time-Resolved Fluorescence Spectroscopy
2.4. Nanosecond Time-Resolved Transient Absorption Spectroscopy
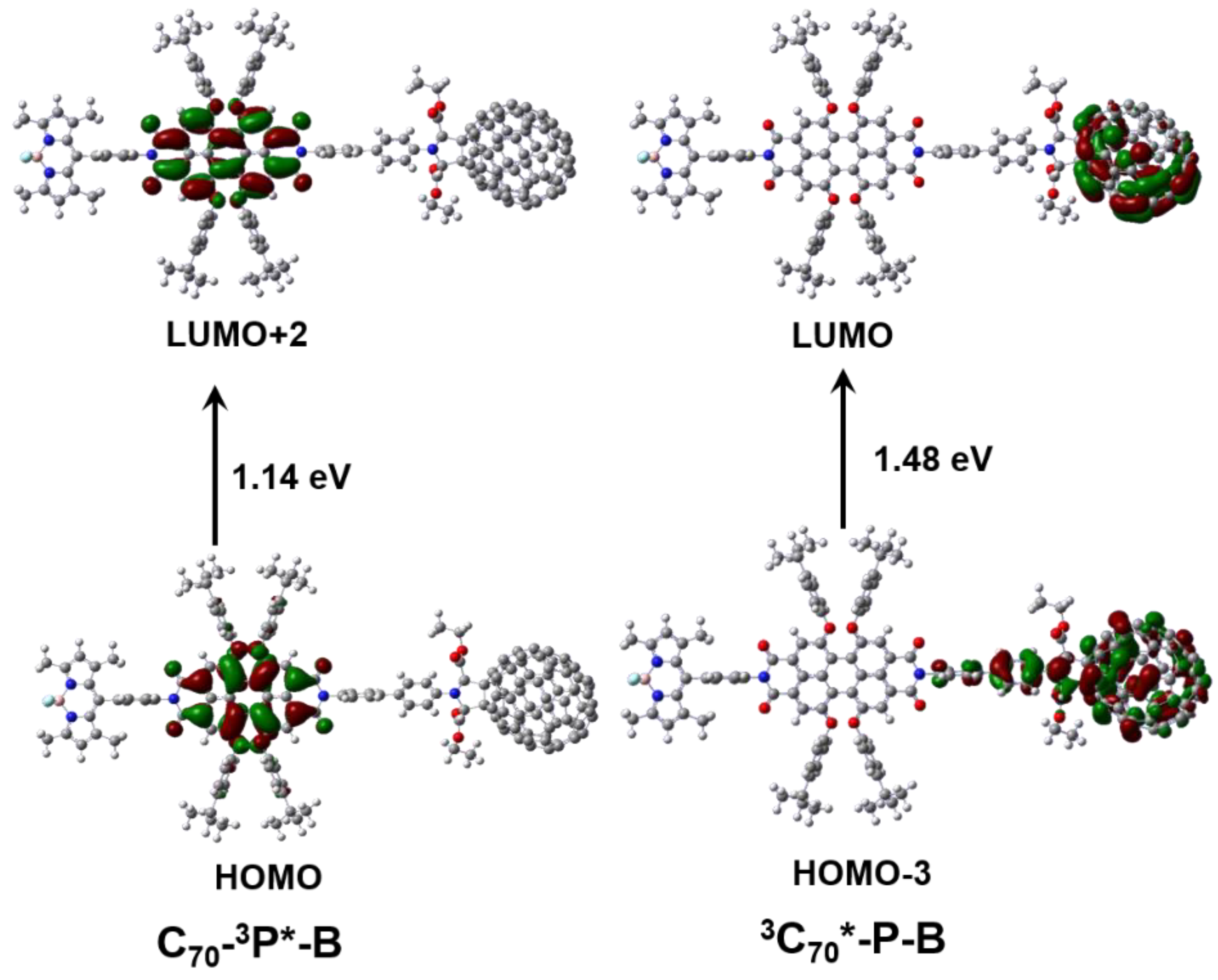
2.5. TD-DFT Calculations
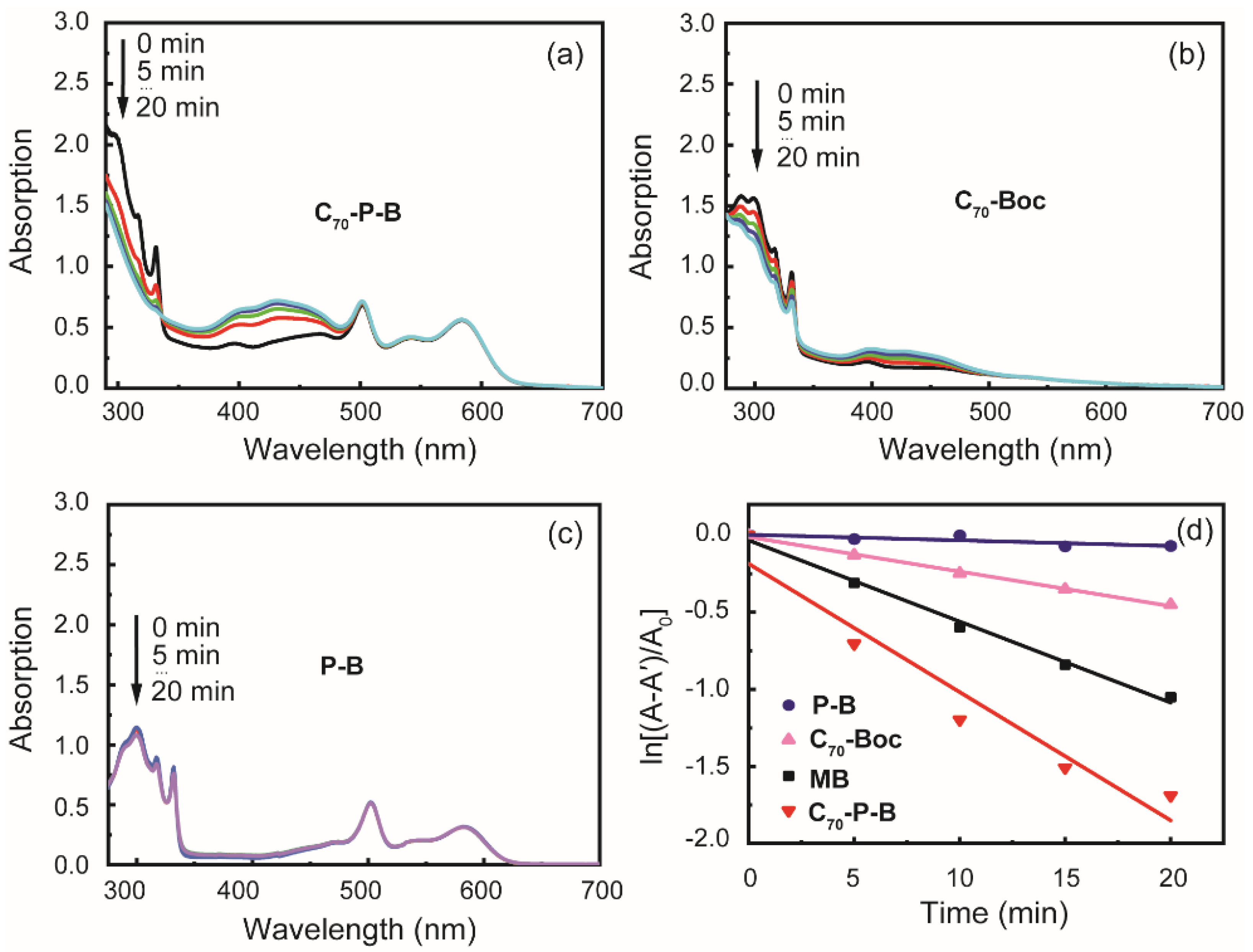
2.6. Photooxidation of 1,5-Dihydroxy Naphthalene Mediated by 1O2
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of C70-Boc
3.2.2. Synthesis of Compound 2
3.2.3. Synthesis of P-B
3.2.4. Synthesis of C70-P-B
3.3. Photooxidation Experiment
3.4. Photostability Experiment
3.5. Measurement of Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yaraki, M.T.; Liu, B.; Tan, Y.N. Emerging strategies in enhancing singlet oxygen generation of nano-photosensitizers toward advanced phototherapy. Nanomicro Lett. 2022, 14, 123. [Google Scholar]
- Cao, W.-W.; Zhu, Y.; Wu, F.-P.; Tian, Y.; Chen, Z.-M.; Xu, W.-J.; Liu, S.-Y.; Liu, T.-T.; Xiong, H. Three birds with one stone: Acceptor engineering of hemicyanine dye with NIR-II emission for synergistic photodynamic and photothermal anticancer therapy. Small 2022, 18, 2204851. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.Y.; Park, S.; Yoon, J. Molecular design of highly efficient heavy-atom-free triplet BODIPY derivatives for photodynamic therapy and bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef]
- Yokoi, K.; Yasuda, Y.; Kanbe, A.; Imura, T.; Aoki, S. Development of wireless power-transmission-based photodynamic therapy for the induction of cell death in cancer cells by cyclometalated iridium (III) complexes. Molecules 2023, 28, 1433. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cui, X.-N.; Therrien, B.; Zhao, J.-Z. Energy-funneling-based broadband visible-light-absorbing bodipy-C60 triads and tetrads as dual functional heavy-atom-free organic triplet photosensitizers for photocatalytic organic reactions. Chem. Eur. J. 2013, 19, 17472–17482. [Google Scholar] [CrossRef]
- Zhao, J.-Z.; Wu, W.-H.; Sun, J.-F.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Zhang, J.-H.; Gong, Y.; Dou, L.-F.; Mao, L.-H.; Lu, H.-D.; Wei, C.-X.; Chen, H.; Wang, X.-F.; Yang, W. Broadband visible light-absorbing [70]fullerene-BODIPY-triphenylamine triad: Synthesis and application as heavy atom-free organic triplet photosensitizer for photooxidation. Molecules 2021, 26, 1243. [Google Scholar] [CrossRef]
- Namkoong, G.; Mamun, A.A.; Ava, T.T. Impact of PCBM/C60 electron transfer layer on charge transports on ordered and disordered perovskite phases and hysteresis-free perovskite solar cells. Org. Electron. 2018, 56, 163–169. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, X.-M.; Wang, Y.; Che, Y.-Y.; Sun, L.; Zhao, J.-Z.; Xu, H.-J. Truxene-linked coumarin-corrole triad: Synthesis, photophysical properties and application in triplet-triplet annihilation upconversion. Dye. Pigment. 2022, 208, 110865. [Google Scholar] [CrossRef]
- Yu, X.-K.; Gao, F.-R.; Zhao, W.-Y.; Lai, H.-X.; Wei, L.-L.; Yang, C.; Wu, W.-H. BODIPY-conjugated bis-terpyridine Ru (II) complexes showing ultra-long luminescence lifetimes and applications to triplet-triplet annihilation upconversion. Dalton Trans. 2022, 51, 9314–9322. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yanai, N.; Kimizuka, N. Osmium complex-chromophore conjugates with both singlet-to-triplet absorption and long triplet lifetime through tuning of the heavy-atom effect. Inorg. Chem. 2022, 61, 5982–5990. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-B.; Liu, M.-Y.; Zhang, X.-F.; Shi, M.; Jia, M.-X.; Hu, X.-F.; Liu, L.-L.; Wang, T. Minimizing the electron donor size of donor-acceptor-type photosensitizer: Twisted intramolecular charge-transfer-induced triplet state and singlet oxygen formation. J. Phys. Chem. 2020, 124, 23558–23566. [Google Scholar] [CrossRef]
- Dong, Y.; Dick, B.; Zhao, J.-Z. Twisted Bodipy derivative as a heavy-atom-free triplet photosensitizer showing strong absorption of yellow light, intersystem crossing, and a high-energy long-lived triplet state. Org. Lett. 2020, 22, 5535–5539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Zhao, J.-Z.; Barbon, A.; Toffoletti, A.; Liu, Y.; An, Y.-L.; Xu, L.; Karatay, A.; Yaglioglu, H.G.; Yildiz, E.A.; et al. Radical-enhanced intersystem crossing in new Bodipy derivatives and application for efficient triplet-triplet annihilation upconversion. J. Am. Chem. Soc. 2017, 139, 7831–7842. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.-F.; Huo, Y.-Y.; Yao, G.-X.; Feng, Y.; Weng, J.-J.; Zhao, W.; Guo, W. Heavy atom-free, mitochondria-targeted, and activatable photosensitizers for photodynamic therapy with real-time in-situ therapeutic monitoring. Angew. Chem. Int. Ed. 2022, 61, e202201815. [Google Scholar] [CrossRef]
- Lv, M.; Lu, X.-C.; Jiang, Y.-R.; Sandoval-Salinas, M.E.; Casanova, D.; Sun, H.-T.; Sun, Z.-R.; Xu, J.-H.; Yang, Y.-J.; Chen, J.-Q. Near-unity triplet generation promoted via spiro-conjugation. Angew. Chem. Int. Ed. 2022, 61, e202113190. [Google Scholar] [CrossRef]
- Joseph, J.; Bauroth, S.; Charisiadis, A.; Charalambidis, G.; Coutsolelos, A.G.; Guldi, D.M. Cascades of energy and electron transfer in a panchromatic absorber. Nanoscale 2022, 14, 9304–9312. [Google Scholar] [CrossRef]
- Che, Y.-Y.; Yuan, X.-M.; Sun, L.; Xu, H.-J.; Zhao, X.-Y.; Cai, F.-J.; Liu, L.; Zhao, J.-Z. Truxene-bridged Bodipy fullerene tetrads without precious metals: Study of the energy transfer and application in triplet–triplet annihilation upconversion. J. Mater. Chem. C 2020, 8, 15839–15851. [Google Scholar] [CrossRef]
- Siposova, K.; Petrenko, V.I.; Ivankov, O.I.; Musatov, A.; Bulavin, L.A.; Avdeev, M.V.; Kyzyma, O.A. Fullerenes as an Effective Amyloid Fibrils Disaggregating Nanomaterial. ACS Appl. Mater. Inter. 2020, 12, 32410–32419. [Google Scholar] [CrossRef]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2016, 33, 777–794. [Google Scholar] [CrossRef]
- Arbogast, J.W.; Foote, C.S. Photophysical Properties of C70. J. Am. Chem. Soc. 1991, 113, 8886–8889. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Guan, M.-R.; Xu, L.; Shu, C.-Y.; Jin, C.; Zheng, J.-P.; Fang, X.-H.; Yang, Y.-J.; Wang, C.-R. Structural effect and mechanism of C70-carboxyfullerenes as efficient sensitizers against cancer cells. Small 2012, 8, 2070–2077. [Google Scholar] [CrossRef]
- Doi, Y.; Ikeda, A.; Akiyama, M.; Nagano, M.; Shigematsu, T.; Ogawa, T.; Takeya, T.; Nagasaki, T. Intracellular uptake and photodynamic activity of water-soluble [60]- and [70]fullerenes incorporated in liposomes. Chem. Eur. J. 2008, 14, 8892–8897. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Kim, J.H.; Snow, S.; Kim, J.H. [70]Fullerene-sensitized triplet-triplet annihilation upconversion. Chem. Commun. 2013, 49, 10829–10831. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-X.; Zheng, M.; Zhou, Q.-H.; Zhou, X.-G.; Liu, S.-L. Application of a bodipy-C70 dyad in triplet-triplet annihilation upconversion of perylene as a metal-free photosensitizer. Org. Biomol. Chem. 2018, 16, 5598–5608. [Google Scholar] [CrossRef]
- Zhang, X.-F. BODIPY photosensitizers based on PET and heavy atom effect: A comparative study on the efficient formation of excited triplet state and singlet oxygen in BODIPY dimers and monomers. J. Photochem. Photobiol. A Chem. 2018, 355, 431–443. [Google Scholar] [CrossRef]
- Nagarajan, K.; Mallia, A.R.; Muraleedharan, K.; Hariharan, M. Enhanced intersystem crossing in core-twisted aromatics. Chem. Sci. 2017, 8, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-E.; Liu, K.-Q.; Wang, X.-F.; Xia, A.-D.; Wang, G.-W. Synthesis and properties of axially symmetrical rigid visible light-harvesting systems containing [60]fullerene and perylenebisimide. J. Org. Chem. 2016, 81, 12223–12231. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Zhang, J.-H.; Dou, L.-F.; Li, N.; Hu, K.-H.; Gao, T.-Y.; Lu, H.-D.; Si, J.-Y.; Wang, X.-F.; Yang, W. Rigid axially symmetrical C60-BODIPY triplet photosensitizers: Effect of bridge length on singlet oxygen generation. New J. Chem. 2020, 44, 20419–20427. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Wang, F.-D.; Liu, J.-J.; Wang, L.-L.; Wang, C.; Yuen, A.C.Y.; Chen, T.B.Y.; Kabir, I.I.; Yeoh, G.H.; Lu, H.-D.; et al. BODIPY coated on MXene nanosheets for improving mechanical and fire safety properties of ABS resin. Compos. B Eng. 2021, 223, 109130–109142. [Google Scholar] [CrossRef]
- Yin, J.-J.; Jin, L.-M.; Liu, R.-L.; Li, Q.-N.; Fan, C.-H.; Li, Y.; Li, W.-X.; Chen, Q.-Y. Reactions of fullerenes with reactive methylene organophosphorus reagents: Efficient synthesis of organophosphorus group substituted C60 and C70 derivatives. J. Org. Chem. 2006, 71, 2267–2271. [Google Scholar] [CrossRef]
- Catalán, J.; Elguero, J. Fluorescence of C60 and C70. J. Am. Chem. Soc. 1993, 115, 9249–9252. [Google Scholar] [CrossRef]
- Ma, B.; Sun, Y.P. Fluorescence spectra and quantum yields of [60]fullerene and [70]fullerene under different solvent conditions. A quantitative examination using a near-infrared-sensitive emission spectrometer. J. Chem. Soc. Perkin Trans. 1996, 2, 2157–2162. [Google Scholar]
- Kim, D.; Lee, M. Observation of fluorescence emission from solutions of C60 and C70 and measurement of their excited-state lifetmes. J. Am. Chem. Soc. 1992, 114, 4429–4430. [Google Scholar] [CrossRef]
- Rani, K.; Sengupta, S. Metal-free FRET macrocycles of perylenediimide and aza-BODIPY for multifunctional sensing. Chem. Commun. 2023, 59, 1042–1045. [Google Scholar] [CrossRef]
- Mayländer, M.; Nolden, O.; Franz, M.; Chen, S.; Bancroft, L.; Qiu, Y.; Wasielewski, M.R.; Gilch, P.; Richert, S. Accessing the triplet state of perylenediimide by radical-enhanced intersystem crossing. Chem. Sci. 2022, 13, 6732–6743. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Zhang, X.-R.; Wu, Y.-S.; Zhang, J.-P.; Ai, X.-C.; Wang, Y.; Sun, M.-T. Two-photon photophysical properties of tri-9-anthrylborane. Chem. Phys. Lett. 2007, 436, 280–286. [Google Scholar] [CrossRef]
- Bisht, H.; Singh, A.P.; Jit, S.; Mishra, H. Effect of concentration on the photophysics of solution of [6,6]-phenyl C61 butyric acid methyl ester (PCBM) in chloroform. J. Lumin. 2023, 258, 119808. [Google Scholar] [CrossRef]




| Photosensitizers | λabs a | λF b | τF c | τT d | kobs e/min−1 | υi f | Φ∆ g |
|---|---|---|---|---|---|---|---|
| C70-P-B | 306, 399, 503, 575 | 607 | 3.7 | 175, 23 | 83.3 | 8.33 | 0.82 |
| C70-Boc | 398, 465, 535 | - | - | 42 | 22.5 | 2.25 | 0.81 h |
| B-P | 503, 575 | 607 | 5.7 | - | 3.7 | 0.37 | - |
| MB | - | - | - | - | 52.7 | 5.27 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, L.; Li, Y.; Dong, L.; Zhang, S.; Wu, Y.; Gong, Y.; Yang, W.; Lu, H.; Zhu, S.; Zhou, X. Panchromatic Light-Absorbing [70]Fullerene-Perylene-BODIPY Triad with Cascade of Energy Transfer as an Efficient Singlet Oxygen Sensitizer. Molecules 2023, 28, 3534. https://doi.org/10.3390/molecules28083534
Dou L, Li Y, Dong L, Zhang S, Wu Y, Gong Y, Yang W, Lu H, Zhu S, Zhou X. Panchromatic Light-Absorbing [70]Fullerene-Perylene-BODIPY Triad with Cascade of Energy Transfer as an Efficient Singlet Oxygen Sensitizer. Molecules. 2023; 28(8):3534. https://doi.org/10.3390/molecules28083534
Chicago/Turabian StyleDou, Lifeng, Yuanming Li, Lei Dong, Shuao Zhang, Yuanqi Wu, Yu Gong, Wei Yang, Hongdian Lu, Sane Zhu, and Xiaoguo Zhou. 2023. "Panchromatic Light-Absorbing [70]Fullerene-Perylene-BODIPY Triad with Cascade of Energy Transfer as an Efficient Singlet Oxygen Sensitizer" Molecules 28, no. 8: 3534. https://doi.org/10.3390/molecules28083534
APA StyleDou, L., Li, Y., Dong, L., Zhang, S., Wu, Y., Gong, Y., Yang, W., Lu, H., Zhu, S., & Zhou, X. (2023). Panchromatic Light-Absorbing [70]Fullerene-Perylene-BODIPY Triad with Cascade of Energy Transfer as an Efficient Singlet Oxygen Sensitizer. Molecules, 28(8), 3534. https://doi.org/10.3390/molecules28083534





