Untargeted Lipidomics Method for the Discrimination of Five Crab Species by Ultra-High-Performance Liquid Chromatography High-Resolution Mass Spectrometry Combined with Chemometrics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Lipid Content of Crab Muscle and Edible Viscera
2.2. Validation of the UHPLC-HRAM/MS Method
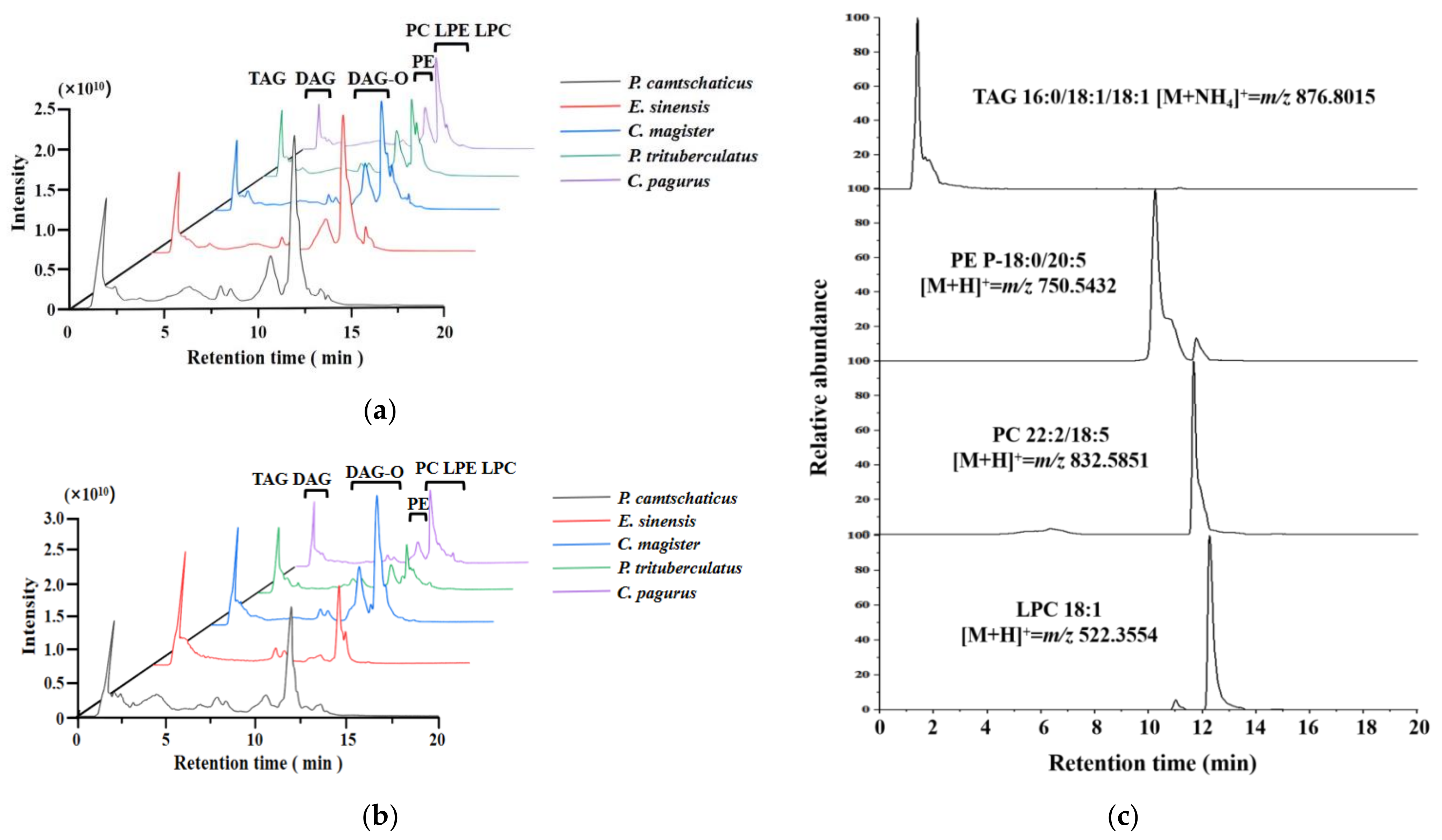
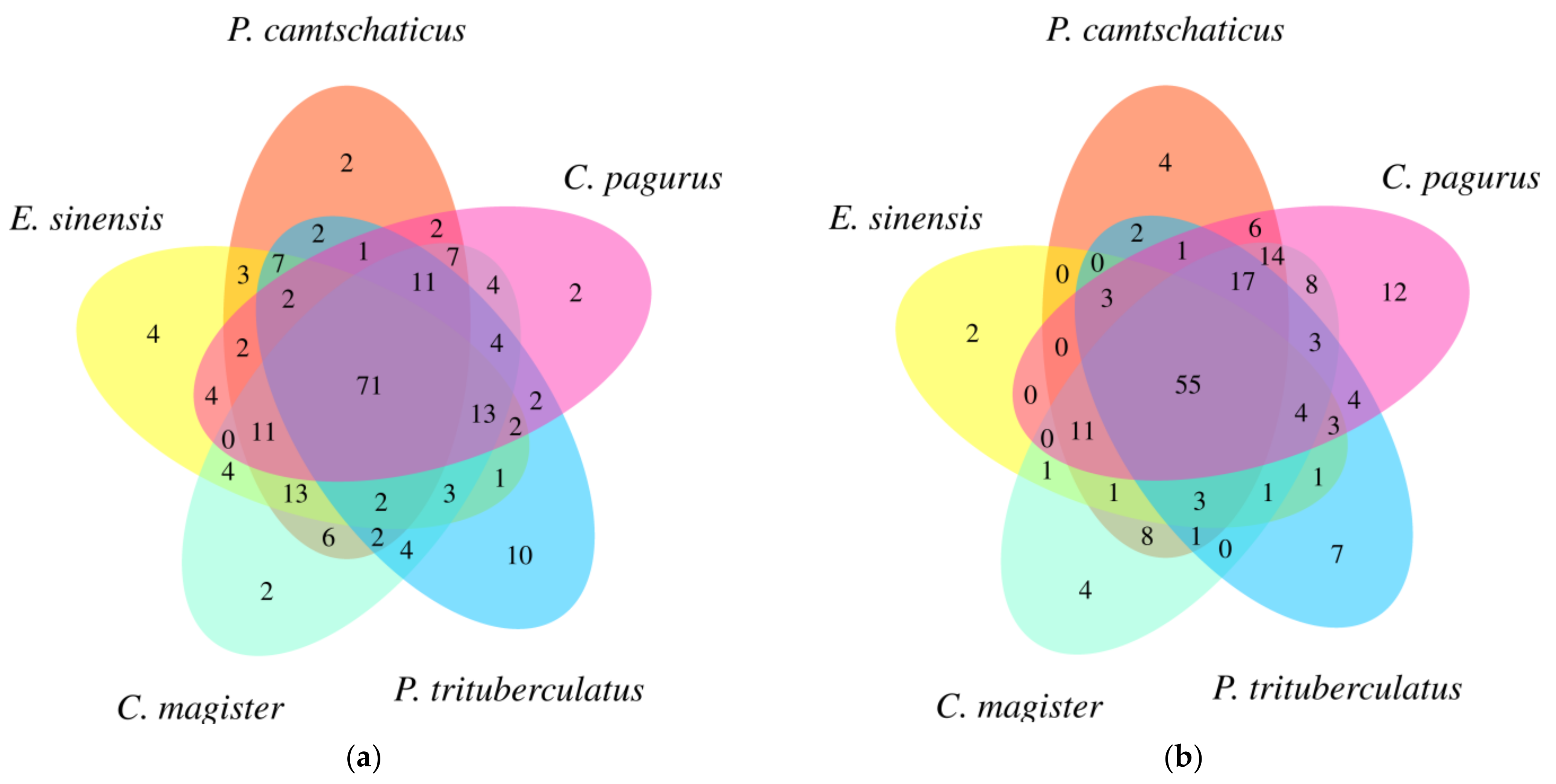
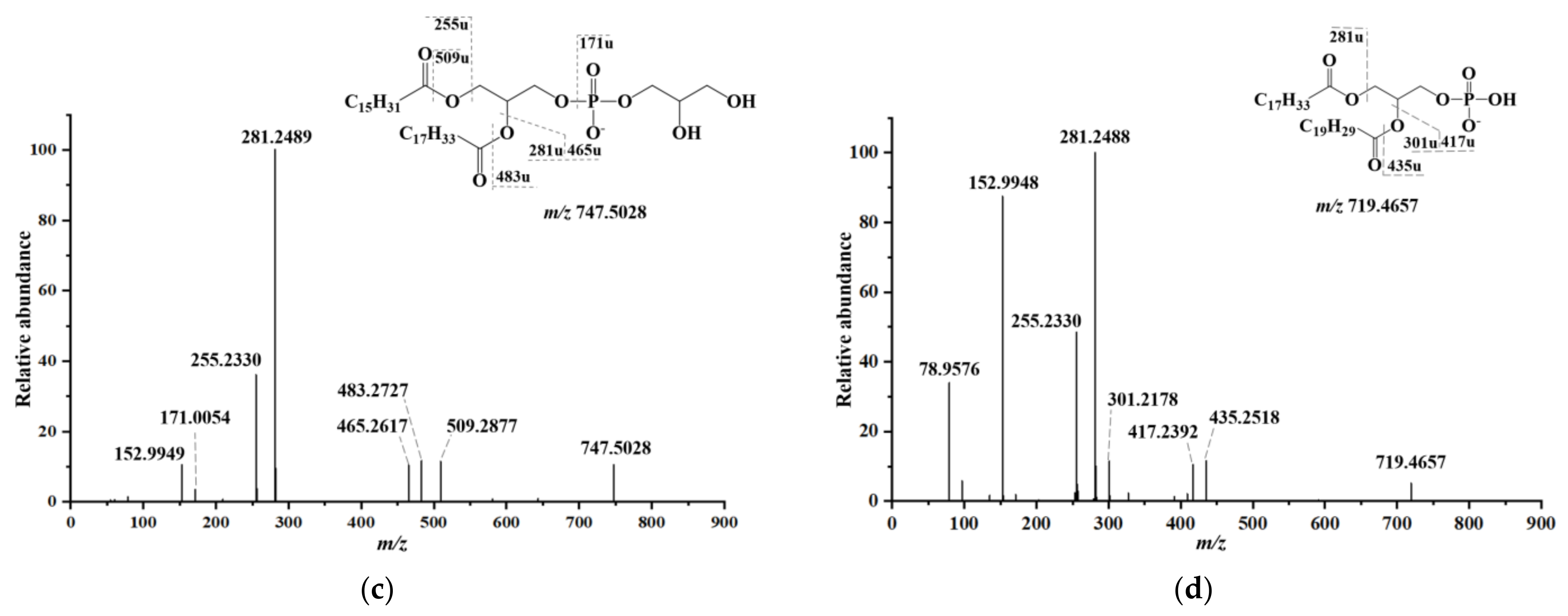
2.3. Lipid Identification
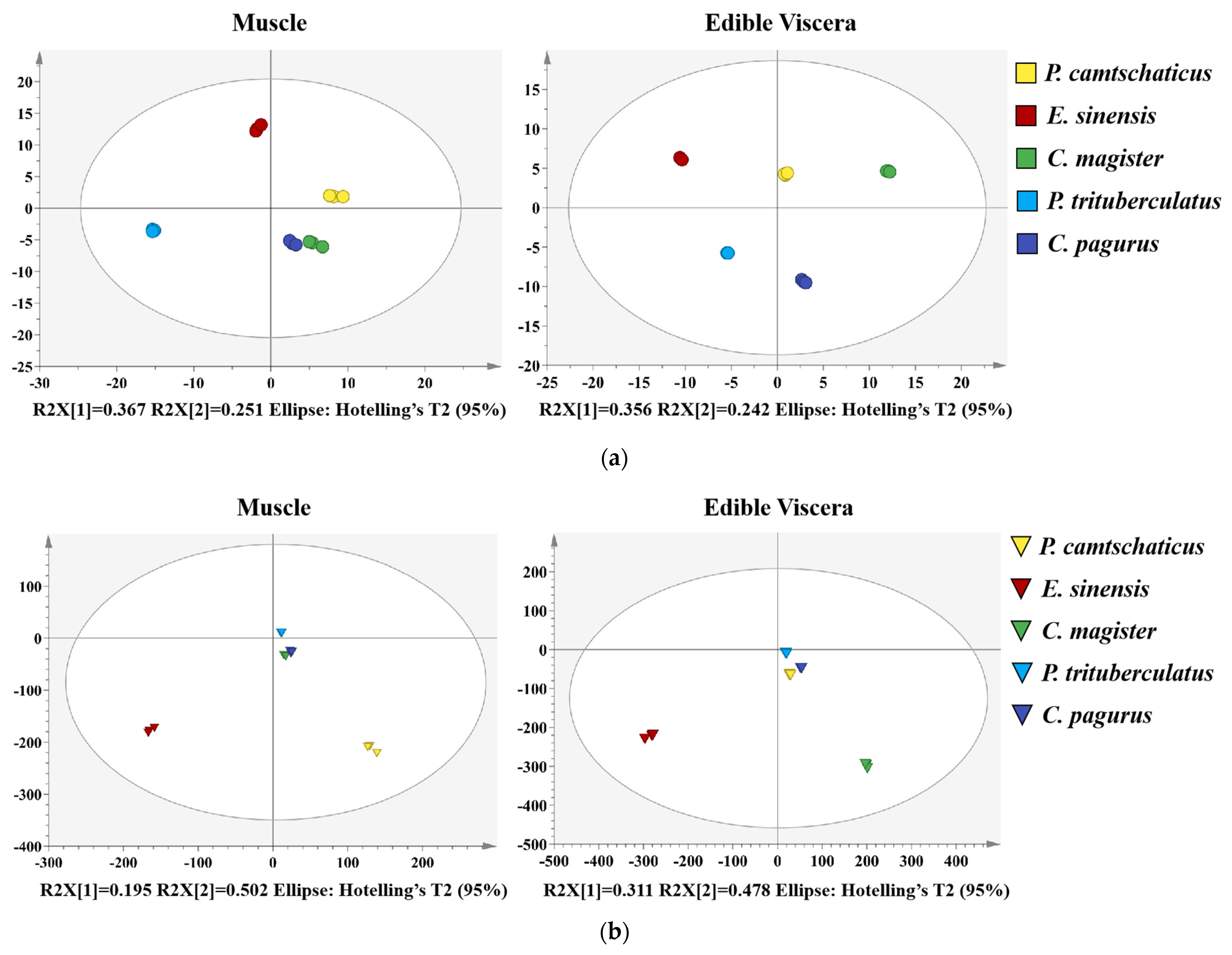
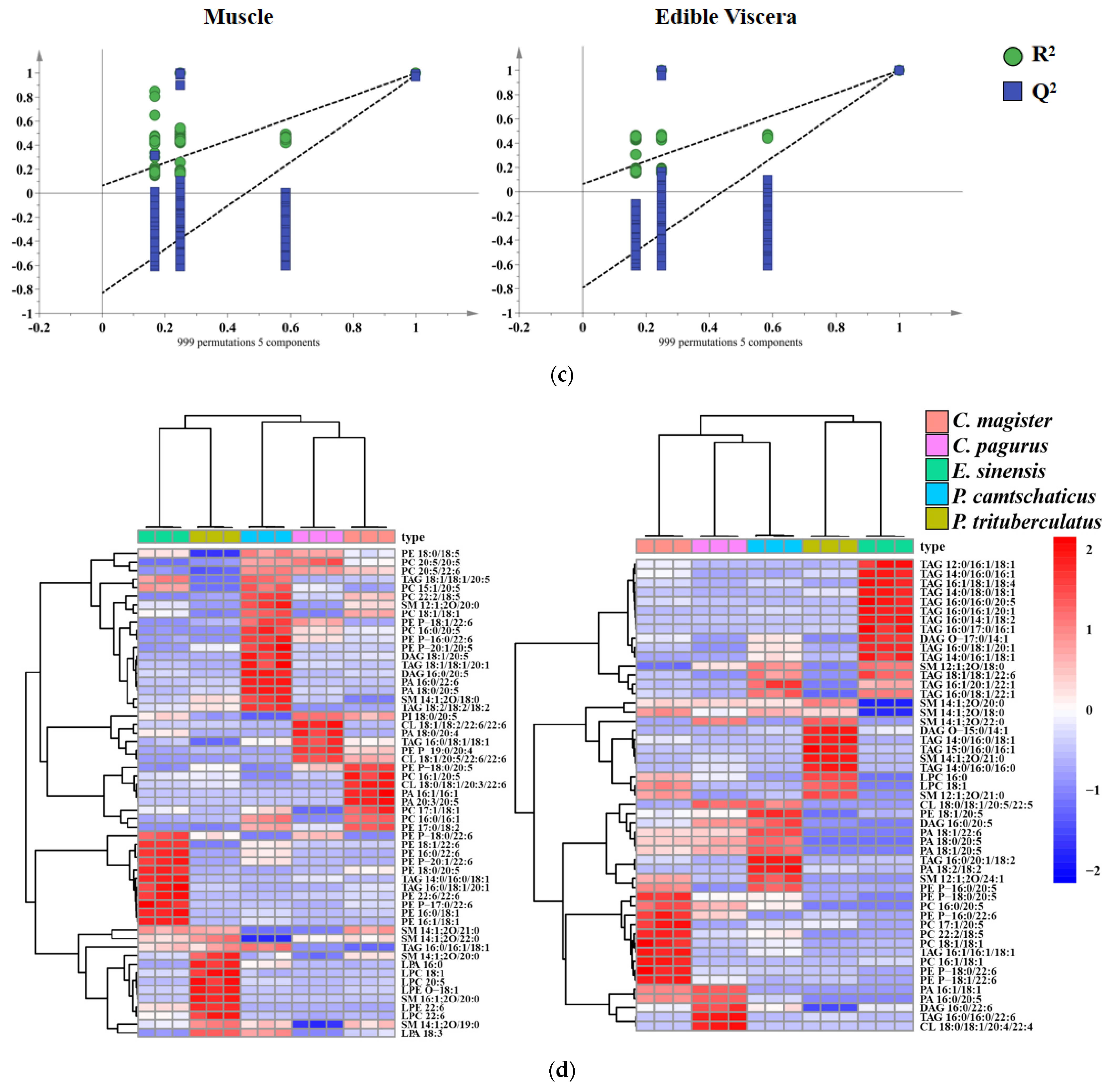
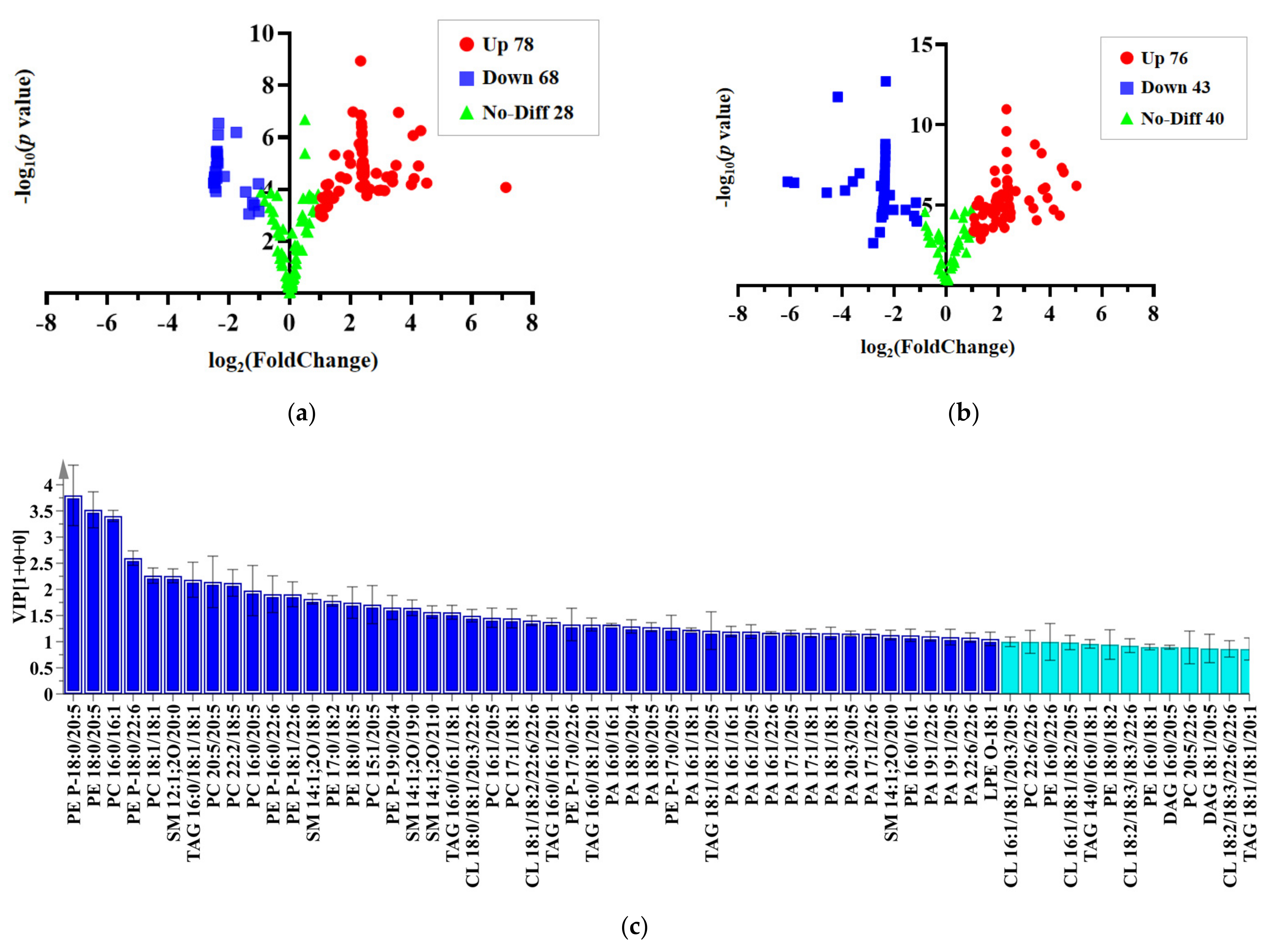
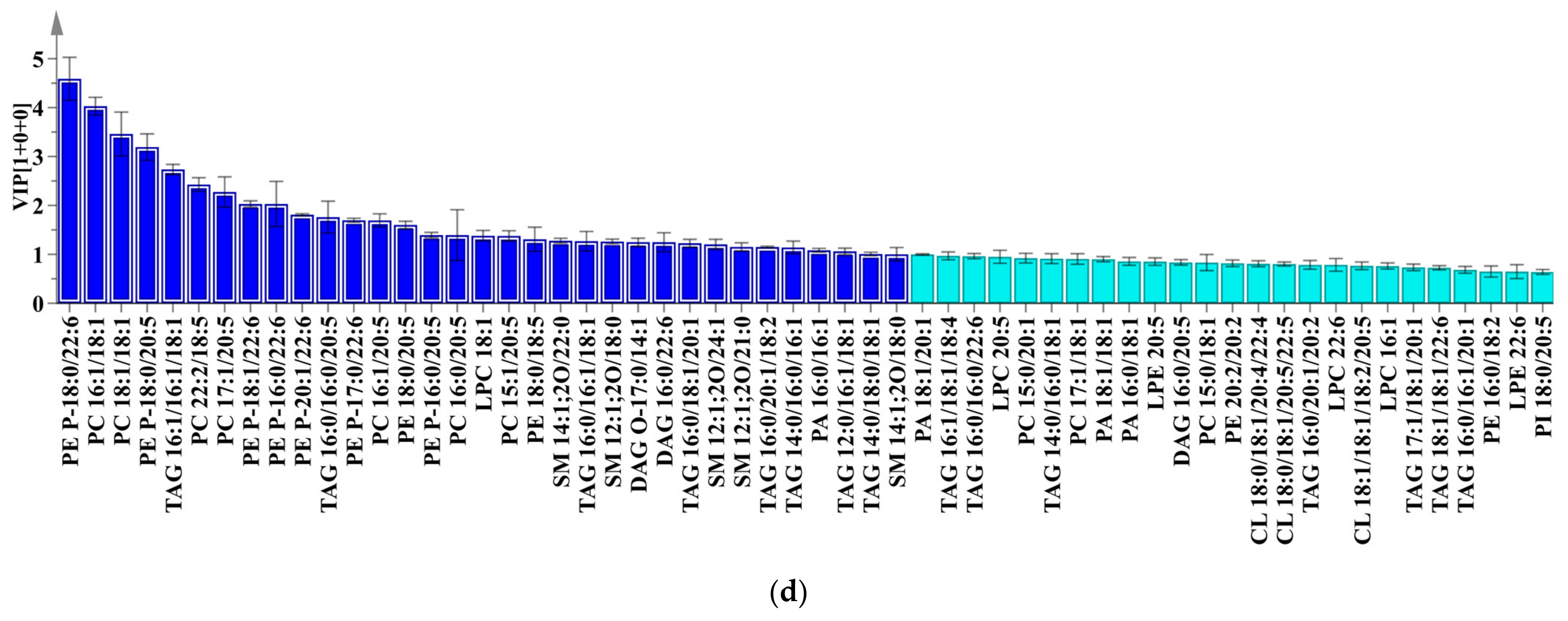
2.4. Multivariate Analysis
2.5. Orthogonal Partial Least Squares Discrimination Analysis
3. Materials and Methods
3.1. Ethical Statement
3.2. Sample Preparation
3.3. Standards and Reagents
3.4. Lipid Extraction
3.5. Lipid Identification and Semi-Quantification
3.6. UHPLC-HRAM/MS Analysis
3.6.1. UHPLC Conditions
3.6.2. Quadrupole-Exactive High-Resolution Accurate Mass Spectrometry
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, F.; Guo, H.; Gao, P.; Yu, D.; Xu, Y.; Jiang, Q.; Yu, P.; Xia, W. Comparison of methodological proposal in sensory evaluation for Chinese mitten crab (Eriocheir sinensis) by data mining and sensory panel. Food Chem. 2021, 356, 129698. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Shen, Y.; Cui, G.; Yin, F.; Yu, Z.; Zhou, D. Detailed Analysis of Lipids in Edible Viscera and Muscles of Cooked Crabs Portunus trituberculatus and Portunus pelagicus. J. Aquat. Food Prod. Technol. 2020, 29, 391–406. [Google Scholar] [CrossRef]
- Barrento, S.; Marques, A.; Teixeira, B.; Anacleto, P.; Vaz-Pires, P.; Nunes, M.L. Effect of season on the chemical composition and nutritional quality of the edible crab Cancer pagurus. J. Agric. Food Chem. 2009, 57, 10814–10824. [Google Scholar] [CrossRef]
- Nanda, P.K.; Das, A.K.; Dandapat, P.; Dhar, P.; Bandyopadhyay, S.; Dib, A.L.; Lorenzo, J.M.; Gagaoua, M. Nutritional aspects, flavour profile and health benefits of crab meat based novel food products and valorisation of processing waste to wealth: A review. Trends Food Sci. Technol. 2021, 112, 252–267. [Google Scholar] [CrossRef]
- Narayanasamy, A.; Balde, A.; Raghavender, P.; Shashanth, D.; Abraham, J.; Joshi, I.; Nazeer, R.A. Isolation of marine crab (Charybdis natator) leg muscle peptide and its anti-inflammatory effects on macrophage cells. Biocatal. Agric. Biotechnol. 2020, 25, 101577. [Google Scholar] [CrossRef]
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.C.J.; Ng, P.K.L.; Cumberlidge, N.; Magalhães, C.; Daniels, S.R.; Campos, M.R. Global diversity of crabs (Crustacea: Decapoda: Brachyura) in freshwater. Hydrobiologia 2007, 595, 275–286. [Google Scholar] [CrossRef]
- Ng, P.K.L. Collecting and processing freshwater shrimps and crabs. J. Crust. Biol. 2017, 37, 115–122. [Google Scholar] [CrossRef]
- Ferdoushi, Z.; Zhang, X.G.; Hasan, M.R. Mud crab (Scylla sp.) marketing system in Bangladesh. Asian J. Food Agro-Ind. 2010, 3, 248–265. [Google Scholar]
- Yao, Y.; Ding, L.; Huang, X. Diverse Functions of Lipids and Lipid Metabolism in Development. Small Methods 2020, 4, 1900564. [Google Scholar] [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862 Pt B, 1260–1272. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; Cherubini, A.; Andres-Lacueva, C. Lipids and physical function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Bondia-Pons, I.; Orešič, M. Lipidomics in nutrition and food research. Mol. Nutr. Food Res. 2013, 57, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alves, V.; Orešič, M.; Hyötyläinen, T. Lipidomics in nutrition research. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Imbs, A.B.; Ermolenko, E.V.; Grigorchuk, V.P.; Sikorskaya, T.V.; Velansky, P.V. Current Progress in Lipidomics of Marine Invertebrates. Mar. Drugs 2021, 19, 660. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, F.; Jin, M.; Wang, X.; Hu, X.; Zhao, M.; Cheng, X.; Luo, J.; Jiao, L.; Betancor, M.; et al. Untargeted lipidomics reveals metabolic responses to different dietary n-3 PUFA in juvenile swimming crab (Portunus trituberculatus). Food Chem. 2021, 354, 129570. [Google Scholar] [CrossRef]
- Ding, Z. Lipid metabolism disorders contribute to the pathogenesis of Hepatospora eriocheir in the crab Eriocheir sinensis. J. Fish Dis. 2021, 44, 305–313. [Google Scholar] [CrossRef]
- Cherif, S.; Frikha, F.; Gargouri, Y.; Miled, N. Fatty acid composition of green crab (Carcinus mediterraneus) from the Tunisian mediterranean coasts. Food Chem. 2008, 111, 930–933. [Google Scholar] [CrossRef]
- Nguyen, T.P.L.; Nguyen, V.T.A.; Do, T.T.T.; Nguyen Quang, T.; Pham, Q.L.; Le, T.T. Fatty Acid Composition, Phospholipid Molecules, and Bioactivities of Lipids of the Mud Crab Scylla paramamosain. J. Chem. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Qiu, J.; Zhang, L.; Zhang, Y.; Qiu, X.; Huang, Z.; Xu, W. Comprehensive Comparison on the Chemical Profile of Guang Chen Pi at Different Ripeness Stages Using Untargeted and Pseudotargeted Metabolomics. J. Agric. Food Chem. 2020, 68, 8483–8495. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition of the Barents Sea red king crab (Paralithodes camtschaticus) leg meat. J. Food Compost. Anal. 2021, 98, 103826. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, X.; Regenstein, J.M.; Wu, W.; Zhou, Y.; Wang, S.; Cheng, Y.; Wu, X.; Bao, B. Effect on lipid metabolism of mice continuously fed a crab-containing diet. Food Biosci. 2019, 30, 100422. [Google Scholar] [CrossRef]
- Stoner, A.W.; Ottmar, M.L.; Copeman, L.A. Temperature effects on the molting, growth, and lipid composition of newly-settled red king crab. J. Exp. Mar. Biol. Ecol. 2010, 393, 138–147. [Google Scholar] [CrossRef]
- Wu, H.; Ge, M.; Zhou, X.; Jiang, S.; Lin, L.; Lu, J. Nutritional qualities of normal and precocious adult male Chinese mitten crabs (Eriocheir sinensis). Aquac. Res. 2019, 50, 2267–2275. [Google Scholar] [CrossRef]
- Han, T.; Wang, J.; Hu, S.; Li, X.; Jiang, Y.; Wang, C. Effects of different dietary lipid sources on growth performance and tissue fatty acid composition of juvenile swimming crab Portunus trituberculatus. Chin. J. Oceanol. Limnol. 2015, 33, 957–965. [Google Scholar] [CrossRef]
- Liu, F.; Ren, D.; Guo, D.A.; Pan, Y.; Zhang, H.; Hu, P. Method Development for Gypenosides Fingerprint by High Performance Liquid Chromatography with Diode-Array Detection and the Addition of Internal Standard. Chem. Pharm. Bull. 2008, 56, 389–393. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wang, K.; Xi, J.; Xu, Y.; Si, X.; Pei, D.; Lyu, S.; Xia, G.; Wang, J.; et al. Analysis of lipidomics profile of Carya cathayensis nuts and lipid dynamic changes during embryonic development. Food Chem. 2022, 370, 130975. [Google Scholar] [CrossRef]
- Wang, F.; Lin, W.; Lv, S.; Jiang, S.; Lin, L.; Lu, J. Comparison of Lipids Extracted by Different Methods from Chinese Mitten Crab (Eriocheir sinensis) Hepatopancreas. J. Food Sci. 2019, 84, 3594–3600. [Google Scholar] [CrossRef]
- Jobling, M.; Johansen, S.; Foshaug, H.; Burkow, I.C.; JøRgensen, E.H. Lipid dynamics in anadromous Arctic charr, Salvelinus alpinus (L.): Seasonal variations in lipid storage depots and lipid class composition. Fish Physiol. Biochem. 1998, 18, 225–240. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Liu, Z.; Zheng, H.; Cheng, Y. Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus: Gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS ONE 2014, 9, e84921. [Google Scholar] [CrossRef]
- Lu, Z.; Shi, C.; Liu, L.; Mu, C.; Ye, Y.; Wang, C. Phospholipid Compositions in Portunus trituberculatus Larvae at Different Developmental Stages. J. Ocean. Univ. China 2022, 21, 152–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Dong, L.; Chang, J.; Wang, H.; Shen, Q. Lipidomics Screening of Polyunsaturated Phospholipid Molecular Species in Crab (Portunus trituberculatus) Muscular Tissue: A Nontarget Approach by HILIC-MS. Eur. J. Lipid Sci. Technol. 2021, 124, 2100097. [Google Scholar] [CrossRef]
- Suprayudi, M.A.; Takeuchi, T.; Hamasaki, K. Phospholipids Effect on Survival and Molting Synchronicity of Larvae Mud Crab Scylla serrata. HAYATI J. Biosci. 2012, 19, 163–168. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Han, T.; Li, X.; Zheng, P.; Yin, F.; Wang, C. Effects of dietary phospholipids levels on growth performance, lipid metabolism, and antioxidant capacity of the early juvenile green mud crab, Scylla paramamosain (Estampador). Aquac. Res. 2018, 50, 513–520. [Google Scholar] [CrossRef]
- Fitzpatrick, J. Subjectivity and objectivity: Polanyi and Lonergan. High. Educ. Q. 1982, 36, 183–195. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Hosokawa, M.; Takahashi, K. Growth inhibition and induction of apoptosis of colon cancer cell lines by applying marine phospholipid. Nutr. Cancer 2009, 61, 123–130. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ding, L.; Shi, H.H.; Xu, J.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Eicosapentaenoic acid in the form of phospholipids exerts superior anti-atherosclerosis effects to its triglyceride form in ApoE(-/-) mice. Food Funct. 2019, 10, 4177–4188. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, J.; Wang, Y.M.; Xue, C.H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef]
- Donovan, E.L.; Pettine, S.M.; Hickey, M.S.; Hamilton, K.L.; Miller, B.F. Lipidomic analysis of human plasma reveals ether-linked lipids that are elevated in morbidly obese humans compared to lean. Diabetol. Metab. Syndr. 2013, 5, 24. [Google Scholar] [CrossRef]
- Rangholia, N.; Leisner, T.M.; Holly, S.P. Bioactive Ether Lipids: Primordial Modulators of Cellular Signaling. Metabolites 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Aboshi, T.; Nishida, R.; Mori, N. Identification of plasmalogen in the gut of silkworm (Bombyx mori). Insect Biochem. Mol. Biol. 2012, 42, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Zemski Berry, K.A.; Murphy, R.C. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J. Am. Soc. Mass. Spectrom. 2004, 15, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Zhou, D.Y.; Wu, Z.X.; Yin, F.W.; Zhao, Q.; Xie, H.K.; Zhang, J.R.; Qin, L.; Shahidi, F. Extraction and detailed characterization of phospholipid-enriched oils from six species of edible clams. Food Chem. 2018, 239, 1175–1181. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-Based Metabolomics Data for Identification of Biochemically Interesting Compounds Using OPLS Class Models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Liu, H.; Hui, T.; Zheng, X.; Li, S.; Wei, X.; Li, P.; Zhang, D.; Wang, Z. Characterization of key lipids for binding and generating aroma compounds in roasted mutton by UPLC-ESI-MS/MS and Orbitrap Exploris GC. Food Chem. 2022, 374, 131723. [Google Scholar] [CrossRef]
- He, C.; Cao, J.; Bao, Y.; Sun, Z.; Liu, Z.; Li, C. Characterization of lipid profiling in three parts (muscle, head and viscera) of tilapia (Oreochromis niloticus) using lipidomics with UPLC-ESI-Q-TOF-MS. Food Chem. 2021, 347, 129057. [Google Scholar] [CrossRef]
- Roth, B.; Ines, S. Stunning and killing of edible crabs (Cancer pagurus). Anim. Welf. 2010, 19, 287–294. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, M.; Zhou, L.; Zhang, M.; Liu, J.; Marchioni, E. Identification and Differentiation of Wide Edible Mushrooms Based on Lipidomics Profiling Combined with Principal Component Analysis. J. Agric. Food Chem. 2021, 69, 9991–10001. [Google Scholar] [CrossRef]
- Song, S.; Cheong, L.Z.; Wang, H.; Man, Q.Q.; Pang, S.J.; Li, Y.Q.; Ren, B.; Wang, Z.; Zhang, J. Characterization of phospholipid profiles in six kinds of nut using HILIC-ESI-IT-TOF-MS system. Food Chem. 2018, 240, 1171–1178. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H.; Chen, D.; Tan, H.; Huang, Y.; Cozzolino, D. Lipidomic Changes in Banana (Musa cavendish) during Ripening and Comparison of Extraction by Folch and Bligh-Dyer Methods. J. Agric. Food Chem. 2020, 68, 11309–11316. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, H.; Wu, T.; Tao, N.; Wang, X.; Zhong, J. Effect of three types of thermal processing methods on the lipidomics profile of tilapia fillets by UPLC-Q-Extractive Orbitrap mass spectrometry. Food Chem. 2019, 298, 125029. [Google Scholar] [CrossRef] [PubMed]

| Muscle | Edible Viscera | ||||||
|---|---|---|---|---|---|---|---|
| Compound | VIP | log2(FC) | p-Value | Compounds | VIP | log2(FC) | p-Value |
| PE 18:0/20:5 | 3.28 | 2.41 | <0.01 | PE P-18:0/22:6 | 4.43 | 2.49 | <0.01 |
| PC 16:0/16:1 | 3.28 | 4.06 | <0.01 | PC 16:1/18:1 | 3.97 | 2.39 | <0.01 |
| PE P-18:0/22:6 | 2.37 | −1.75 | <0.01 | PC 18:1/18:1 | 3.37 | 3.49 | <0.01 |
| SM 12:1;2O/20:0 | 2.21 | 2.39 | <0.01 | PE P-18:0/20:5 | 3.16 | 1.20 | <0.01 |
| PC 18:1/18:1 | 2.08 | 1.94 | <0.01 | TAG 16:1/16:1/18:1 | 2.68 | 5.02 | <0.01 |
| PC 20:5/20:5 | 1.96 | −1.16 | <0.01 | PC 22:2/18:5 | 2.33 | 2.14 | <0.01 |
| PC 22:2/18:5 | 1.93 | 1.20 | <0.01 | PC 17:1/20:5 | 2.23 | 4.38 | <0.01 |
| PE P-18:1/22:6 | 1.78 | −2.46 | <0.01 | PE P-16:0/22:6 | 2.04 | 1.14 | <0.01 |
| SM 14:1;2O/18:0 | 1.78 | −2.40 | <0.01 | PE P-18:1/22:6 | 1.95 | 1.88 | <0.01 |
| PE 17:0/18:2 | 1.61 | 1.48 | <0.01 | PE P-20:1/22:6 | 1.76 | 3.68 | <0.01 |
| PC 15:1/20:5 | 1.56 | −1.22 | <0.01 | TAG 16:0/16:0/20:5 | 1.70 | 1.81 | <0.01 |
| SM 14:1;2O/19:0 | 1.56 | 2.85 | <0.01 | PE P-17:0/22:6 | 1.65 | 3.42 | <0.01 |
| PE P-19:0/20:4 | 1.52 | −1.03 | <0.01 | PC 16:1/20:5 | 1.65 | 3.36 | <0.01 |
| SM 14:1;2O/21:0 | 1.51 | 4.24 | <0.01 | PE 18:0/20:5 | 1.54 | 1.92 | <0.01 |
| CL 18:0/18:1/20:3/22:6 | 1.46 | 2.44 | <0.01 | PE P-16:0/20:5 | 1.36 | 3.73 | <0.01 |
| TAG 16:0/16:1/18:1 | 1.45 | −2.36 | <0.01 | LPC 18:1 | 1.33 | 1.91 | <0.01 |
| PC 17:1/18:1 | 1.41 | 7.12 | <0.01 | PC 15:1/20:5 | 1.33 | 2.21 | <0.01 |
| CL 18:1/18:2/22:6/22:6 | 1.37 | −2.39 | <0.01 | ||||
| PC 16:1/20:5 | 1.36 | 2.33 | <0.01 | ||||
| TAG 16:0/16:1/20:1 | 1.35 | 2.40 | <0.01 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Zhu, J.; Zhao, M.; Zhou, L.; Marchioni, E. Untargeted Lipidomics Method for the Discrimination of Five Crab Species by Ultra-High-Performance Liquid Chromatography High-Resolution Mass Spectrometry Combined with Chemometrics. Molecules 2023, 28, 3653. https://doi.org/10.3390/molecules28093653
Yao J, Zhu J, Zhao M, Zhou L, Marchioni E. Untargeted Lipidomics Method for the Discrimination of Five Crab Species by Ultra-High-Performance Liquid Chromatography High-Resolution Mass Spectrometry Combined with Chemometrics. Molecules. 2023; 28(9):3653. https://doi.org/10.3390/molecules28093653
Chicago/Turabian StyleYao, Jiaxu, Jinrui Zhu, Minjie Zhao, Li Zhou, and Eric Marchioni. 2023. "Untargeted Lipidomics Method for the Discrimination of Five Crab Species by Ultra-High-Performance Liquid Chromatography High-Resolution Mass Spectrometry Combined with Chemometrics" Molecules 28, no. 9: 3653. https://doi.org/10.3390/molecules28093653
APA StyleYao, J., Zhu, J., Zhao, M., Zhou, L., & Marchioni, E. (2023). Untargeted Lipidomics Method for the Discrimination of Five Crab Species by Ultra-High-Performance Liquid Chromatography High-Resolution Mass Spectrometry Combined with Chemometrics. Molecules, 28(9), 3653. https://doi.org/10.3390/molecules28093653






