Supramolecular β-Cyclodextrin-Quercetin Based Metal–Organic Frameworks as an Efficient Antibiofilm and Antifungal Agent
Abstract
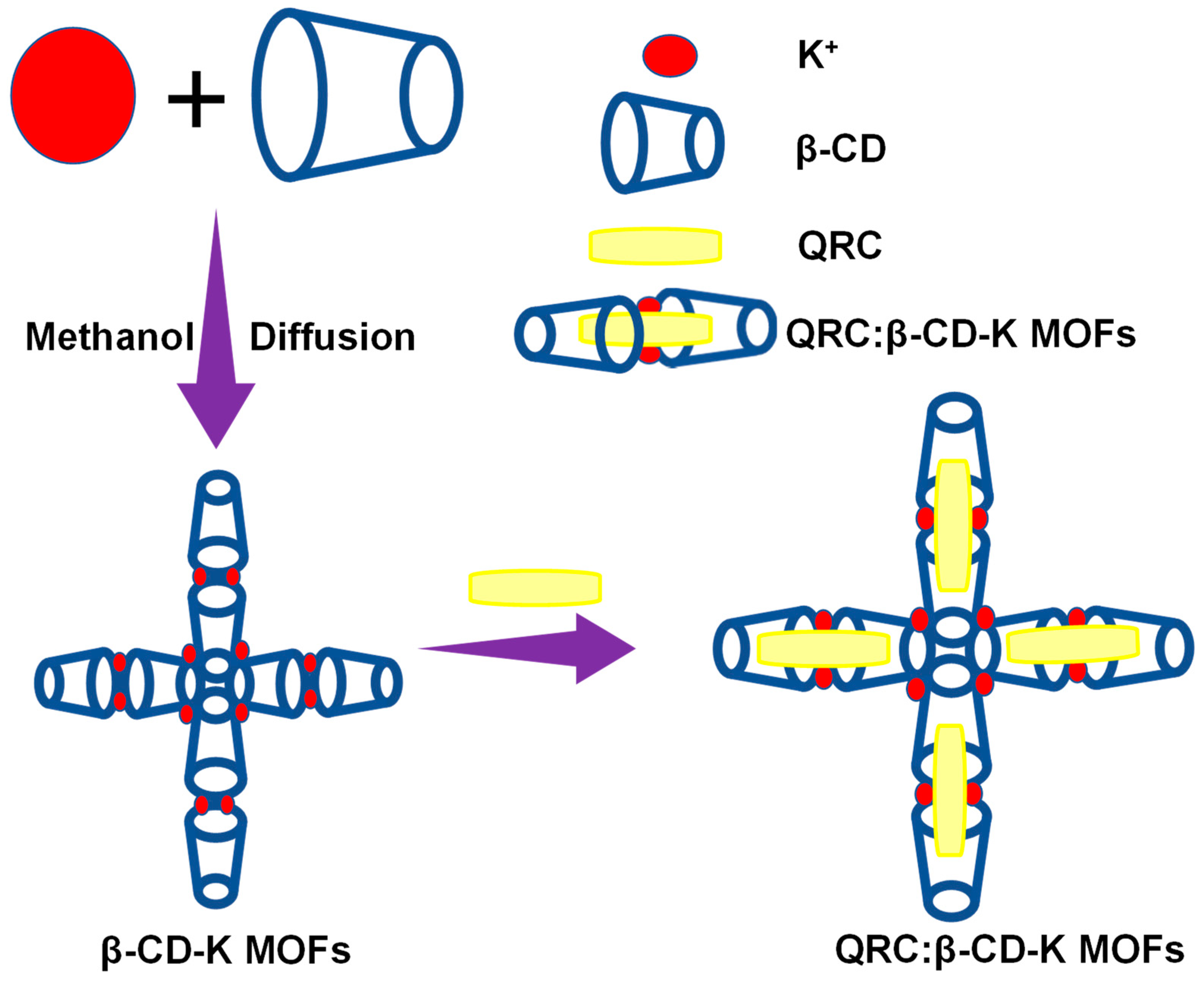
:1. Introduction
2. Results and Discussion
2.1. Interaction of QRC:β-CD in the Virtual State
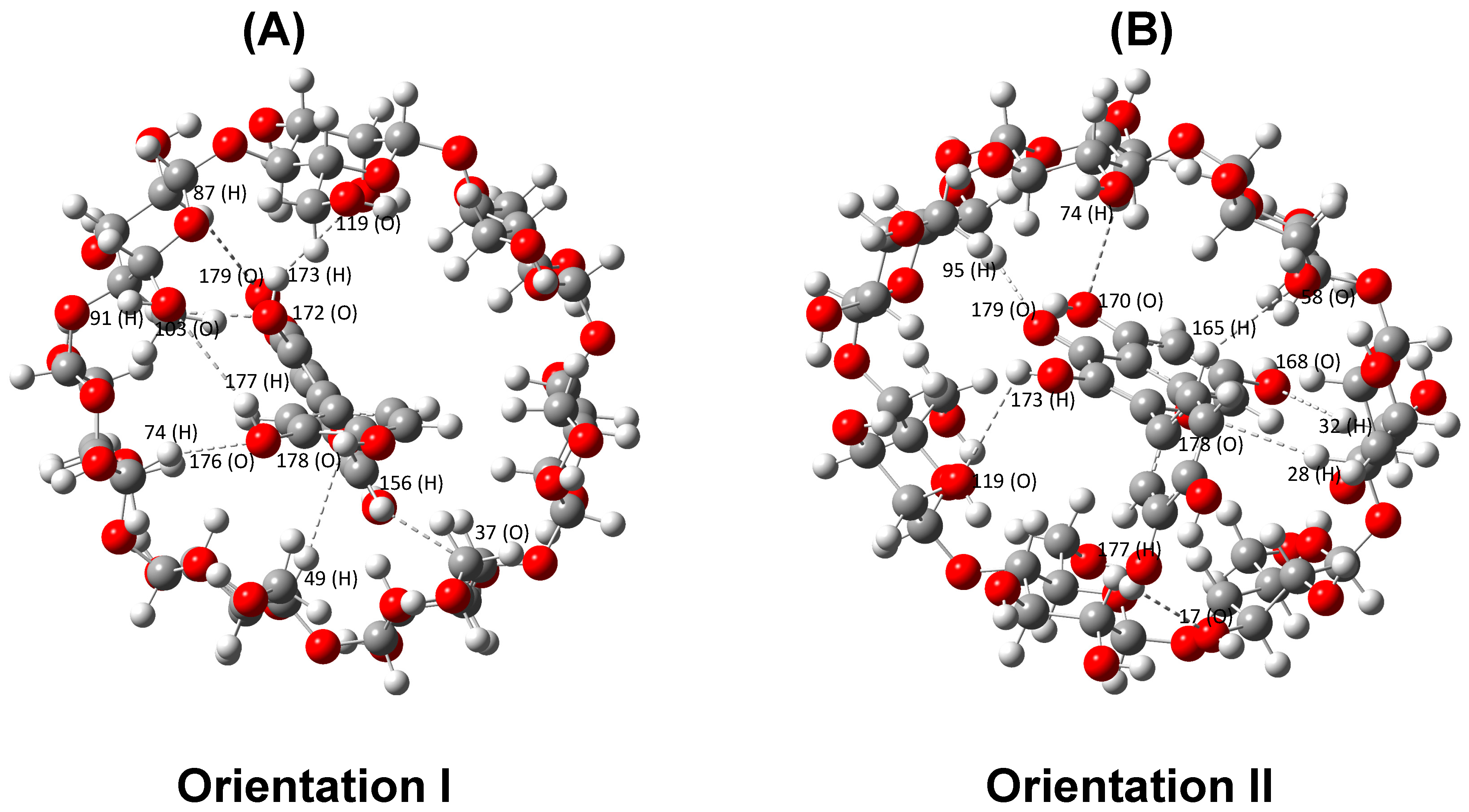
2.1.1. Single-Point Energy Computation for the Energetically Favorable Model
2.1.2. EHOMO-ELUMO of Frontier Molecular Orbitals of ICs
2.2. Spectral Analysis of ICs Based MOFs
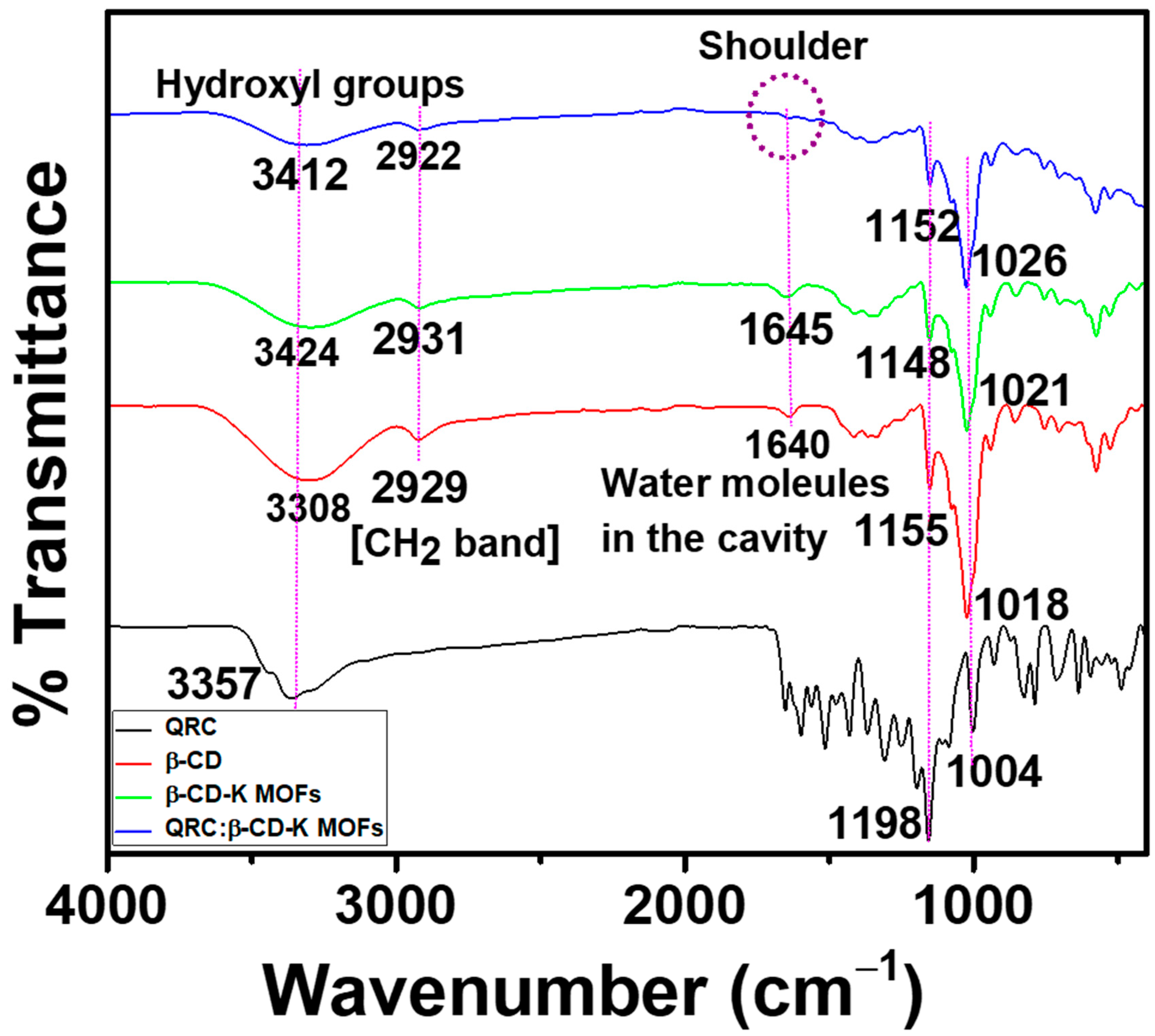
2.2.1. FT-IR Spectral Analysis
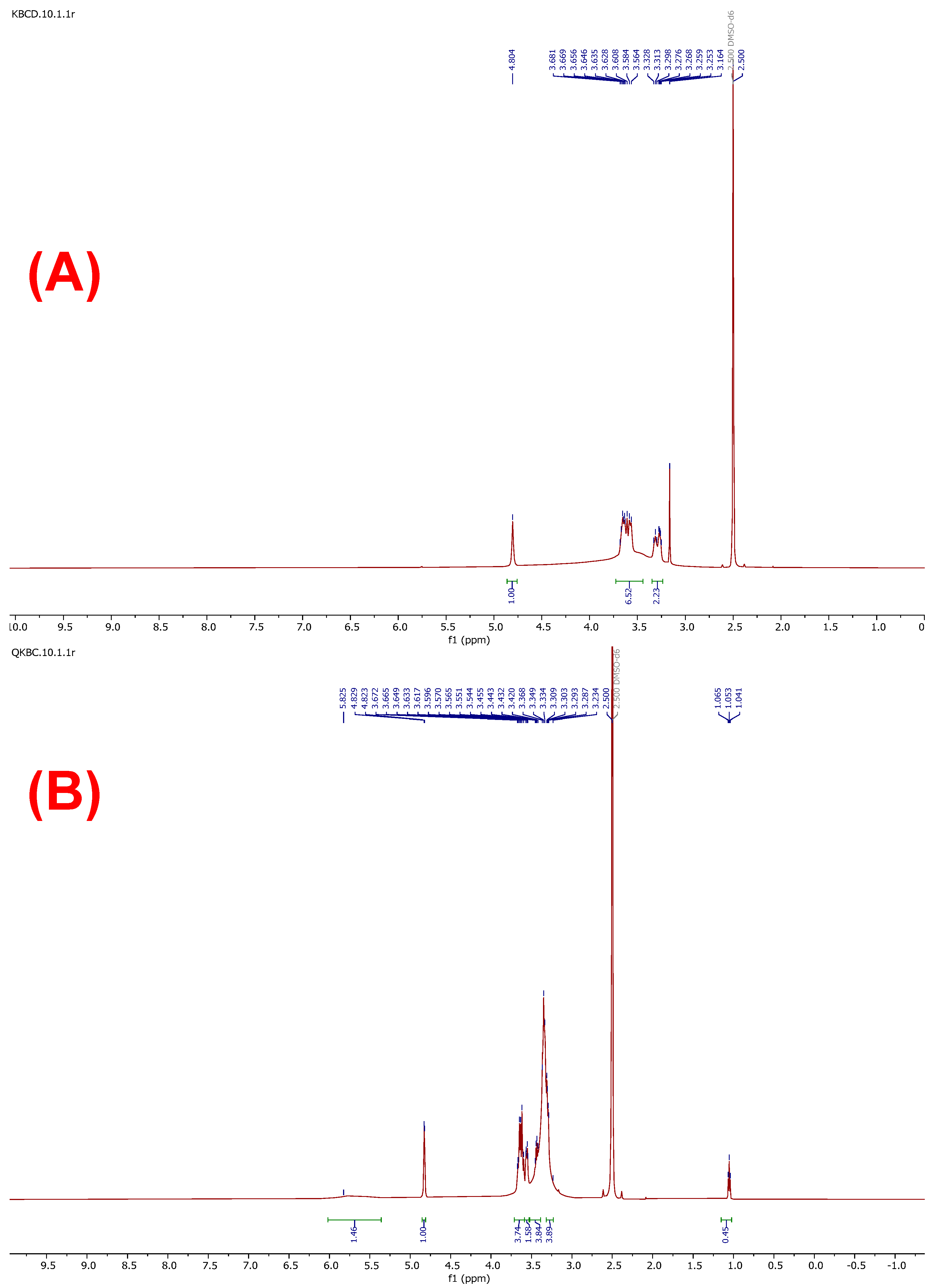
2.2.2. NMR Spectral Analysis
2.2.3. XRD Analysis
2.2.4. FE-SEM Image Analysis
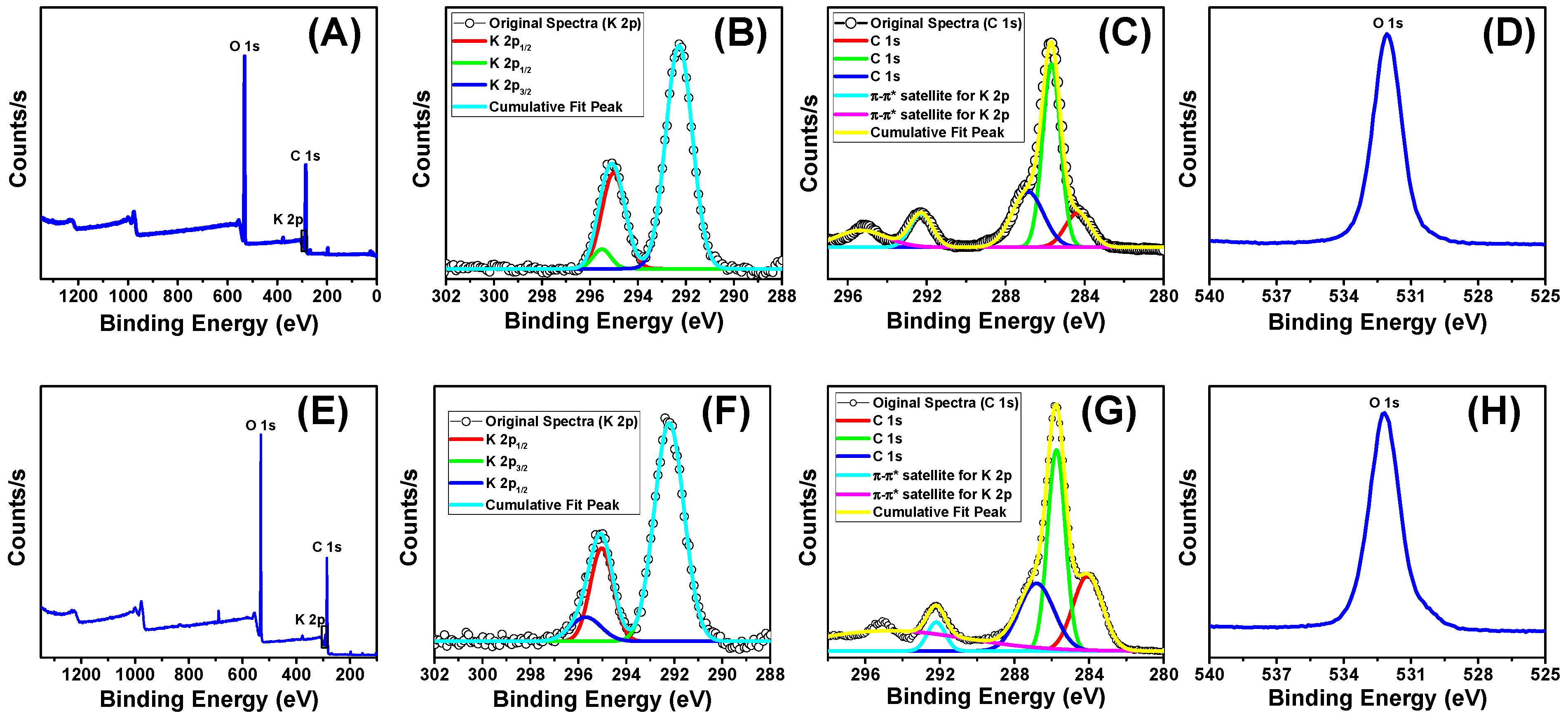
2.2.5. XPS Analysis
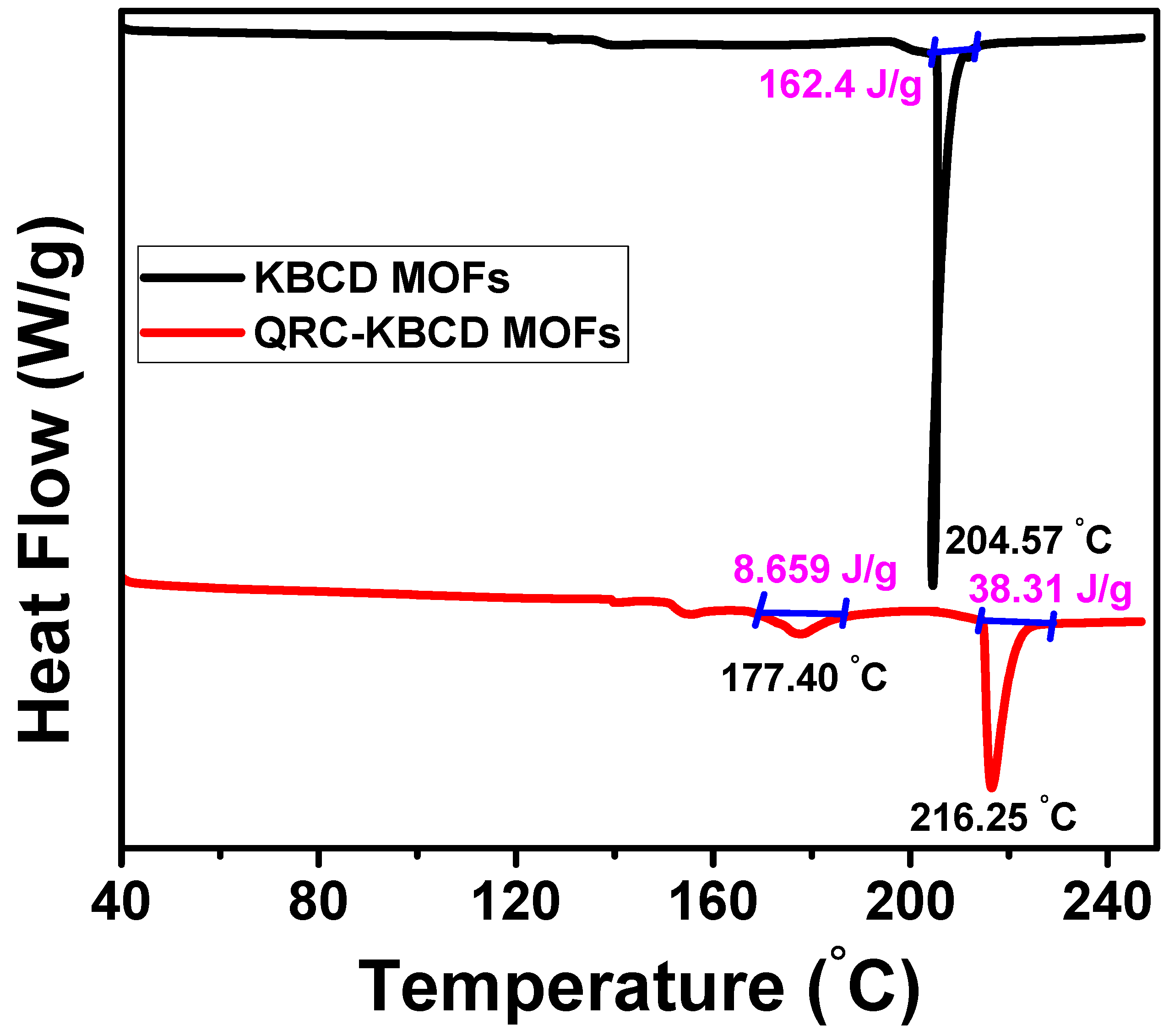
2.2.6. DSC Analysis
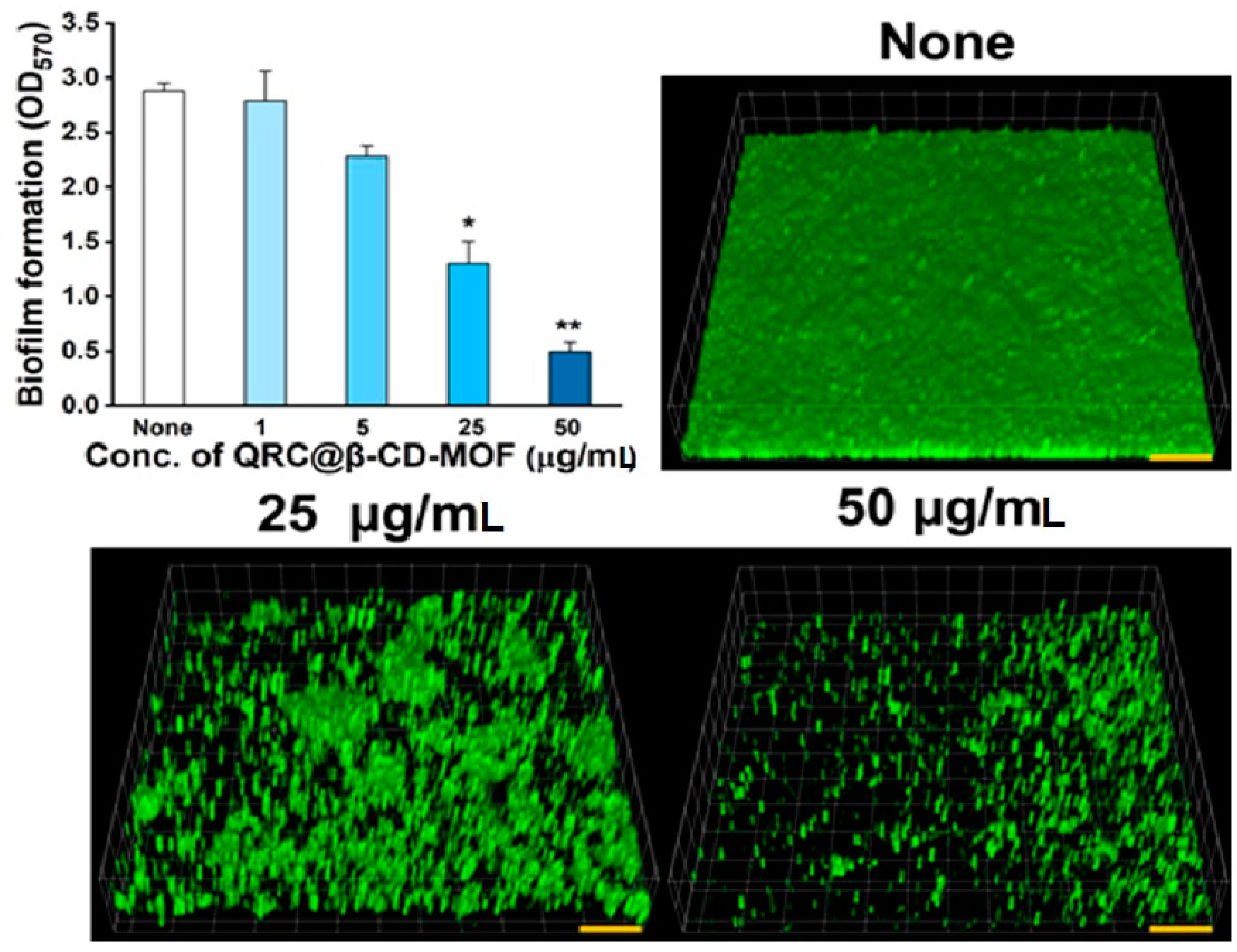
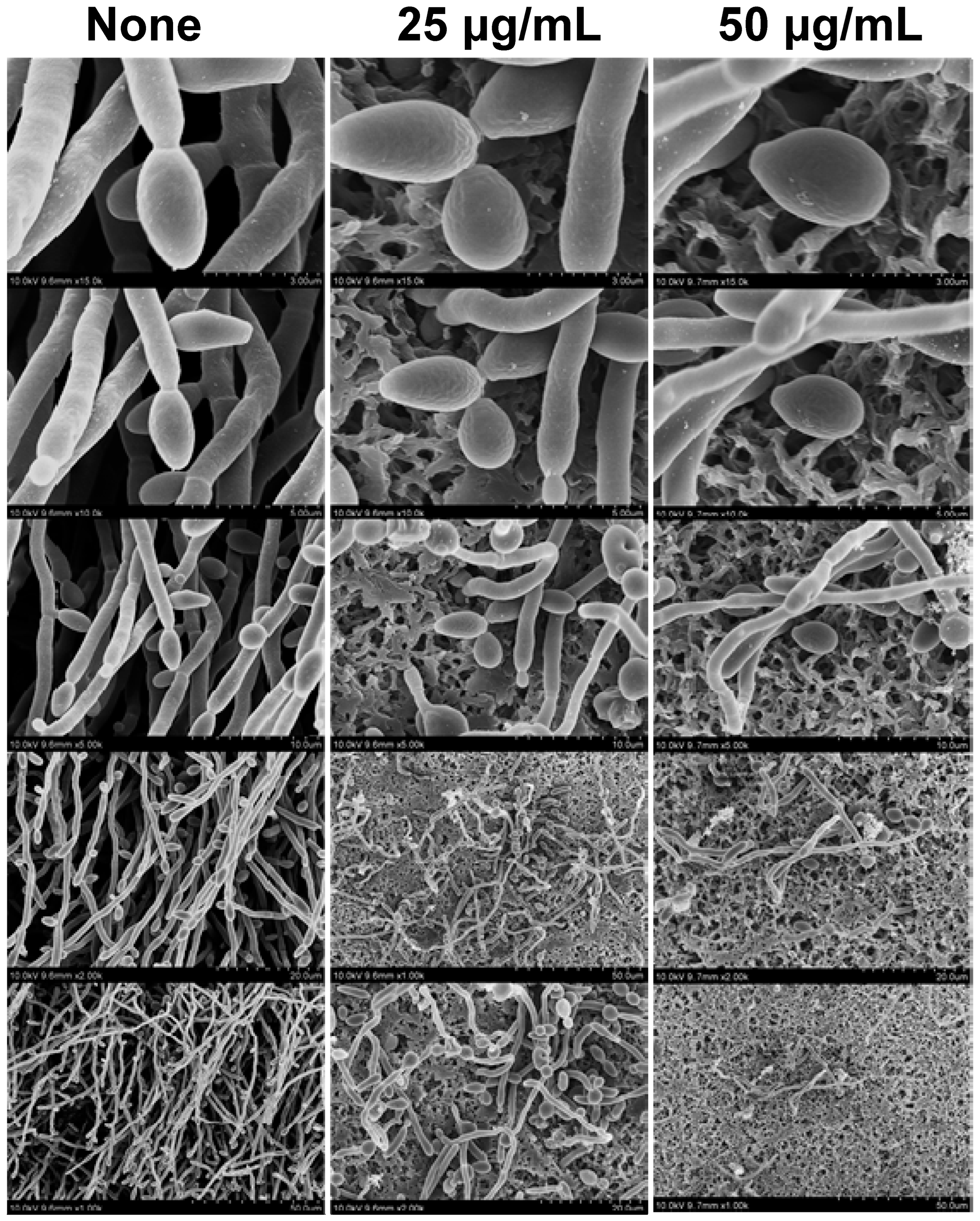
2.3. Antibiofilm Potency and SEM Analysis of QRC:β-CD-K MOFs Treated C. albicans
3. Materials and Methods
3.1. Materials
3.2. Preparation of β-CD-K MOFs
3.3. QRC Adsorption Process (QRC:β-CD-K MOFs)
3.4. Quantum Mechanical Calculations
3.5. Experimental Section and Materials for Biofilm
3.6. Antibiofilm Potency of QRC:β-CD-K MOF against C. albicans
3.7. Architecture of C. albicans Biofilm
3.8. Biofilm Observations by Confocal Laser Scanning Microscopy
3.9. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xue, L.; Tao, G.; Laurent, L.; Francesco, M.; Mario, M.-M.; Ilse, M.; Zhen, G.; Li, W.; Jiwen, Z.; Ruxandra, G. Cyclodextrin-based metal-organic frameworks particles as efficient carriers for lansoprazole: Study of morphology and chemical composition of individual particles. Int. J. Pharm. 2017, 531, 424–432. [Google Scholar]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal—Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Della, R.; Liu, D.; Lin, W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Su, H.; Sun, F.; Jia, J.; He, H.; Wang, A.; Zhu, G. A highly porous medical metal– organic framework constructed from bioactive curcumin. Chem. Commun. 2015, 51, 5774–5777. [Google Scholar] [CrossRef]
- Sontz, P.A.; Bailey, J.B.; Ahn, S.; Tezcan, F.A. A Metal Organic Framework with Spherical Protein Nodes: Rational Chemical Design of 3D Protein Crystals. J. Am. Chem. Soc. 2015, 137, 11598–11601. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal-organic frameworks for biological and medical applications. Ang. Chem. Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Sophie, F.; Grégorio, C.; Eric, L. Fundamentals and applications of cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis Environmental Chemistry for a Sustainable World; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Liu, B.; Li, H.; Xu, X.; Li, X.; Lv, N.; Singh, V.; Stoddart, F.; Yorka, P.; Xu, X.; Gref, R.; et al. Optimized synthesis and crystalline stability of c-cyclodextrin metal-organic frameworks for drug adsorption. Int. J. Pharm. 2016, 514, 212–219. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal-organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Liu, J.; Bao, T.-Y.; Yang, X.-Y.; Zhu, P.-P.; Wu, L.-H.; Sha, J.-Q.; Zhang, L.; Dong, L.-Z.; Cao, X.-L.; Lan, Y.-Q. Controllable porosity conversion of metal-organic frameworks composed of natural ingredients for drug delivery. Chem. Commun. 2017, 53, 7804–7807. [Google Scholar] [CrossRef]
- Ding, H.; Wu, L.; Guo, T.; Zhang, Z.; Bello, M.G.; Gao, G.; He, S.; Zhang, W.; Chen, Y.; Lin, Y.; et al. CD-MOFs crystal transformation from dense to highly porous form for efficient drug loading. Cryst. Growth Des. 2019, 19, 3888–3894. [Google Scholar] [CrossRef]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal-organic frameworks from edible natural products. Ang. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef]
- Abucafy, M.P.; Caetano, B.L.; Chiari-Andreo, B.G.; Fonseca-Santos, B.; do Santos, A.M.; Chorilli, M.; Chiavacci, L.A. Supramolecular cyclodextrin-based metal-organic frameworks as efficient carrier for anti-inflammatory drugs. Eur. J. Pharm. Biopharm. 2018, 127, 112–119. [Google Scholar] [CrossRef]
- Li, X.; Porcino, M.; Martineau-Corcos, C.; Guo, T.; Xiong, T.; Zhu, W.; Patriarche, G.; Péchoux, C.; Perronne, B.; Hassan, A.; et al. Efficient incorporation and protection of lansoprazole in cyclodextrin metal-organic frameworks. Int. J. Pharm. 2020, 585, 119442. [Google Scholar] [CrossRef]
- Sha, J.; Yang, X.; Sun, L.; Zhang, X.; Li, S.; Li, J.; Sheng, N. Unprecedented a-cyclodextrin metal-organic frameworks with chirality: Structure and drug adsorptions. Polyhedron 2017, 127, 396–402. [Google Scholar] [CrossRef]
- Lu, H.; Yang, X.; Li, S.; Zhang, Y.; Sha, J.; Li, C.; Sun, J. Study on a new cyclodextrin based metal–organic framework with chiral helices. Inorg. Chem. Comm. 2015, 61, 48–52. [Google Scholar] [CrossRef]
- Magri, A.; Petriccione, M.; Gutiérrez, T.J. Metal-organic frameworks for food applications: A review. Food Chem. 2021, 354, 129533. [Google Scholar] [CrossRef]
- Mofei, S.; Jianwei, Z.; Mohamed, E.; Yunlei, X.; Jinsong, F.; Donghong, L.; Tian, D. Cyclodextrin metal–organic framework by ultrasound-assisted rapid synthesis for caffeic acid loading and antibacterial application. Ultrason. Sonochem. 2022, 86, 106003. [Google Scholar]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal–organic frameworks and their composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef]
- Hamedi, A.; Anceschi, A.; Patrucco, A.; Hasanzadeh, M. A γ-cyclodextrin-based metal-organic framework (γ-CD-MOF): A review of recent advances for drug delivery application. J. Drug Target. 2022, 30, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rashwan, A.K.; Osman, A.I.; Abd El-Monaem, E.M.; Elgarahy, A.M.; Eltaweil, A.S.; Omar, M.; Li, Y.; Mehanni, A.E.; Chen, W.; et al. Synthesis and potential applications of cyclodextrin-based metal-organic frameworks: A review. Environ. Chem. Lett. 2023, 21, 447–477. [Google Scholar] [CrossRef] [PubMed]
- Alexander Victor, A.D.; Radhakrishnan, A.; Subramani, P. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. Biomed. Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F. Flavonols (Kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Zizkova, P.; Stefek, M.; Rackova, L.; Prnova, M.; Horakova, L. Novel quercetin derivatives: From redox properties to promising treatment of oxidative stress related diseases. Chem. Biol. Interact. 2017, 265, 36–46. [Google Scholar] [CrossRef]
- Konstantina, M.; Paraskevi, P.; Maria, C.; Dimitrios, A.D.; Dimitrios, S.; Vasiliki, V.; Nikolaos, N.; Maria, V.C.; Ioannis, A.; Kalliopi, M.; et al. Preparation and Biophysical Characterization of Quercetin Inclusion Complexes with β-Cyclodextrin Derivatives to be Formulated as Possible Nose-to-Brain Quercetin Delivery Systems. Mol. Pharm. 2020, 17, 4241–4255. [Google Scholar]
- Zeynep, A.; Semran, I.K.; Engin, D.; Tamer, U. Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chem. 2016, 197 Pt A, 864–871. [Google Scholar]
- Greice, S.B.; Ivana, S.L.; Ruben, D.S.; Valquiria, L.B. Quercetin/β-Cyclodextrin Solid Complexes Prepared in Aqueous Solution Followed by Spray-drying or by Physical Mixture. AAPS PharmSciTech 2009, 10, 235–242. [Google Scholar]
- Nutsarun, W.; Chasuda, C.; Sonthaya, C.; Pongpol, E.; Orawan, S.; Piyachat, C.; Supanna, T.; Pitt, S. Quercetin/Hydroxypropyl-β-Cyclodextrin Inclusion Complex-Loaded Hydrogels for Accelerated Wound Healing. Gels 2022, 8, 573. [Google Scholar]
- Arumugam, P.; Samikannu, P.; Rajaram, R. Encapsulation of quercetin in β-cyclodextrin and (2-hydroxypropyl)-β-cyclodextrin cavity: In-vitro cytotoxic evaluation. J. Macromol. Sci. Part A 2017, 54, 894–901. [Google Scholar]
- Zhenjiong, W.; Yiding, M.A.; Ying, J.; Feng, Z.; Yulong, W.; Haitao, J.; Renlei, W.; Qing, X.; Chun, H. Encapsulating quercetin in cyclodextrin metal-organic frameworks improved its solubility and bioavailability. J. Sci. Food Agric. 2022, 102, 3887–3896. [Google Scholar]
- Sivakumar, K.; Komathi, V.; Murali Krishnan, M. Dinitrophenylhydrazine: β-cyclodextrin inclusion complex as a novel fluorescent chemosensor probe for Ce4+. Res. Chem. Intermed. 2018, 44, 5301–5327. [Google Scholar] [CrossRef]
- Sivakumar, K.; Parinamachivayam, G.; Murali Krishnan, M.; Sujay, C.; Bharathi, A. Preparation, characterization and molecular modeling studies of the beta-cyclodextrin inclusion complex with benzoguanamine and its analytical application as chemosensor for the selective sensing of Ce4+. Spectrochim. Acta A 2018, 200, 212–225. [Google Scholar] [CrossRef]
- Arumugam, P.; Samikannu, P.; Fatiha, M.; Rajaram, R. Theoretical Investigation of Inclusion Complexes of 3-Hydroxyflavone and Quercetin as Guests with Native and Modified β-Cyclodextrins as Hosts. Pol. Aromat. Compd. 2023, 43, 141–153. [Google Scholar]
- Moorthiraman, M.; Rajaram, R.; Arumugam, A.; Madi, F. Non-Covalent Bonding Interaction between Primaquine as Guest and 2-(Hydroxypropyl)-β-Cyclodextrin as Host. Pol. Aromat. Compd. 2022, 42, 1861–1878. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Tatyana, V.; Artem, S.; Irina, T. Metal–organic frameworks based on b-cyclodextrin: Design and selective entrapment of non-steroidal antiinflammatory drugs. J. Mat. Sci. 2020, 55, 13193–13205. [Google Scholar]
- Meta, K.L.; Janka, S.; Eva, T.B.; Samo, K. FT-IR-based method for rutin, quercetin and quercitrin quantification in different buckwheat (Fagopyrum) species. Sci. Rep. 2017, 7, 7226. [Google Scholar]
- Aohui, Y.; Huijun, L.; Zhendong, L.; Liuxing, L.; Wei, L.; Kai, L. Green synthesis of β-cyclodextrin metal–organic frameworks and the adsorption of quercetin and emodin. Polyhedron 2019, 159, 116–126. [Google Scholar]
- Desislava, K.; Yordan, S.; Stela, S.-A.; Margarita, D. Quercetin Hybrids-Synthesis, Spectral Characterization and Radical Scavenging Potential. Molbank 2022, 2022, M1329. [Google Scholar]
- Hasmukh, A.P.; Timur, I.; Zhichang, L.; Siva Krishna Mohan, N.; Avik, S.; Ommid, A.; Christos, D.M.; Omar, K.F.; Fraser, S. Noninvasive Substitution of K+ Sites in Cyclodextrin Metal−Organic Frameworks by Li+ Ions. J. Am. Chem. Soc. 2017, 139, 11020–11023. [Google Scholar]
- Li, Y.; Haobin, H.; Chongwei, D.; Xiaoping, Z.; Hong, L. β-Cyclodextrin-based metal-organic framework as a carrier for zero-order drug delivery. Mat. Lett. 2021, 300, 129766. [Google Scholar] [CrossRef]
- Chang, L.; Peng, W.; Xueke, L.; Xiaotong, Y.; Zhiqiang, Z.; Donghui, L. Multifunctional β-Cyclodextrin MOF-Derived Porous Carbon as Efficient Herbicides Adsorbent and Potassium Fertilizer. ACS Sustain. Chem. Eng. 2019, 7, 14479–14489. [Google Scholar]
- Huiping, C.; Yongpan, S.; Chunli, X.; Muhammad, B.; Pengyue, Z.; Chong, C.; Hongjun, Z.; Qiliang, H.; Lidong, C. Multifunctional γ-Cyclodextrin-Based Metal–Organic Frameworks as Avermectins Carriers for Controlled Release and Enhanced Acaricidal Activity. ACS Agric. Sci. Technol. 2023, 3, 190–202. [Google Scholar]
- Chao, Q.; Jinpeng, W.; Huang, Z.; Yang, Q.; Xueming, X.; Zhengyu, J. Novel Approach with Controlled Nucleation and Growth for Green Synthesis of Size-Controlled Cyclodextrin-Based Metal–Organic Frameworks Based on Short-Chain Starch Nanoparticles. J. Agric. Food Chem. 2018, 66, 9785–9793. [Google Scholar]
- Hao, P.; Zhu, T.; Su, Q.; Lin, J.; Cui, R.; Cao, X.; Wang, Y.; Pan, A. Electrospun Single Crystalline Fork-Like K2V8O21 as High-Performance Cathode Materials for Lithium-Ion Batteries. Front. Chem. 2018, 6, 195. [Google Scholar] [CrossRef]
- Wang, S.; Shao, G.; Zhao, H.; Yang, L.; Zhu, L.; Liu, H.; Cui, B.; Zhu, D.; Li, J.; He, Y. Covering soy polysaccharides gel on the surface of β-cyclodextrin-based metal–organic frameworks. J. Mater. Sci. 2021, 56, 3049–3061. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, J. In Situ Growth of Cyclodextrin-Based Metal Organic Framework Air Filters for Reusable SO2 Adsorbent Applications. Macromol. Mater. Eng. 2023, 2200645. [Google Scholar] [CrossRef]
- Sousa Filho, L.F.; Santos, M.M.B.; dos Passos Menezes, P.; dos Santos Lima, B.; de Souza Araújo, A.A.; de Oliveira, E.D. A novel quercetin/β-cyclodextrin transdermal gel, combined or not with therapeutic ultrasound, reduces oxidative stress after skeletal muscle injury. RSC Adv. 2021, 11, 27837–27844. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.G.; Li, D.; Tan, H.Q.; Zhao, Z.; Xie, Z.G.; Sun, Z.C. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef] [PubMed]
- Jinxin, C.; Keqin, C.; Jaorao, S.; Ying, T.; Okwong, O.R.; Xiumei, C.; Nengguo, T. Fabrication of γ-cyclodextrin-Based metal-organic frameworks as a carrier of cinnamaldehyde and its application in fresh-cut cantaloupes. Curr. Res. Food Sci. 2022, 5, 2114–2124. [Google Scholar]
- Koontz, J.L.; Marcy, J.E.; O’Keefe, S.F.; Duncan, S.E. Cyclodextrin Inclusion Complex Formation and Solid-State Characterization of the Natural Antioxidants α-Tocopherol and Quercetin. J. Agric. Food Chem. 2009, 57, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, S.; Potrzebowski, M.J. Solid state NMR studies and density functional theory (DFT) calculations of conformers of quercetin. Org. Biomol. Chem. 2004, 2, 2315–2322. [Google Scholar] [CrossRef]
- Sri, K.V.; Kondaiah, A.; Ratna, J.V.; Annapurna, A. Preparation and characterization of quercetin and rutin cyclodextrin inclusion complexes. Drug Dev. Ind. Pharm. 2007, 33, 245–253. [Google Scholar] [CrossRef]
- Passos Menezes, P.; dos Santos, P.B.P.; Doria, G.A.A.; de Sousa, B.M.H.; Serafini, M.R.; Nunes, P.S.; Quintas-Junior, L.J.; de Matos, L.I.; Alves, P.B.; Bezerra, D.P.; et al. Molecular Modeling and Physicochemical Properties of Supramolecular Complexes of Limonene with α- and β-Cyclodextrins. AAPS PharmSciTech 2017, 18, 49–57. [Google Scholar] [CrossRef]
- Raorane, C.J.; Lee, J.-H.; Kim, Y.-G.; Rajasekharan, S.; Rodolfo, G.-C.; Jintae, L. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microb. 2019, 10, 990. [Google Scholar] [CrossRef]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-gum arabic embedded alizarin nanocarriers inhibit biofilm formation of multispecies microorganisms. Carb. Pol. 2021, 284, 118959. [Google Scholar] [CrossRef]
- Raorane, C.J.; Lee, J.-H.; Lee, J. Rapid killing and biofilm inhibition of multidrug-resistant Acinetobacter baumannii strains and other microbes by iodoindoles. Biomolecules 2020, 10, 1186. [Google Scholar] [CrossRef]
- Runci, F.; Bonchi, C.; Frangipani, E.; Visaggio, D.; Visca, P. Acinetobacter baumannii biofilm formation in human serum and disruption by gallium. Antimicrob. Agents Chemother. 2017, 61, e01563-16. [Google Scholar] [CrossRef]
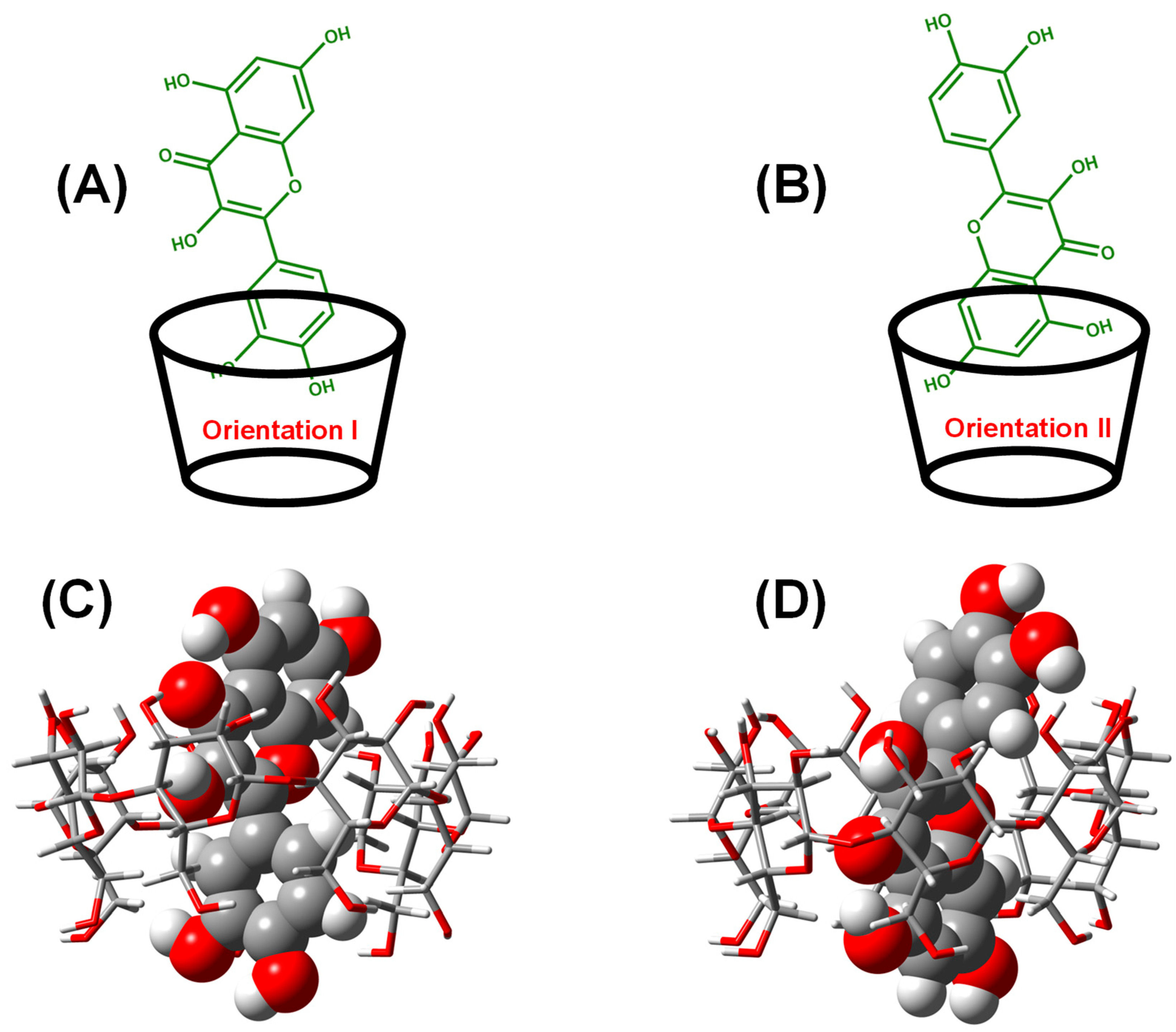




| Parameter | EOpt (kJ/mol) | ΔE (kJ/mol) | |
|---|---|---|---|
| QRC | PM3 | −945.967 | - |
| β-CD | −6072.256 | - | |
| Orientation I | −7070.472 | −52.249 | |
| Orientation II | −7052.093 | −33.870 | |
| ICs—Orientation I | ICs—Orientation II | ||
|---|---|---|---|
| Bonding Parameter | Distance (Å) | Bonding Parameter | Distance (Å) |
| 119 (O)–173 (H) | 3.069 | 74 (H)–170 (O) | 3.078 |
| 87 (H)–179 (O) | 3.022 | 95 (H)–179(O) | 2.628 |
| 91 (H)–172 (O) | 2.663 | 119 (O)–173 (H) | 2.900 |
| 103 (O)–177 (H) | 3.006 | 17 (O)–177 (H) | 2.601 |
| 74 (H)–176 (O) | 2.600 | 32 (H)–168 (O) | 3.012 |
| 49 (H)–178 (O) | 3.129 | 28 (H)–178 (O) | 3.097 |
| 156 (H)–37 (O) | 2.772 | 58 (O)–165 (H) | 2.941 |
| Molecule | HOMO (eV) | LUMO (eV) | EHOMO-ELUMO (eV) |
|---|---|---|---|
| QRC | −8.951 | −0.921 | 8.030 |
| Orientation I | −9.133 | −1.223 | 7.910 |
| Orientation II | −9.371 | −1.121 | 8.250 |
| Materials | C 1s | O 1s | K 2p | |||
|---|---|---|---|---|---|---|
| Binding Energy (eV) | Atomic Percentage | Binding Energy (eV) | Atomic Percentage | Binding Energy (eV) | Atomic Percentage | |
| β-CD-K MOFs | 285.68 | 54.90 | 532.08 | 42.80 | 292.28 | 1.57 |
| QRC:β-CD-K MOFs | 285.88 | 55.63 | 532.18 | 43.07 | 292.38 | 2.04 |
| Biofilm Biomasses (µm3 µm−2) | Mean Thicknesses (µm) | Substratum Coverages (%) | ||||
|---|---|---|---|---|---|---|
| None | 50 µg/mL | None | 50 µg/mL | None | 50 µg/mL | |
| C. albicans DAY185 | 123.02 ± 12.4 | 9.11 ± 0.6 | 106.38 ± 11.1 | 14.36 ± 2.0 | 100 ± 0.9 | 8.04 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajamohan, R.; Raorane, C.J.; Kim, S.-C.; Krishnan, M.M.; Lee, Y.R. Supramolecular β-Cyclodextrin-Quercetin Based Metal–Organic Frameworks as an Efficient Antibiofilm and Antifungal Agent. Molecules 2023, 28, 3667. https://doi.org/10.3390/molecules28093667
Rajamohan R, Raorane CJ, Kim S-C, Krishnan MM, Lee YR. Supramolecular β-Cyclodextrin-Quercetin Based Metal–Organic Frameworks as an Efficient Antibiofilm and Antifungal Agent. Molecules. 2023; 28(9):3667. https://doi.org/10.3390/molecules28093667
Chicago/Turabian StyleRajamohan, Rajaram, Chaitany Jayprakash Raorane, Seong-Cheol Kim, Mani Murali Krishnan, and Yong Rok Lee. 2023. "Supramolecular β-Cyclodextrin-Quercetin Based Metal–Organic Frameworks as an Efficient Antibiofilm and Antifungal Agent" Molecules 28, no. 9: 3667. https://doi.org/10.3390/molecules28093667






