Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts
Abstract
1. Introduction
2. Results
2.1. Preparation of Vegetal Extracts
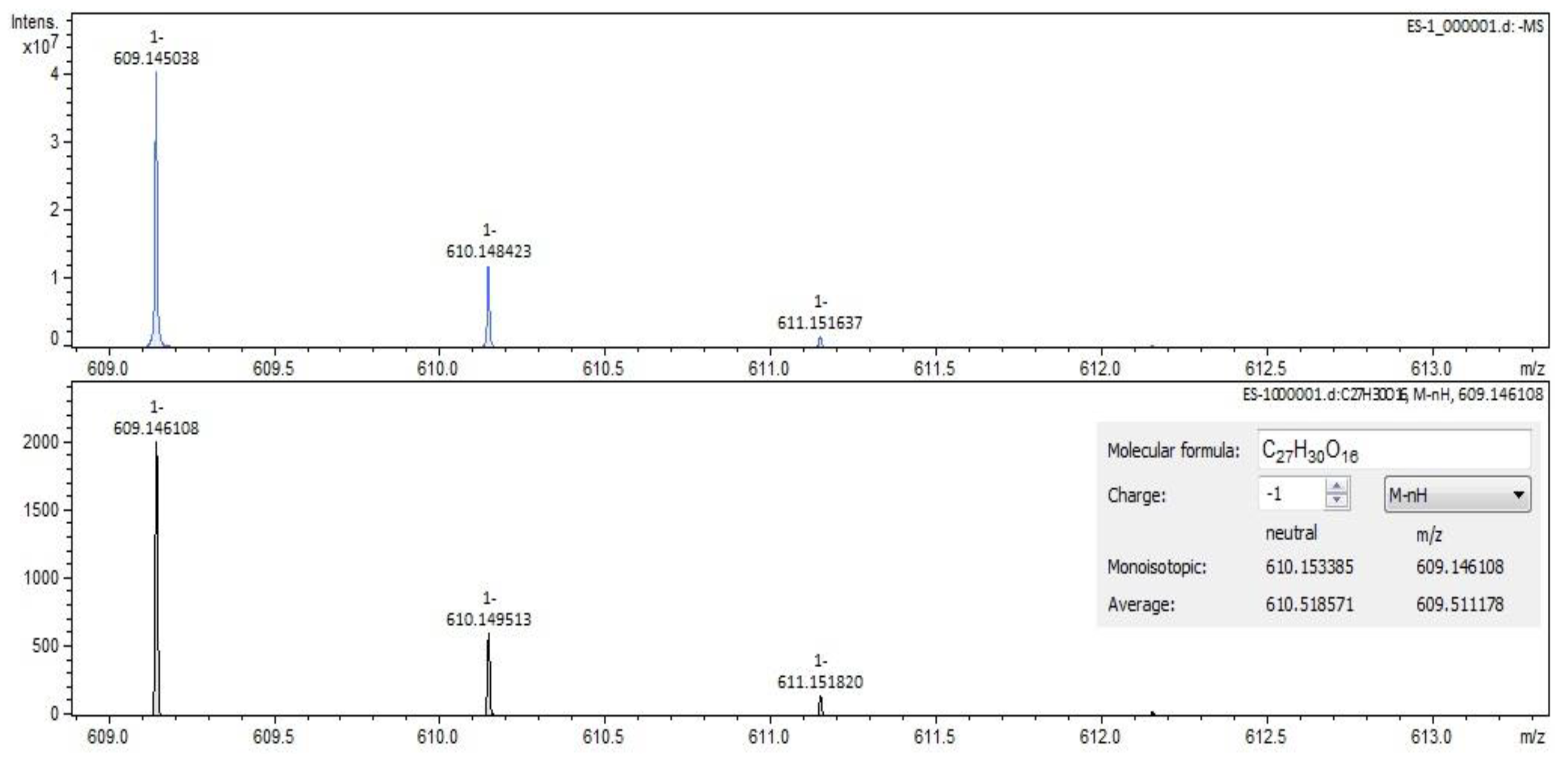
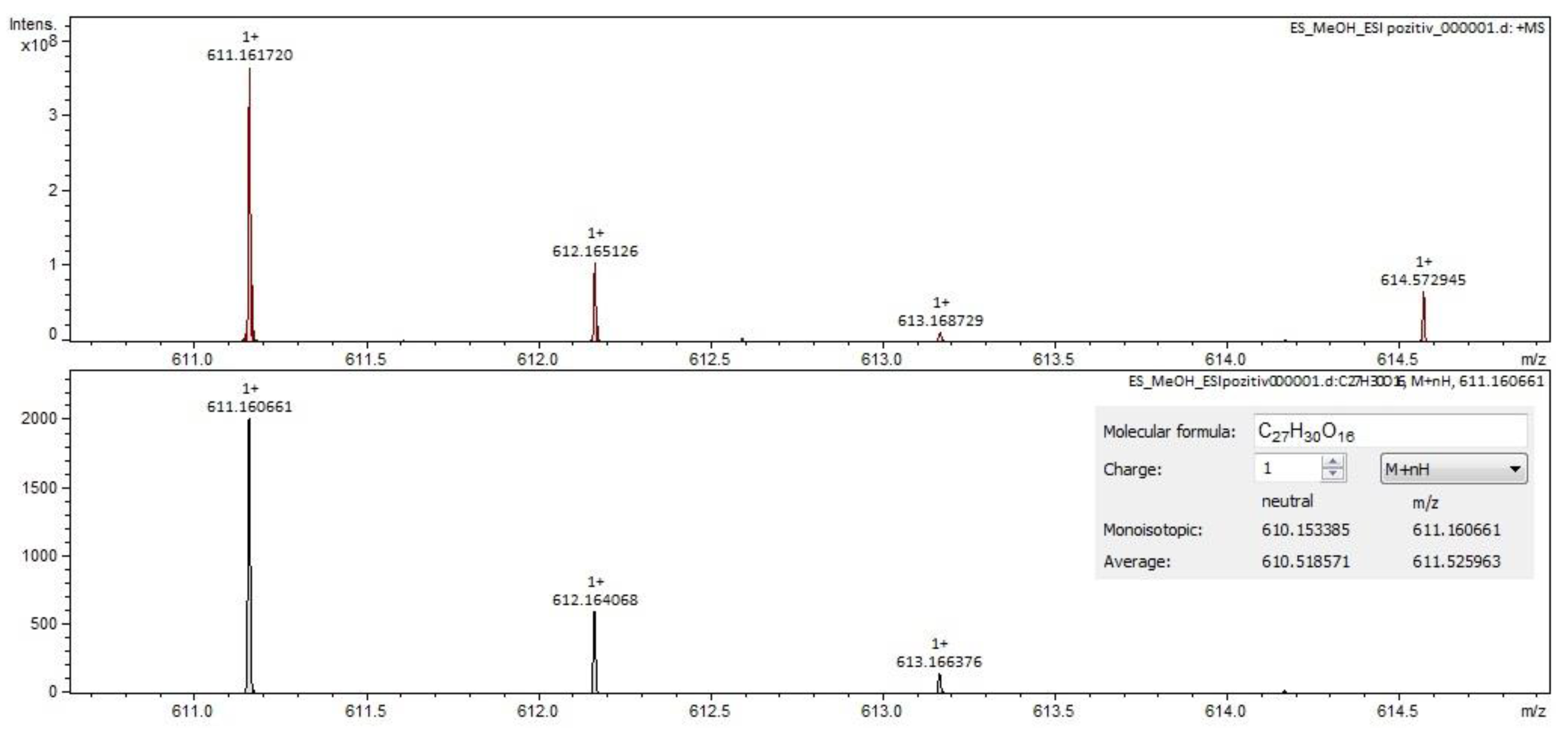
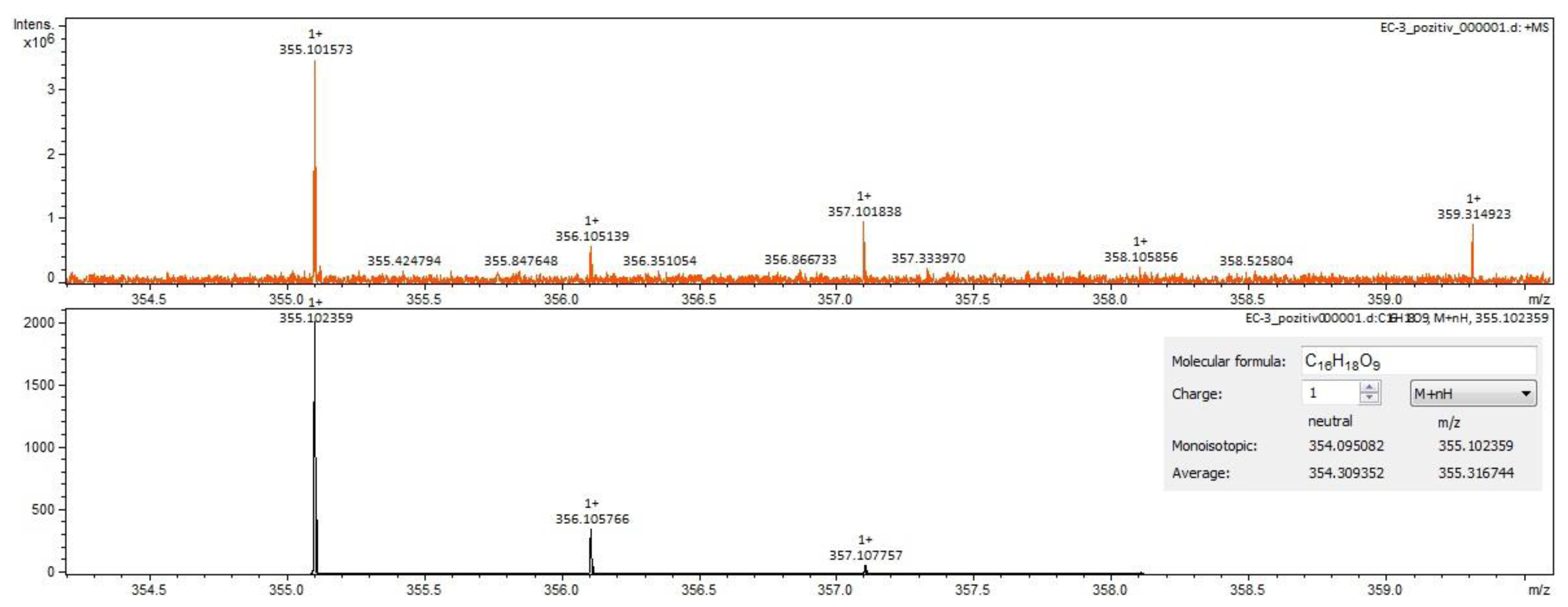
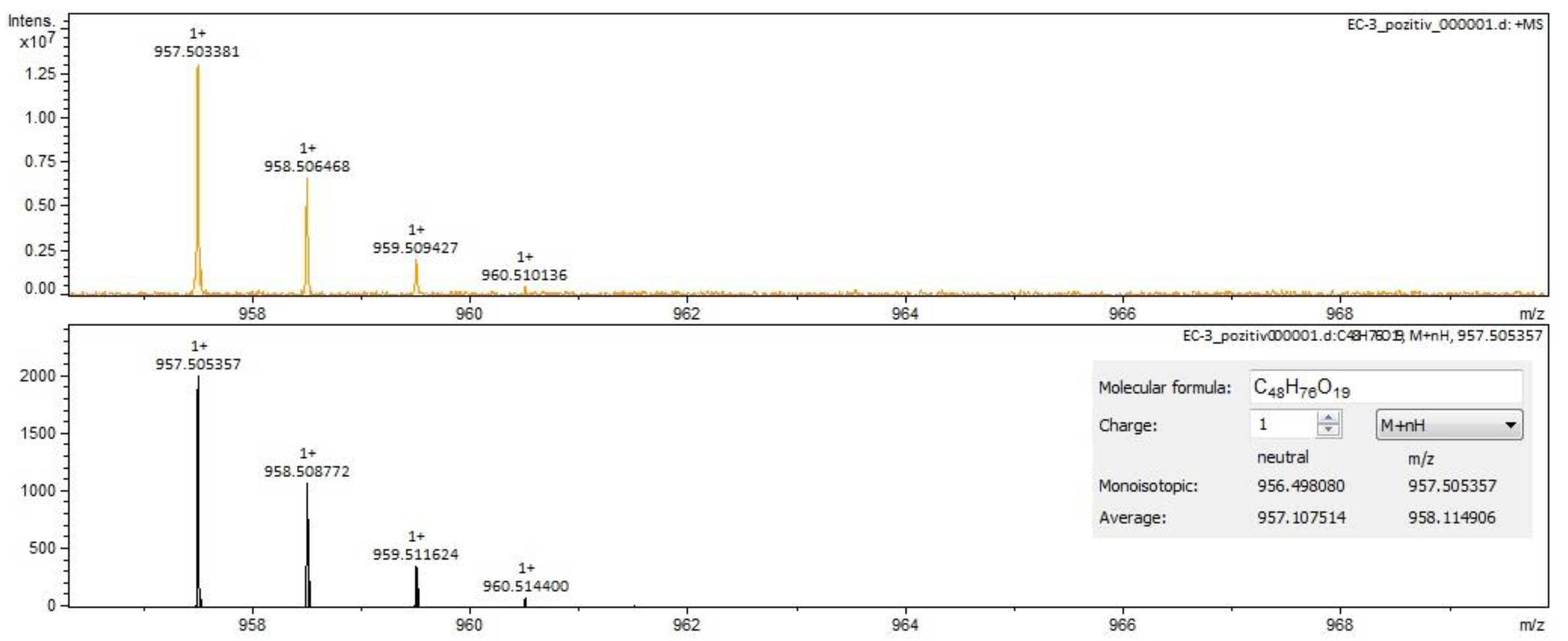
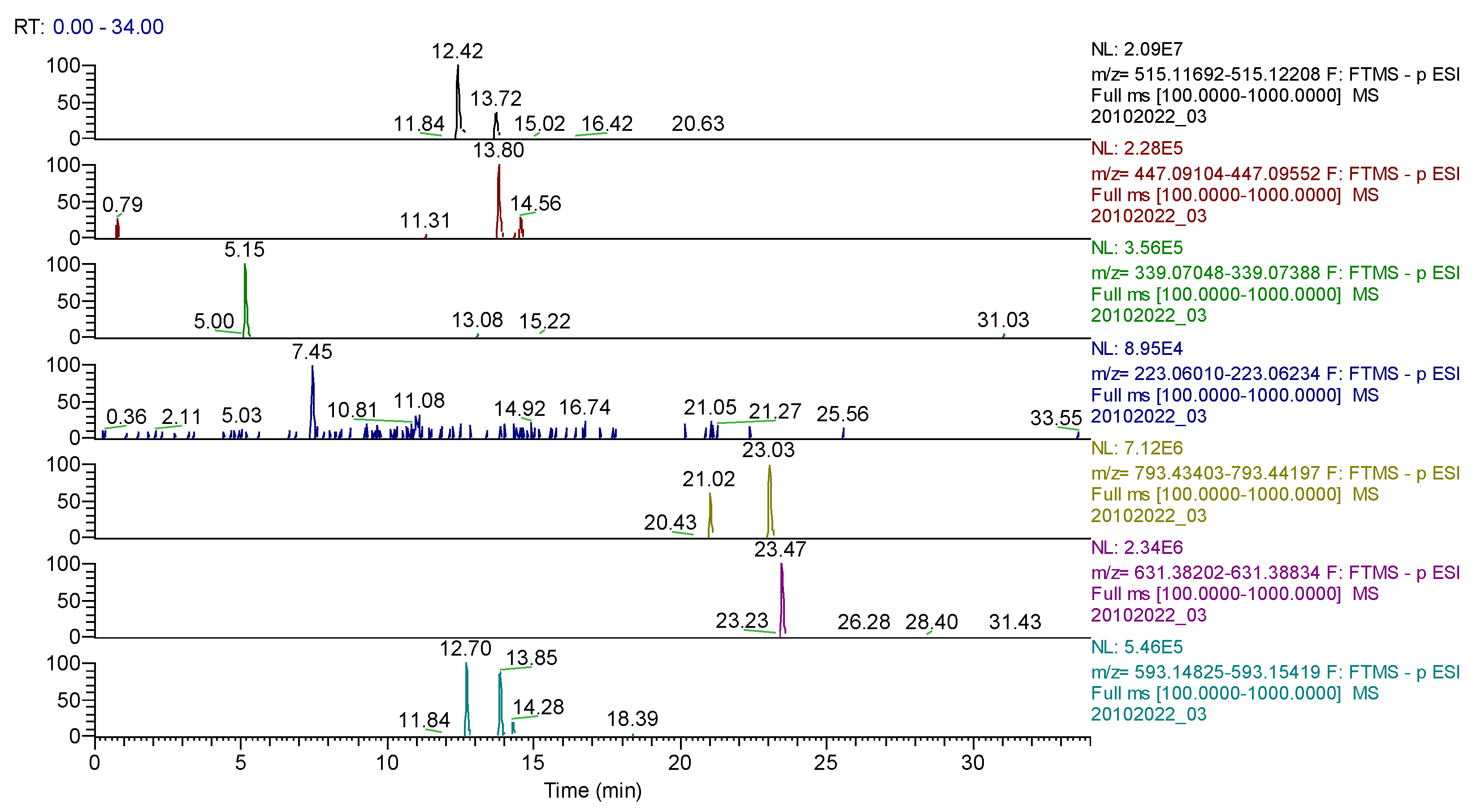
2.2. Qualitative Analysis FT-ICR-MS (Fourier-Transform Ion-Cyclotron-Resonance High-Resolution Mass Spectrometry)
2.3. Quantitative Spectrophotometric Analysis
2.4. UHPLC-HRMS/MS Analysis
2.5. Antioxidant Activity and Correlation Analysis
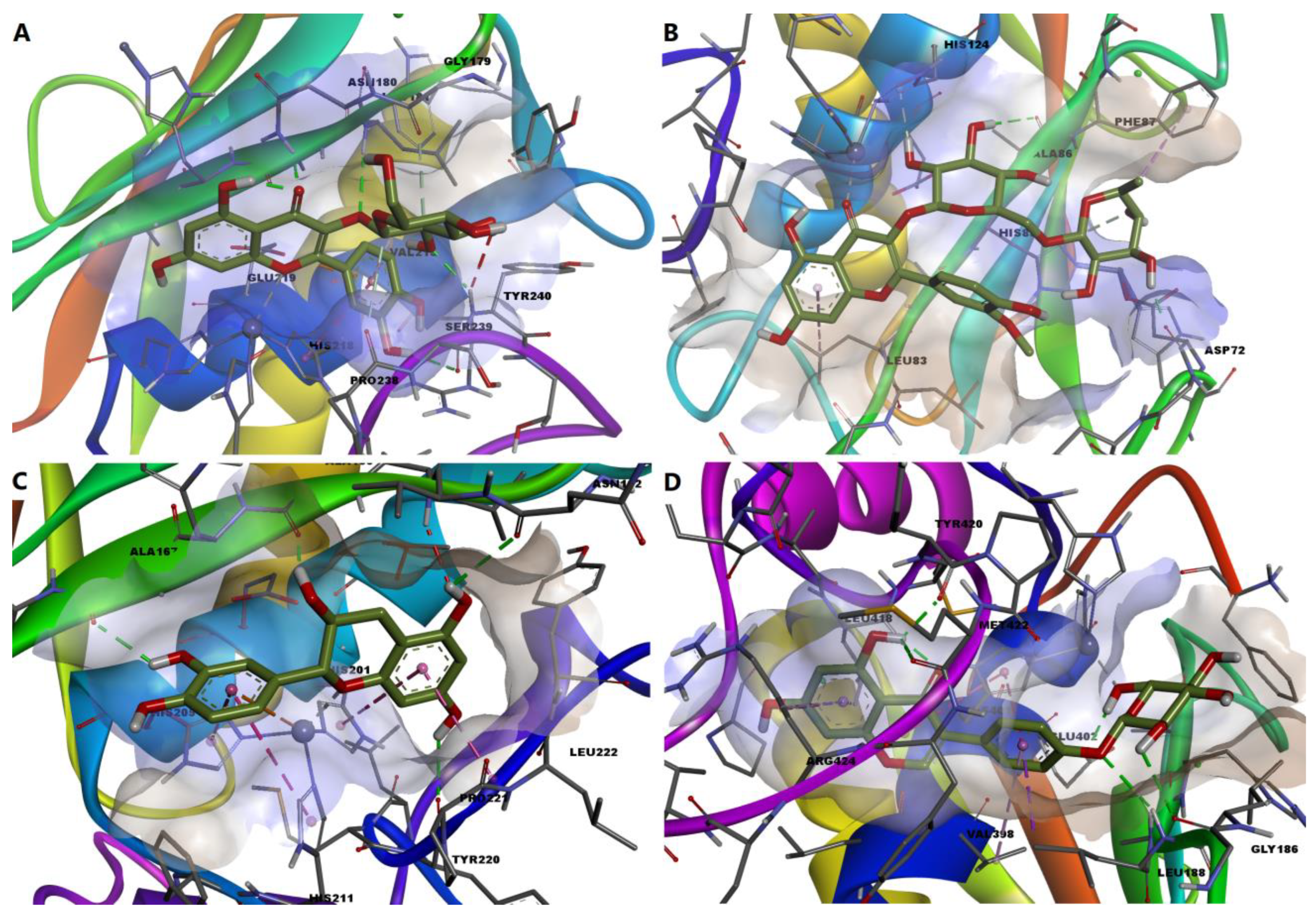
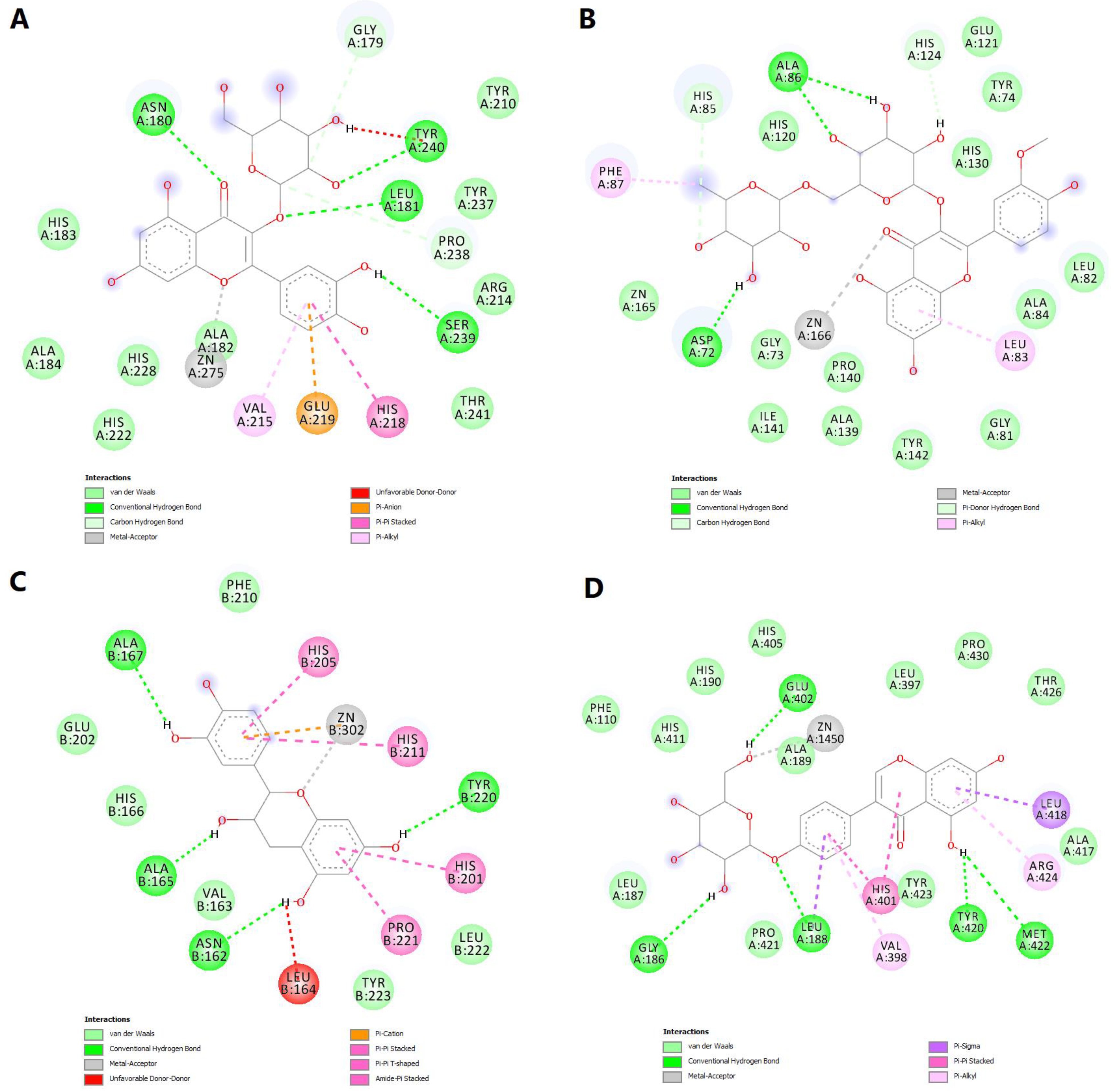
2.6. Molecular Docking Simulations
3. Discussion
4. Materials and Methods
4.1. Preparation of Vegetal Extracts
4.2. FT-ICR-MS (Fourier-Transform Ion–Cyclotron-Resonance High-Resolution Mass Spectrometry) Method
4.3. Quantitative Spectrophotometric Analysis
4.3.1. Phenolcarboxylic Acids Content
4.3.2. Flavonoid Content
4.3.3. Polyphenolic Content
4.4. UHPLC-HRMS/MS Analysis
4.5. In Vitro Antioxidant Activity
4.5.1. Ferric Reducing Antioxidant Power Assay (FRAP)
4.5.2. 2,2-Diphenyl-1-Picrylhydrazyl Free Radical Scavenging Assay (DPPH)
4.5.3. 2,20-Azinobis-3-Ethylbenzotiazoline-6-Sulfonic Acid Assay (ABTS)
4.6. Molecular Docking
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. JCM 2021, 10, 3239. [Google Scholar] [CrossRef] [PubMed]
- Wittens, C.; Davies, A.H.; Bækgaard, N.; Broholm, R.; Cavezzi, A.; Chastanet, S.; de Wolf, M.; Eggen, C.; Giannoukas, A.; Gohel, M.; et al. Editor’s Choice—Management of Chronic Venous Disease. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 678–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Maeseneer, M.G.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar] [CrossRef] [PubMed]
- MacColl, E.; Khalil, R.A. Matrix Metalloproteinases as Regulators of Vein Structure and Function: Implications in Chronic Venous Disease. J. Pharmacol. Exp. Ther. 2015, 355, 410–428. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Peng, W.; Raffetto, J.D.; Khalil, R.A. Matrix Metalloproteinases in Remodeling of Lower Extremity Veins and Chronic Venous Disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 267–299. [Google Scholar] [CrossRef][Green Version]
- Raffetto, J.; Khalil, R. Matrix Metalloproteinases in Venous Tissue Remodeling and Varicose Vein Formation. CVP 2008, 6, 158–172. [Google Scholar] [CrossRef]
- Saito, S.; Trovato, M.J.; You, R.; Lal, B.K.; Fasehun, F.; Padberg, F.T.; Hobson, R.W.; Durán, W.N.; Pappas, P.J. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase-1 in chronic venous insufficiency. J. Vasc. Surg. 2001, 34, 930–938. [Google Scholar] [CrossRef][Green Version]
- Herouy, Y.; Simkin, R.; Ulloa, J.; Pearson, I.C.; Mortimer, P.S.; Ciucci, J.L. The role of matrix metalloproteinases (MMPs) and their inhibitors in venous leg ulcer healing. Phlebolymphology 2004, 44, 231–243. [Google Scholar]
- Antignani, P.L. Medical Treatment of Chronic Venous Disease. SM J. Pharmacol. Ther. 2017, 3, 1015. [Google Scholar]
- Perrin, M.; Comerota, A.J.; Blanchemaison, P.; Pichot, O.; Menez, C.; Mattassi, R. An update on operative treatments of primary superficial vein incompetence: Part 2. Phlebolymphology 2016, 23, 57–120. [Google Scholar]
- Shoab, S.S.; Porter, J.B.; Scurr, J.H.; Coleridge-Smith, P.D. Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: A pilot study. J. Vasc. Surg. 2000, 31, 456–461. [Google Scholar] [CrossRef]
- Scallon, C.; Bell-Syer, S.E.; Aziz, Z. Flavonoids for treating venous leg ulcers. Cochrane Database Syst. Rev. 2013, CD006477. [Google Scholar] [CrossRef]
- Perrin, M.; Ramelet, A.A. Pharmacological Treatment of Primary Chronic Venous Disease: Rationale, Results and Unanswered Questions. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 117–125. [Google Scholar] [CrossRef][Green Version]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Peng, F.; Xu, P.; Zhao, B.-Y.; Zong, M.-H.; Lou, W.-Y. The application of deep eutectic solvent on the extraction and in vitro antioxidant activity of rutin from Sophora japonica bud. J. Food Sci. Technol. 2018, 55, 2326–2333. [Google Scholar] [CrossRef]
- Chen, H.-N.; Hsieh, C.-L. Effects of Sophora japonica flowers (Huaihua) on cerebral infarction. Chin. Med. 2010, 5, 34. [Google Scholar] [CrossRef][Green Version]
- Lim, H.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of Anti-inflammatory Biflavonoid, Ginkgetin, on Chronic Skin Inflammation. Biol. Pharm. Bull. 2006, 29, 1046–1049. [Google Scholar] [CrossRef][Green Version]
- Piazza, S.; Pacchetti, B.; Fumagalli, M.; Bonacina, F.; Dell’Agli, M.; Sangiovanni, E. Comparison of Two Ginkgo biloba L. Extracts on Oxidative Stress and Inflammation Markers in Human Endothelial Cells. Mediat. Inflamm. 2019, 2019, 6173893. [Google Scholar] [CrossRef][Green Version]
- Wu, T.-C.; Chen, J.-S.; Wang, C.-H.; Huang, P.-H.; Lin, F.-Y.; Lin, L.-Y.; Lin, S.-J.; Chen, J.-W. Activation of heme oxygenase-1 by Ginkgo biloba extract differentially modulates endothelial and smooth muscle-like progenitor cells for vascular repair. Sci. Rep. 2019, 9, 17316. [Google Scholar] [CrossRef][Green Version]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Rep. Reg. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179. [Google Scholar] [CrossRef][Green Version]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 2021, 8, 31. [Google Scholar] [CrossRef]
- Parente, L.M.L.; Lino Júnior, R.d.S.; Tresvenzol, L.M.F.; Vinaud, M.C.; de Paula, J.R.; Paulo, N.M. Wound Healing and Anti-Inflammatory Effect in Animal Models of Calendula officinalis L. Growing in Brazil. Evid.-Based Complement. Altern. Med. 2012, 2012, 375671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naeini, A.T.; Miri, R.; Shafiei, N.; Tabandeh, M.R.; Oryan, A.; Nazifi, S. Effects of topical application of Calendula officinalis gel on collagen and hydroxyproline content of skin in rats. Comp. Clin. Pathol. 2012, 21, 253–257. [Google Scholar] [CrossRef]
- Kim, B.H.; Chung, E.Y.; Min, B.-K.; Lee, S.H.; Kim, M.-K.; Min, K.R.; Kim, Y. Anti-Inflammatory Action of Legume Isoflavonoid Sophoricoside through Inhibition on Cyclooxygenase-2 Activity. Planta Med. 2003, 69, 474–476. [Google Scholar] [CrossRef]
- Kim, B.H.; Chung, E.Y.; Ryu, J.-C.; Jung, S.-H.; Min, K.R.; Kim, Y. Anti-inflammatory mode of isoflavone glycoside sophoricoside by inhibition of lnterleukin-6 and cyclooxygenase-2 in inflammatory response. Arch. Pharm. Res. 2003, 26, 306–311. [Google Scholar] [CrossRef]
- Yun, J.; Lee, C.-K.; Chang, I.-M.; Takatsu, K.; Hirano, T.; Min, K.R.; Lee, M.K.; Kim, Y. Differential inhibitory effects of sophoricoside analogs on bioactivity of several cytokines. Life Sci. 2000, 67, 2855–2863. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Hou, Y.; Tian, Y.; Chen, L.; Liu, S.; Zhang, N.; Dong, J. Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264.7 macrophages. Phytother. Res. 2019, 33, 989–997. [Google Scholar] [CrossRef]
- Chen, T.-R.; Wei, L.-H.; Guan, X.-Q.; Huang, C.; Liu, Z.-Y.; Wang, F.-J.; Hou, J.; Jin, Q.; Liu, Y.-F.; Wen, P.-H.; et al. Biflavones from Ginkgo biloba as inhibitors of human thrombin. Bioorganic Chem. 2019, 92, 103199. [Google Scholar] [CrossRef]
- Janssens, D.; Michiels, C.; Delaive, E.; Eliaers, F.; Drieu, K.; Remacle, J. Protection of hypoxia-induced ATP decrease in endothelial cells by ginkgo beloba extract and bilobalide. Biochem. Pharmacol. 1995, 50, 991–999. [Google Scholar] [CrossRef]
- Chen, K.; Sun, W.; Jiang, Y.; Chen, B.; Zhao, Y.; Sun, J.; Gong, H.; Qi, R. Ginkgolide B Suppresses TLR4-Mediated Inflammatory Response by Inhibiting the Phosphorylation of JAK2/STAT3 and p38 MAPK in High Glucose-Treated HUVECs. Oxid. Med. Cell. Longev. 2017, 2017, 9371602. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Wang, S.; Shang, H.; Wang, W.-Q.; Wang, B.-Q.; Zhang, X.; Xu, X.-D.; Sun, G.-B.; Sun, X.-B. The clickable activity-based probe of anti-apoptotic calenduloside E. Pharm. Biol. 2019, 57, 133–139. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Wang, S.; Shang, H.; Wang, M.; Sun, G.; Xu, X.; Sun, X. The proteomic profiling of calenduloside E targets in HUVEC: Design, synthesis and application of biotinylated probe BCEA. RSC Adv. 2017, 7, 6259–6265. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Bu, Y.; Li, B.; Zhang, H.; Guo, J.; Hu, J.; Zhang, Y. Calenduloside E alleviates cerebral ischemia/reperfusion injury by preserving mitochondrial function. J. Mol. Histol. 2022, 53, 713–727. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Sthijns, M.M.J.P.E.; Schiffers, P.M.; Janssen, G.M.; Lemmens, K.J.A.; Ides, B.; Vangrieken, P.; Bouwman, F.G.; Mariman, E.C.; Pader, I.; Arnér, E.S.J.; et al. Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in Human Umbilical Vein Endothelial Cells exposed to oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1177–1189. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, D.-W.; Park, S.-E.; Lee, H.-J.; Kim, K.-M.; Kim, K.-J.; Kim, M.-K.; Kim, S.-J.; Kim, S. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015, 120, 181–186. [Google Scholar] [CrossRef]
- Chen, T.-L.; Zhu, G.-L.; Wang, J.-A.; Zhang, G.-D.; Liu, H.-F.; Chen, J.-R.; Wang, Y.; He, X.-L. Protective effects of isorhamnetin on apoptosis and inflammation in TNF-α-induced HUVECs injury. Int. J. Clin. Exp. Pathol. 2015, 8, 2311–2320. [Google Scholar] [PubMed]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.K.; Kar, H.K. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, J.-Y.; Jeong, M.S.; Park, K.Y.; Park, K.H.; Lee, M.W.; Joo, S.S.; Seo, S.J. Effect of topical application of quercetin-3-O-(2″-gallate)-α-l-rhamnopyranoside on atopic dermatitis in NC/Nga mice. J. Dermatol. Sci. 2015, 77, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Fu, L.; Li, C.-Y.; Xu, L.-P.; Zhang, L.-X.; Zhang, W.-M. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects: Phenolics’ endothelial protective effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Hung, C.-H.; Chan, S.-H.; Hsieh, P.-L.; Ou, H.-C.; Cheng, Y.-H.; Chu, P.-M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1700928. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Mechanisms of endothelial cell protection by hydroxycinnamic acids. Vasc. Pharmacol. 2014, 63, 155–161. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Cambridge, UK, 2018; pp. 3–44. ISBN 978-0-12-813572-3. [Google Scholar]
- Hobeika, M.J.; Thompson, R.W.; Muhs, B.E.; Brooks, P.C.; Gagne, P.J. Matrix metalloproteinases in peripheral vascular disease. J. Vasc. Surg. 2007, 45, 849–857. [Google Scholar] [CrossRef][Green Version]
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar] [CrossRef]
- Lee, E.; Park, H.; Kim, H.; Jung, H.; Kang, I.; Cho, Y. Isolated isoquercitrin from Green ball apple peel inhibits photoaging in CCD-986Sk fibroblasts cells via modulation of the MMPs signaling. J. Cosmet. Dermatol. 2021, 20, 2932–2939. [Google Scholar] [CrossRef]
- Kim-Park, W.K.; Allam, E.S.; Palasuk, J.; Kowolik, M.; Park, K.K.; Windsor, L.J. Green tea catechin inhibits the activity and neutrophil release of Matrix Metalloproteinase-9. J. Tradit. Complement. Med. 2016, 6, 343–346. [Google Scholar] [CrossRef][Green Version]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef]
- Costea, L.; Chițescu, C.L.; Boscencu, R.; Ghica, M.; Lupuliasa, D.; Mihai, D.P.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; Luță, E.-A.; et al. The Polyphenolic Profile and Antioxidant Activity of Five Vegetal Extracts with Hepatoprotective Potential. Plants 2022, 11, 1680. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Spurlino, J.C.; Smallwood, A.M.; Carlton, D.D.; Banks, T.M.; Vavra, K.J.; Johnson, J.S.; Cook, E.R.; Falvo, J.; Wahl, R.C.; Pulvino, T.A.; et al. 1.56 Å structure of mature truncated human fibroblast collagenase. Proteins 1994, 19, 98–109. [Google Scholar] [CrossRef]
- Feng, Y.; Likos, J.J.; Zhu, L.; Woodward, H.; Munie, G.; McDonald, J.J.; Stevens, A.M.; Howard, C.P.; De Crescenzo, G.A.; Welsch, D.; et al. Solution structure and backbone dynamics of the catalytic domain of matrix metalloproteinase-2 complexed with a hydroxamic acid inhibitor. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2002, 1598, 10–23. [Google Scholar] [CrossRef]
- Belviso, B.D.; Caliandro, R.; Siliqi, D.; Calderone, V.; Arnesano, F.; Natile, G. Structure of matrix metalloproteinase-3 with a platinum-based inhibitor. Chem. Commun. 2013, 49, 5492. [Google Scholar] [CrossRef]
- Rowsell, S.; Hawtin, P.; Minshull, C.A.; Jepson, H.; Brockbank, S.M.V.; Barratt, D.G.; Slater, A.M.; McPheat, W.L.; Waterson, D.; Henney, A.M.; et al. Crystal Structure of Human MMP9 in Complex with a Reverse Hydroxamate Inhibitor. J. Mol. Biol. 2002, 319, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. In Protein Engineering; Bornscheuer, U.T., Höhne, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1685, pp. 43–67. ISBN 978-1-4939-7364-4. [Google Scholar]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization And Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Compound | ESI+ | ESI− | ||
|---|---|---|---|---|
| w | met | w | met | |
| rutin | + | + | + | + |
| quercetin | + | + | + | + |
| isorhamnetin/rhamnetin | + | + | – | + |
| kaempferol/fisetin | + | + | – | + |
| isoquercitrin | + | + | – | + |
| sophoricoside | + | + | – | – |
| kaikasaponin I | – | + | – | + |
| kaikasaponin III | – | + | – | – |
| Compound | ESI+ | ESI– | ||
|---|---|---|---|---|
| w | met | w | met | |
| rutin | + | + | + | + |
| bilobalid | + | + | + | + |
| quercetin | + | + | + | – |
| ginkgolide A | + | + | – | – |
| ginkgolide B | + | + | – | + |
| ginkgolide C | + | + | – | – |
| kaempferol | + | + | – | – |
| isoquercitrin | + | + | – | – |
| isorhamnetin/rhamnetin | + | + | – | – |
| catechin | + | + | – | – |
| bilobetin | – | + | – | – |
| caffeic acid | – | + | – | – |
| Compound | ESI+ | ESI– | ||
|---|---|---|---|---|
| w | met | w | met | |
| rutin | + | + | + | + |
| narcissin/calendoflavoside | + | + | + | + |
| typhaneoside | + | + | + | + |
| chlorogenic acid | + | + | + | + |
| isoquercitrin | + | + | – | + |
| isorhamnetin/rhamnetin | + | + | – | – |
| quercetin | + | + | – | – |
| calenduloside E | – | + | – | – |
| calenduloside F/G | – | + | – | + |
| calenduloside H | – | + | + | + |
| Extract | Phenolcarboxylic Acids Content (g Chlorogenic Acid/100 g Extract) | Flavonoid Content (g Rutin/100 g Extract) | Polyphenolic Content (g Tannic Acid/100 g Extract) |
|---|---|---|---|
| SE | 17.1399 | 37.4473 | 28.4903 |
| [16.5139; 17.7659] * | [37.1959; 37.6987] * | [28.2607; 28.7198] * | |
| GE | 2.1722 [2.1007; 2.2437] * | 2.3765 [2.3108; 2.4421] * | 7.2344 [7.1773; 7.2915] * |
| CE | 3.8073 [3.8638; 3.7509] * | 2.0129 [1.9924; 2.0334] * | 4.9029 [4.8385; 4.9672] * |
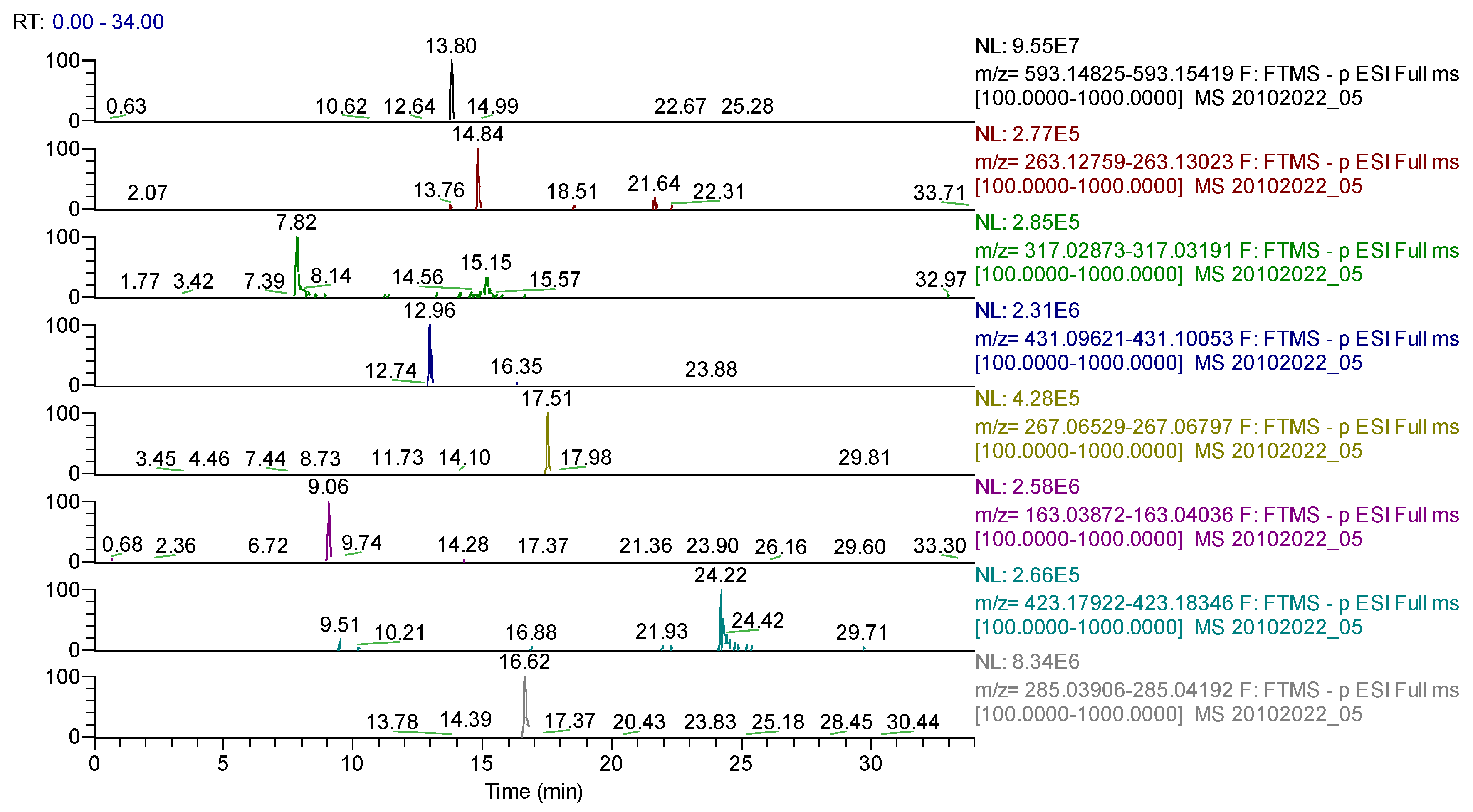
| Compound | Molecular Formula | Adduct Ion (m/z) Monitored Negative Ion | Retention Time (min) | Phytochemical Class |
|---|---|---|---|---|
| quinic acid | C7H12O6 | 191.05611 | 0.66 | Cyclohexanecarboxylic acid |
| hidroxyferulic acid | C16H20O10 | 371.09839 | 6.77 | phenolcarboxylic acids |
| caffeoylshikimic acid | C10H15O9 | 278.06435 | 7.65 | phenolcarboxylic acids |
| myricetin | C15H10O8 | 317.03032 | 7.82 | flavonoids |
| quercetagetin (6-hydroxyquercetin) | C15H10O8 | 317.03032 | 7.82 | flavonoids |
| syringic acid | C9H10O5 | 197.04555 | 8.86 | phenolcarboxylic acids |
| p-coumaric acid | C9H8O3 | 163.03954 | 9.06 | phenolcarboxylic acids |
| hyperoside (quercetin-3-hexoside) | C21H20O12 | 463.08768 | 9.63 | flavonoids |
| phlorizin | C21H24O10 | 435.12969 | 10.27 | flavonoids |
| quercetin-3-O-rutinoside | C33H40O21 | 771.19896 | 10.37 | flavonoids |
| isorhamnetin-3-O-hexoside | C22H22O12 | 477.10387 | 11.97/13.86 | flavonoids |
| sissotrin(biochanin A7-O-β-D-hexoside) | C22H22O10 | 445.11404 | 12.5/15.91 | flavonoids |
| rutin (quercetin-3-rutinoside) | C27H30O16 | 609.14613 | 12.58 | flavonoids |
| robinin | C33H40O19 | 739.20913 | 12.67 | flavonoids |
| genistin | C21H20O10 | 431.09837 | 12.96 | flavonoids |
| naringin | C27H32O14 | 579.17185 | 13.00 | flavonoids |
| chryosoeriol-7-hexoside | C22H22O11 | 461.10893 | 13.40 | flavonoids |
| apigenin–7-rutinoside | C27H30O14 | 577.15630 | 13.49 | flavonoids |
| hispidulin-O-hexoside/isomers | C22H22O11 | 461.10893 | 13.64 | flavonoids |
| kaempferol (or luteolin)-O-hexoside/isomers | C21H20O11 | 447.09331 | 13.75 | flavonoids |
| cynaroside (luteolin-7-hexoside) | C21H20O11 | 447.09328 | 13.75 | flavonoids |
| isoquercitrin/quercitrin (quercetin-3-rhamnoside) | C21H20O11 | 447.09331 | 13.75 | flavonoids |
| apigenin-7-O-glucosylhexoside | C27H30O15 | 593.15122 | 13.80 | flavonoids |
| hispidulin-7-rutinoside/isomers | C28H32O15 | 607.16684 | 13.82 | flavonoids |
| isorhamnetin-3-O-rutinoside | C28H32O16 | 623.16178 | 13.96 | flavonoids |
| azelaic acid | C9H16O4 | 187.09761 | 14.10 | dicarboxylic acids |
| vitexin 2-O-rhamnoside | C27H30O14 | 577.15630 | 14.29 | flavonoids |
| abscisic acid | C15H20O4 | 263.12891 | 14.84 | sesquiterpenes |
| quercetin | C15H10O7 | 301.03540 | 15.15 | flavonoids |
| naringenin | C15H12O5 | 271.06122 | 15.63 | flavonoids |
| hesperitin | C16H14O6 | 301.07179 | 15.71 | flavonoids |
| gallocatechin/epigallocatechin | C15H14O7 | 305.06668 | 15.73 | flavonoids |
| afrormosin | C17H14O5 | 297.07687 | 16.39 | flavonoids |
| kaempferol | C15H10O6 | 285.04049 | 16.62 | flavonoids |
| apigenin | C15H10O5 | 269.04502 | 16.83 | flavonoids |
| 6-methoxyluteolin | C16H12O7 | 315.05105 | 16.85 | flavonoids |
| diosmetin | C16H12O6 | 299.05611 | 16.92 | flavonoids |
| pseudobaptigenin | C16H10O5 | 281.04557 | 17.36 | flavonoids |
| formononetin | C16H12O4 | 267.06631 | 17.51 | flavonoids |
| glycitein | C16H12O5 | 283.06122 | 19.65 | flavonoids |
| ginkgetin | C32H22O10 | 565.11404 | 21.37 | flavonoids |
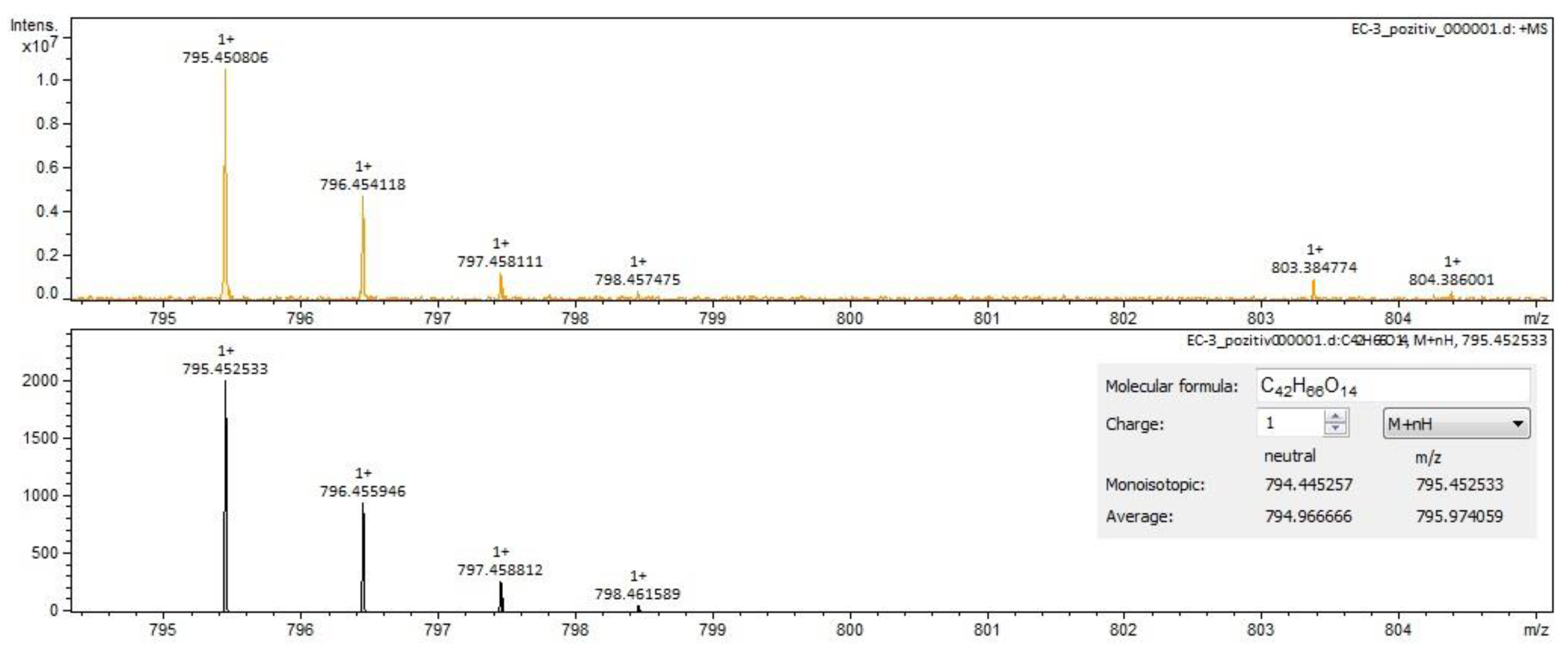
| calenduloside G/isomers | C42H66O14 | 793.43800 | 22.99 | triterpenes |
| sophoraflavanone G/isomers | C25H28O6 | 423.18134 | 24.22 | flavonoids |
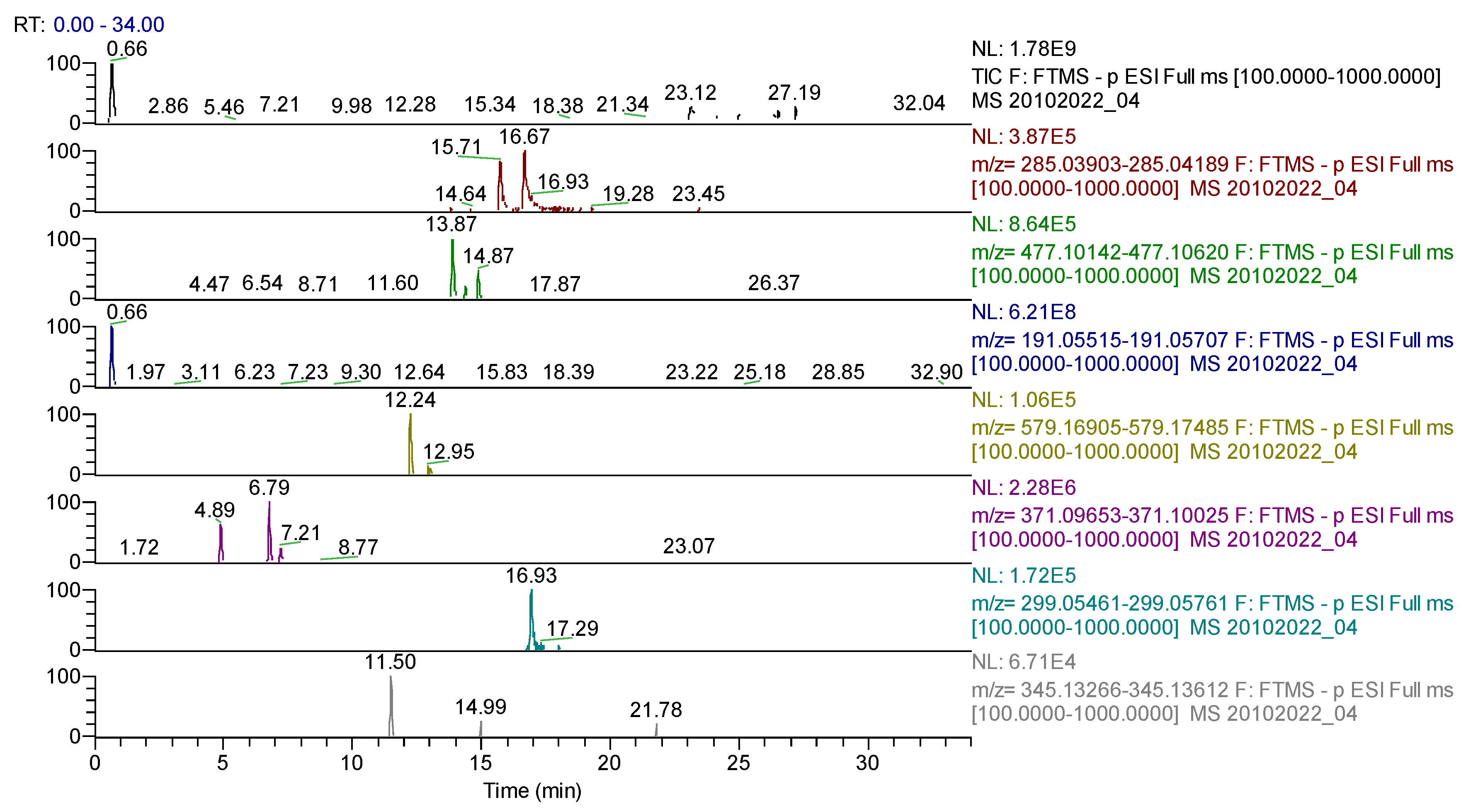
| Compound | Molecular Formula | Adduct Ion (m/z) Monitored Negative Ion | Retention Time (min) | Phytochemical Class |
|---|---|---|---|---|
| quinic acid | C7H12O6 | 191.05611 | 0.66 | cyclohexanecarboxylic acids |
| daphnin | C15H16O9 | 339.072155 | 5.18 | flavonoids |
| gallocatechin/epigallocatechin | C15H14O7 | 305.06668 | 5.47 | flavonoids |
| isorhamnetin-3-O-hexoside | C22H22O12 | 477.10381 | 5.69 | flavonoids |
| chlorogenic acid | C16H18O9 | 353.08783 | 6.23 | phenolcarboxylic acids |
| hidroxyferulic acid | C16H20O10 | 371.09839 | 6.79 | phenolcarboxylic acids |
| caffeoylshikimic acid | C10H15O9 | 278.06435 | 7.70 | phenolcarboxylic acids |
| epicatechin | C15H14O6 | 289.07176 | 8.01 | flavonoids |
| syringic acid | C9H10O5 | 197.04555 | 8.86 | phenolcarboxylic acids |
| p-coumaric acid | C9H8O3 | 163.03954 | 9.09 | phenolcarboxylic acids |
| hyperoside (quercetin-3-hexoside) | C21H20O12 | 463.08768 | 9.64 | flavonoids |
| quercetin-3-O-rutinoside | C33H40O21 | 771.19896 | 10.25 | flavonoids |
| cynaropicrin | C19H22O6 | 345.13439 | 11.50 | sesquiterpenes |
| narirutin (naringenin-7-O-rutinoside) | C27H32O14 | 579.17195 | 12.24 | flavonoids |
| cynarin (1,5-di-O-caffeoylquinic acid) | C25H24O12 | 515.11950 | 12.37 | phenolcarboxylic acids |
| robinin | C33H40O19 | 739.20913 | 12.39 | flavonoids |
| rutin (quercetin-3-rutinoside) | C27H30O16 | 609.14613 | 12.60 | flavonoids |
| naringin | C27H32O14 | 579.17185 | 12.93 | flavonoids |
| chryosoeriol-7-hexoside | C22H22O11 | 461.10893 | 13.33/14.96 | flavonoids |
| vitexin (apigenin-8-C-hexoside)/isovitexin | C21H20O10 | 431.09839 | 13.51/14.89 | flavonoids |
| hispidulin-O-hexoside/isomers | C22H22O11 | 461.10893 | 13.63/14.96 | flavonoids |
| kaempferol (or luteolin)-O-hexoside/isomers | C21H20O11 | 447.09331 | 13.76/14.66 | flavonoids |
| isoquercitrin/quercitrin (quercetin-3-rhamnoside) | C21H20O11 | 447.09331 | 13.76/14.66 | flavonoids |
| cynaroside (luteolin-7-hexoside) | C21H20O11 | 447.09328 | 13.79 | flavonoids |
| apigenin 7-O-glucosylhexoside | C27H30O15 | 593.15122 | 13.82 | flavonoids |
| isorhamnetin-3-O-hexoside | C22H22O12 | 477.10387 | 13.87 | flavonoids |
| isorhamnetin-3-O-rutinoside | C28H32O16 | 623.16178 | 14 | flavonoids |
| azelaic acid | C9H16O4 | 187.09761 | 14.12 | dicarboxylic acids |
| 2′,6-dihydroxyflavone | C15H10O4 | 253.05066 | 14.67 | flavonoids |
| quercetin | C15H10O7 | 301.03540 | 15.22 | flavonoids |
| naringenin | C15H12O5 | 271.06122 | 15.65 | flavonoids |
| baptigenin | C15H10O6 | 285.04046 | 15.71 | flavonoids |
| kaempferol | C15H10O6 | 285.04049 | 16.67 | flavonoids |
| apigenin | C15H10O5 | 269.04502 | 16.86 | flavonoids |
| 6-methoxyluteolin | C16H12O7 | 315.05105 | 16.86 | flavonoids |
| diosmetin | C16H12O6 | 299.05611 | 16.93 | flavonoids |
| tricin | C17H14O7 | 329.06668 | 17.39/17.69 | flavonoids |
| amentoflavone | C30H18O10 | 537.08274 | 18.77 | flavonoids |
| sophoraflavanone G/isomers | C25H28O6 | 423.18134 | 19.48 | flavonoids |
| gallic acid | C7H6O5 | 169.01427 | 19.66 | phenolcarboxylic acids |
| glycitein | C16H12O5 | 283.06122 | 19.66 | flavonoids |
| ginkgetin | C32H22O10 | 565.11404 | 21.39 | flavonoids |
| Compound | Molecular Formula | Adduct Ion (m/z) Monitored Negative Ion | Retention Time (min) | Phytochemical Class |
|---|---|---|---|---|
| quinic acid | C7H12O6 | 191.05611 | 0.66 | cyclohexanecarboxylic acids |
| daphnin | C15H16O9 | 339.072155 | 5.16 | coumarins |
| cichorin | C15H16O9 | 339.07218 | 5.18 | coumarins |
| chlorogenic acid | C16H18O9 | 353.08783 | 6.25 | phenolcarboxylic acids |
| caffeic acid | C9H8O4 | 179.03501 | 6.88 | phenolcarboxylic acids |
| sinapic acid | C11H12O5 | 223.06122 | 7.45 | phenolcarboxylic acids |
| lactucopicrin | C23H22O7 | 409.12930 | 7.67 | sesquiterpenes |
| caffeoylshikimic acid | C10H15O9 | 278.06435 | 7.72 | phenolcarboxylic acids |
| coumaroylquinic acid/isomers | C16H18O8 | 337.09292 | 8.18/10.40 | phenolcarboxylic acids |
| syringic acid | C9H10O5 | 197.04555 | 9.09 | phenolcarboxylic acids |
| p-coumaric acid | C9H8O3 | 163.03954 | 9.13 | phenolcarboxylic acids |
| hyperoside (quercetin-3-hexoside) | C21H20O12 | 463.08768 | 9.61 | flavonoids |
| ferulic acid | C10H10O4 | 193.05066 | 10.22 | phenolcarboxylic acids |
| robinin | C33H40O19 | 739.20913 | 12.42 | flavonoids |
| cynarin (1,5-di-O-caffeoylquinic acid) | C25H24O12 | 515.11950 | 12.42/13.72 | phenolcarboxylic acids |
| rutin (quercetin-3-rutinoside) | C27H30O16 | 609.14613 | 12.56 | flavonoids |
| kaempferol-3-O-rutinoside | C27H30O15 | 593.15122 | 12.7 | flavonoids |
| quercetin-3-(6-malonyl)-hexoside | C24H22O15 | 549.08862 | 13.09 | flavonoids |
| chryosoeriol-7-hexoside | C22H22O11 | 461.10893 | 13.38 | flavonoids |
| cynaroside (luteolin-7-hexoside) | C21H20O11 | 447.09328 | 13.8 | flavonoids |
| isoquercitrin/quercitrin (quercetin-3-rhamnoside) | C21H20O11 | 447.09331 | 13.8 | flavonoids |
| kaempferol (or luteolin)-O-hexoside/isomers | C21H20O11 | 447.09331 | 13.80/14.56 | flavonoids |
| apigenin-7-O-glucosylhexoside | C27H30O15 | 593.15122 | 13.85 | flavonoids |
| isorhamnetin-3-O-hexoside | C22H22O12 | 477.10387 | 13.9 | flavonoids |
| isorhamnetin-3-O-rutinoside | C28H32O16 | 623.16178 | 14 | flavonoids |
| azelaic acid | C9H16O4 | 187.09761 | 14.13 | dicarboxylic acids |
| abscisic acid | C15H20O4 | 263.12891 | 14.87 | sesquiterpenes |
| hispidulin-O-hexoside/isomers | C22H22O11 | 461.10893 | 14.98 | flavonoids |
| quercetin | C15H10O7 | 301.03540 | 15.2 | flavonoids |
| naringenin | C15H12O5 | 271.06122 | 15.6 | flavonoids |
| procyanidine | C30H26O13 | 593.13006 | 16 | flavonoids |
| apigenin | C15H10O5 | 269.04502 | 16.88 | flavonoids |
| carnasol | C20H26O4 | 329.17586 | 18.89 | diterpenes |
| calenduloside G/isomers | C42H66O14 | 793.43800 | 21.02/23.03 | triterpenes |
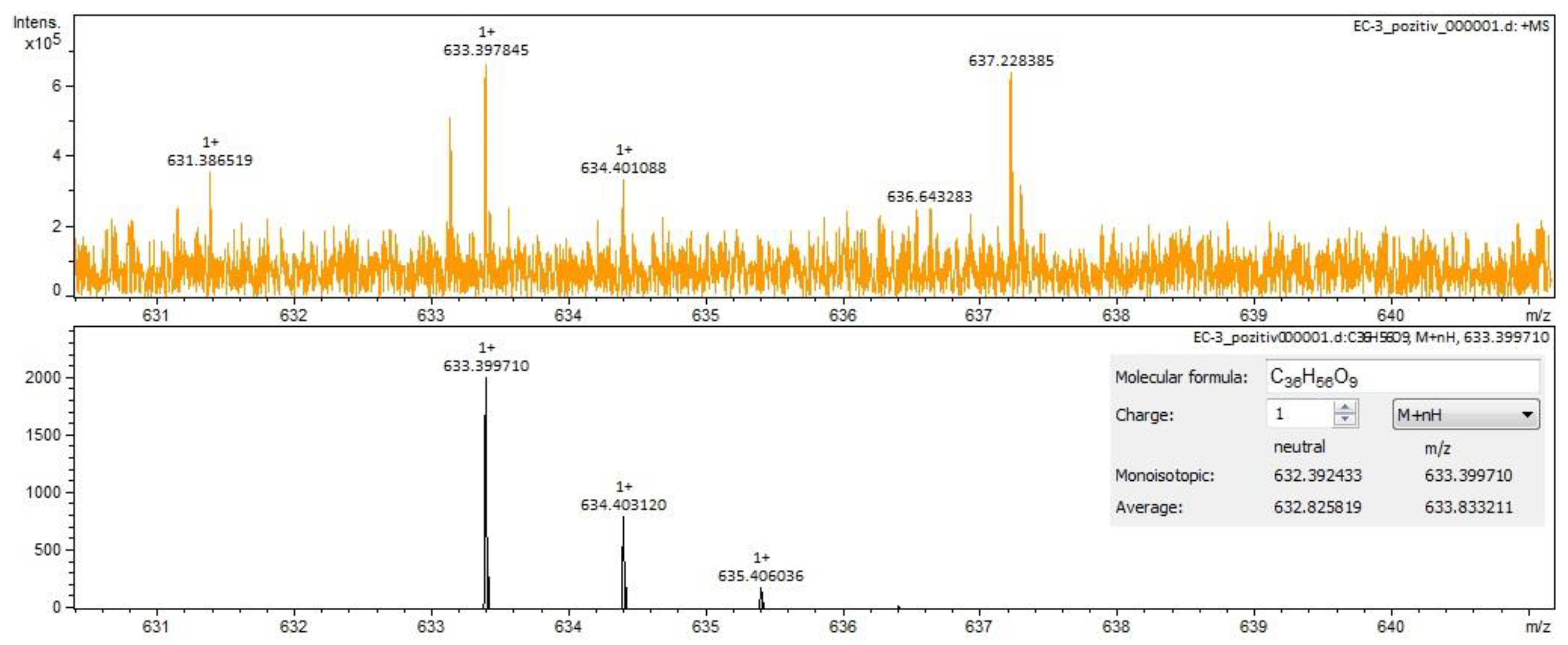
| calenduloside E/isomers | C36H56O9 | 631.38518 | 23.47 | triterpenes |
| Phytochemical Class | Compound | Quantity (μg/g) | ||
|---|---|---|---|---|
| SE | GE | CE | ||
| phenolcarboxylic acids | caffeic acid | / | 3.22 | 12.86 |
| phenolcarboxylic acids | p-coumaric acid | 1241.65 | 909.27 | 7.09 |
| phenolcarboxylic acids | syringic acid | 722.67 | 197.84 | 622.28 |
| phenolcarboxylic acids | chlorogenic acid | / | 386.41 | 20,676.63 |
| phenolcarboxylic acids | ferulic acid | 244.36 | / | 249.23 |
| phenolcarboxylic acids | gallic acid | 4472.70 | 1608.25 | 189.95 |
| flavonoids | epicatechin | / | 918.6 | / |
| flavonoids | genistin | 6974.06 | / | / |
| flavonoids | glicitein | 375.18 | 305.1 | / |
| flavonoids | hyperoside | 2485.75 | 999.62 | 677.76 |
| flavonoids | apigenin | 1.87 | 79.64 | / |
| flavonoids | rutin | 104,186.77 | 3907.47 | 2165.42 |
| flavonoids | formononetin | 6.98 | / | / |
| flavonoids | galangin | 291.14 | / | / |
| flavonoids | kaempherol | 3263.84 | 236.96 | / |
| flavonoids | hesperetin | 8859.90 | / | / |
| flavonoids | naringenin | 14.00 | 15.12 | / |
| flavonoids | quercetin | 46,678.34 | 4504.66 | 352.14 |
| flavonoids | isorhamnetin | 97,049.32 | 5032.60 | 11,286.93 |
| flavonoids | daidzein | 18.24 | 60.51 | / |
| sesquiterpenes | abscinic acid | 164.67 | 151.88 | 144.76 |
| Extract | PCA | FLV | PPC | QHPLC | FRAP | DPPH | ABTS |
|---|---|---|---|---|---|---|---|
| SE | 17.14 | 37.45 | 28.49 | 277.05 | 0.1294 | 0.0948 | 0.0715 |
| GE | 2.17 | 2.37 | 7.23 | 19.32 | 0.4806 | 0.5185 | 0.1939 |
| CE | 3.81 | 2.01 | 4.90 | 36.39 | 0.4370 | 0.8068 | 0.3693 |
| Correlation | r | R2 | R2 (%) |
|---|---|---|---|
| QHPLC vs. PCA * | 0.999 | 0.998 | 99.800 |
| QHPLC vs. FLV * | 0.998 | 0.996 | 99.600 |
| QHPLC vs. PPC | 0.989 | 0.978 | 97.812 |
| Correlation | r | R2 | R2 (%) |
|---|---|---|---|
| FLV vs. FRAP * | −0.997 | 0.994 | 99.400 |
| FLV vs. DPPH | −0.982 | 0.964 | 96.432 |
| FLV vs. ABTS | −0.924 | 0.853 | 85.377 |
| PCA vs. FRAP * | −0.999 | 0.998 | 99.800 |
| PCA vs. DPPH | −0.956 | 0.913 | 91.393 |
| PCA vs. ABTS | −0.878 | 0.770 | 77.088 |
| PPC vs. FRAP * | −0.988 | 0.976 | 97.614 |
| PPC vs. DPPH | −0.994 | 0.988 | 98.803 |
| PPC vs. ABTS | −0.952 | 0.906 | 90.630 |
| QHPLC vs. FRAP * | −1.000 | 1.000 | 100.000 |
| QHPLC vs. DPPH | −0.967 | 0.935 | 93.509 |
| QHPLC vs. ABTS | −0.896 | 0.802 | 80.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, A.R.; Chițescu, C.L.; Luță, E.A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Costea, L.; Ozon, E.A.; Fița, A.C.; Balaci, T.D.; et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules 2023, 28, 3668. https://doi.org/10.3390/molecules28093668
Ungureanu AR, Chițescu CL, Luță EA, Moroșan A, Mihaiescu DE, Mihai DP, Costea L, Ozon EA, Fița AC, Balaci TD, et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules. 2023; 28(9):3668. https://doi.org/10.3390/molecules28093668
Chicago/Turabian StyleUngureanu, Andreea Roxana, Carmen Lidia Chițescu, Emanuela Alice Luță, Alina Moroșan, Dan Eduard Mihaiescu, Dragoș Paul Mihai, Liliana Costea, Emma Adriana Ozon, Ancuța Cătălina Fița, Teodora Dalila Balaci, and et al. 2023. "Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts" Molecules 28, no. 9: 3668. https://doi.org/10.3390/molecules28093668
APA StyleUngureanu, A. R., Chițescu, C. L., Luță, E. A., Moroșan, A., Mihaiescu, D. E., Mihai, D. P., Costea, L., Ozon, E. A., Fița, A. C., Balaci, T. D., Boscencu, R., & Gîrd, C. E. (2023). Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules, 28(9), 3668. https://doi.org/10.3390/molecules28093668












