Programmed Self-Assembly of DNA Nanosheets with Discrete Single-Molecule Thickness and Interfacial Mechanics: Design, Simulation, and Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. DNA Design
2.2. Structure and Planarity of Single-Layer DNA Nanosheets Validated by oxDNA Simulations
2.2.1. Single F-Unit
2.2.2. Single Nanopore of Nanosheet
2.3. Self-Assembly in the Liquid Phase
2.4. Self-Assembly at Solid/Liquid Interface
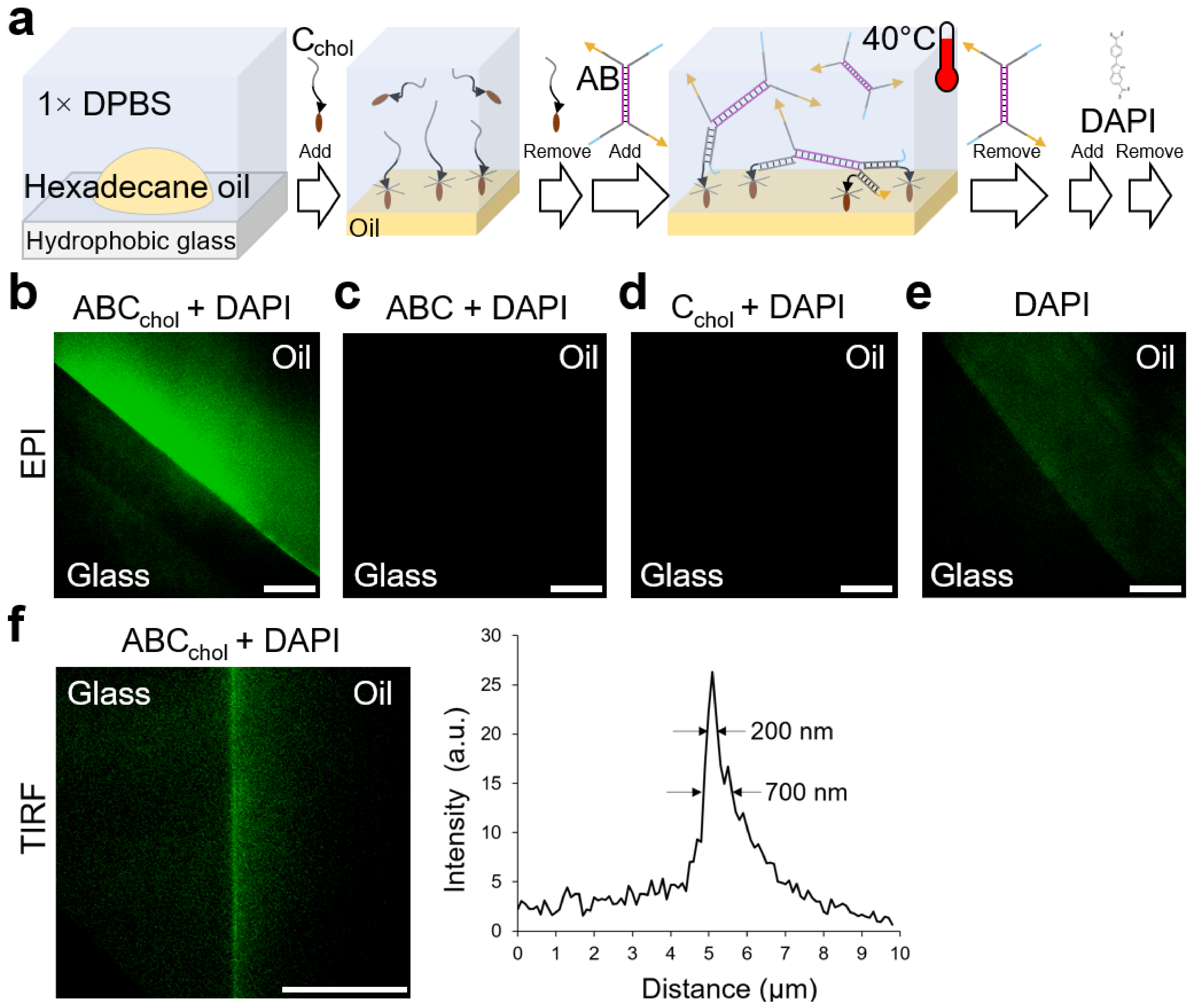
2.5. Self-Assembly at Liquid/Liquid Interface
Interfacial Mechanical Properties
3. Materials and Methods
3.1. F-Unit Design and DNA Sequences
3.2. oxDNA Simulations
3.3. Liquid-Phase Assembly of DNA Nanosheets and AFM Characterization
3.4. Solid/Liquid Interface Assembly of DNA Nanosheets and AFM Characterization
3.5. Liquid/Liquid Interface Assembly of DNA Nanosheets and Fluorescence Microscopy Characterization
3.6. Interface Mechanical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability Statement
References
- Chrysanthou, A.; Kanso, H.; Zhong, W.; Shang, L.; Gautrot, J.E. Supercharged Protein Nanosheets for Cell Expansion on Bioemulsions. ACS Appl. Mater. Interfaces 2023, 15, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Mallya, D.S.; Dumée, L.F.; Muthukumaran, S.; Lei, W.; Baskaran, K. 2D Nanosheet Enabled Thin Film Nanocomposite Membranes for Freshwater Production—A Review. Mater. Adv. 2021, 2, 3519–3537. [Google Scholar] [CrossRef]
- Yoo, S.; Dugasani, S.R.; Chopade, P.; Kesama, M.R.; Gnapareddy, B.; Park, S.H. Metal and Lanthanide Ion-Co-Doped Synthetic and Salmon DNA Thin Films. ACS Omega 2019, 4, 6530–6537. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Barad, H.-N.; Ricardo, A.; Olaya, S.; Alarcón-Correa, M.; Hahn, K.; Richter, G.; Wittstock, G.; Fischer, P. Dry Synthesis of Pure and Ultrathin Nanoporous Metallic Films. ACS Appl. Mater. Interfaces 2023, 15, 5620–5627. [Google Scholar] [CrossRef]
- Rinaldin, M.; ten Haaf, S.L.D.; Vegter, E.J.; van der Wel, C.; Fonda, P.; Giomi, L.; Kraft, D.J. Supported Lipid Membranes with Designed Geometry. arXiv 2021. [Google Scholar] [CrossRef]
- Seeman, N.C.; Sleiman, H.F. DNA Nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Gopinath, A.; Thachuk, C.; Mitskovets, A.; Atwater, H.A.; Kirkpatrick, D.; Rothemund, P.W.K. Absolute and Arbitrary Orientation of Single-Molecule Shapes. Science 2021, 371, eabd6179. [Google Scholar] [CrossRef]
- Cervantes-Salguero, K.; Biaggne, A.; Youngsman, J.M.; Ward, B.M.; Kim, Y.C.; Li, L.; Hall, J.A.; Knowlton, W.B.; Graugnard, E.; Kuang, W. Strategies for Controlling the Spatial Orientation of Single Molecules Tethered on DNA Origami Templates Physisorbed on Glass Substrates: Intercalation and Stretching. Int. J. Mol. Sci. 2022, 23, 7690. [Google Scholar] [CrossRef]
- Huang, D.; Patel, K.; Perez-Garrido, S.; Marshall, J.F.; Palma, M. DNA Origami Nanoarrays for Multivalent Investigations of Cancer Cell Spreading with Nanoscale Spatial Resolution and Single-Molecule Control. ACS Nano 2019, 13, 728–736. [Google Scholar] [CrossRef]
- Cervantes-Salguero, K.; Hamada, S.; Nomura, S.I.M.; Murata, S. Polymorphic Ring-Shaped Molecular Clusters Made of Shape-Variable Building Blocks. Nanomaterials 2015, 5, 208–217. [Google Scholar] [CrossRef]
- Yonamine, Y.; Cervantes-Salguero, K.; Minami, K.; Kawamata, I.; Nakanishi, W.; Hill, J.P.; Murata, S.; Ariga, K. Supramolecular 1-D Polymerization of DNA Origami through a Dynamic Process at the 2-Dimensionally Confined Air–Water Interface. Phys. Chem. Chem. Phys. 2016, 18, 12576–12581. [Google Scholar] [CrossRef]
- Huang, D.; Haddad, L.; Rahman, F.; Palma, M.; Sapelkin, A. Engineering a DNA Origami Mediated Multicolour Quantum Dot Platform for Sub-Diffraction Spectral Separation Imaging. RSC Adv. 2022, 12, 23778–23785. [Google Scholar] [CrossRef]
- Cervantes-Salguero, K.; Freeley, M.; Gwyther, R.E.A.; Jones, D.D.; Chávez, J.L.; Palma, M. Single Molecule DNA Origami Nanoarrays with Controlled Protein Orientation. Biophys. Rev. 2022, 3, 031401. [Google Scholar] [CrossRef]
- Cervantes-Salguero, K.; Freeley, M.; Chávez, J.L.; Palma, M. Single-Molecule DNA Origami Aptasensors for Real-Time Biomarker Detection. J. Mater. Chem. B 2020, 8, 6352–6356. [Google Scholar] [CrossRef]
- Huang, D.; Freeley, M.; Palma, M. DNA-Mediated Patterning of Single Quantum Dot Nanoarrays: A Reusable Platform for Single-Molecule Control. Sci. Rep. 2017, 7, 45591. [Google Scholar] [CrossRef]
- Julin, S.; Keller, A.; Linko, V. Dynamics of DNA Origami Lattices. Bioconjug Chem. 2023, 34, 18–29. [Google Scholar] [CrossRef]
- Parikka, J.M.; Sokołowska, K.; Markešević, N.; Toppari, J.J. Constructing Large 2D Lattices Out of DNA-Tiles. Molecules 2021, 26, 1502. [Google Scholar] [CrossRef]
- Morzy, D.; Tekin, C.; Caroprese, V.; Rubio-Sánchez, R.; di Michele, L.; Bastings, M.M.C. Interplay of the Mechanical and Structural Properties of DNA Nanostructures Determines Their Electrostatic Interactions with Lipid Membranes. Nanoscale 2023, 15, 2849–2859. [Google Scholar] [CrossRef]
- Vittala, S.K.; Saraswathi, S.K.; Ramesan, A.B.; Joseph, J. Nanosheets and 2D-Nanonetworks by Mutually Assisted Self-Assembly of Fullerene Clusters and DNA Three-Way Junctions. Nanoscale Adv. 2019, 1, 4158–4165. [Google Scholar] [CrossRef]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and Self-Assembly of Two-Dimensional DNA Crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef]
- Marras, A.E. Hierarchical Assembly of DNA Origami Nanostructures. MRS Commun. 2022, 12, 543–551. [Google Scholar] [CrossRef]
- Knappe, G.A.; Wamhoff, E.C.; Bathe, M. Functionalizing DNA Origami to Investigate and Interact with Biological Systems. Nat. Rev. Mater. 2023, 8, 123–138. [Google Scholar] [CrossRef]
- Morzy, D.; Schaich, M.; Keyser, U.F. A Surfactant Enables Efficient Membrane Spanning by Non-Aggregating DNA-Based Ion Channels. Molecules 2022, 27, 578. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yao, C.; Zhu, Y.; Yang, L.; Luo, D.; Yang, D. DNA Functional Materials Assembled from Branched DNA: Design, Synthesis, and Applications. Chem. Rev. 2020, 120, 9420–9481. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, C.; Vecchioni, S.; Zhang, T.; Wu, C.; Ohayon, Y.P.; Sha, R.; Seeman, N.C.; Wei, B. Reconfigurable Two-Dimensional DNA Lattices: Static and Dynamic Angle Control. Angew. Chem. Int. Ed. 2021, 60, 25781–25786. [Google Scholar] [CrossRef]
- Sun, X.; Seung, H.K.; Zhang, C.; Ribbe, A.E.; Mao, C. Surface-Mediated DNA Self-Assembly. J. Am. Chem. Soc. 2009, 131, 13248–13249. [Google Scholar] [CrossRef]
- Díaz, S.A.; Pascual, G.; Patten, L.K.; Roy, S.K.; Meares, A.; Chiriboga, M.; Susumu, K.; Knowlton, W.B.; Cunningham, P.D.; Mathur, D.; et al. Towards Control of Excitonic Coupling in DNA-Templated Cy5 Aggregates: The Principal Role of Chemical Substituent Hydrophobicity and Steric Interactions. Nanoscale 2023, 15, 3284–3299. [Google Scholar] [CrossRef]
- Kandatsu, D.; Cervantes-Salguero, K.; Kawamata, I.; Hamada, S.; Nomura, S.I.M.; Fujimoto, K.; Murata, S. Reversible Gel–Sol Transition of a Photo-Responsive DNA Gel. ChemBioChem 2016, 17, 1118–1121. [Google Scholar] [CrossRef]
- Udono, H.; Gong, J.; Sato, Y.; Takinoue, M. DNA Droplets: Intelligent, Dynamic Fluid. Adv. Biol. 2022, 7, 2200180. [Google Scholar] [CrossRef]
- Thomsen, R.P.; Malle, M.G.; Okholm, A.H.; Krishnan, S.; Bohr, S.S.R.; Sørensen, R.S.; Ries, O.; Vogel, S.; Simmel, F.C.; Hatzakis, N.S.; et al. A Large Size-Selective DNA Nanopore with Sensing Applications. Nat. Commun. 2019, 10, 5655. [Google Scholar] [CrossRef]
- Xing, Y.; Dorey, A.; Jayasinghe, L.; Howorka, S. Highly Shape- and Size-Tunable Membrane Nanopores Made with DNA. Nat. Nanotechnol. 2022, 17, 708–713. [Google Scholar] [CrossRef]
- Hamada, S.; Murata, S. Substrate-Assisted Assembly of Interconnected Single-Duplex DNA Nanostructures. Angew. Chem. 2009, 121, 6952–6955. [Google Scholar] [CrossRef]
- Forth, J.; Liu, X.; Hasnain, J.; Toor, A.; Miszta, K.; Shi, S.; Geissler, P.L.; Emrick, T.; Helms, B.A.; Russell, T.P. Reconfigurable Printed Liquids. Adv. Mater. 2018, 30, 1707603. [Google Scholar] [CrossRef]
- Toor, A.; Forth, J.; Bochner De Araujo, S.; Merola, M.C.; Jiang, Y.; Liu, X.; Chai, Y.; Hou, H.; Ashby, P.D.; Fuller, G.G.; et al. Mechanical Properties of Solidifying Assemblies of Nanoparticle Surfactants at the Oil-Water Interface. Langmuir 2019, 35, 13340–13350. [Google Scholar] [CrossRef]
- Kong, D.; Megone, W.; Nguyen, K.D.Q.; di Cio, S.; Ramstedt, M.; Gautrot, J.E. Protein Nanosheet Mechanics Controls Cell Adhesion and Expansion on Low-Viscosity Liquids. Nano Lett. 2018, 18, 1946–1951. [Google Scholar] [CrossRef]
- Peng, L.; Matellan, C.; Bosch-Fortea, M.; Gonzalez-Molina, J.; Frigerio, M.; Salentinig, S.; Hernandez, A.d.R.; Gautrot, J.E. Mesenchymal Stem Cells Sense the Toughness of Nanomaterials and Interfaces. Adv. Healthc. Mater. 2023, 2203297. [Google Scholar] [CrossRef]
- Kong, D.; Peng, L.; Bosch-Fortea, M.; Chrysanthou, A.; Alexis, C.V.J.M.; Matellan, C.; Zarbakhsh, A.; Mastroianni, G.; del Rio Hernandez, A.; Gautrot, J.E. Impact of the Multiscale Viscoelasticity of Quasi-2D Self-Assembled Protein Networks on Stem Cell Expansion at Liquid Interfaces. Biomaterials 2022, 284, 121494. [Google Scholar] [CrossRef]
- Peng, L.; Gautrot, J.E. Long Term Expansion Profile of Mesenchymal Stromal Cells at Protein Nanosheet-Stabilised Bioemulsions for next Generation Cell Culture Microcarriers. Mater. Today Bio. 2021, 12, 100159. [Google Scholar] [CrossRef]
- Kong, D.; Peng, L.; di Cio, S.; Novak, P.; Gautrot, J.E. Stem Cell Expansion and Fate Decision on Liquid Substrates Are Regulated by Self-Assembled Nanosheets. ACS Nano 2018, 12, 9206–9213. [Google Scholar] [CrossRef]
- Wang, T.; Bai, T.; Tan, Z.; Ohayon, Y.P.; Sha, R.; Vecchioni, S.; Seeman, N.C.; Wei, B. Mesojunction-Based Design Paradigm of Structural DNA Nanotechnology. J. Am. Chem. Soc. 2023, 145, 2455–2460. [Google Scholar] [CrossRef]
- Sengar, A.; Ouldridge, T.E.; Henrich, O.; Rovigatti, L.; Šulc, P. A Primer on the OxDNA Model of DNA: When to Use It, How to Simulate It and How to Interpret the Results. Front. Mol. Biosci. 2021, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.A.; Kaufhold, W.T.; Brooks, N.J.; Fodera, V.; di Michele, L. Flexibility Defines Structure in Crystals of Amphiphilic DNA Nanostars. J. Phys. Condens. Matter 2019, 31, 074003. [Google Scholar] [CrossRef] [PubMed]
- Fosado, Y.A.G. Nanostars Planarity Modulates the Elasticity of DNA Hydrogels. arXiv 2022. [Google Scholar] [CrossRef]
- Fuller, G.G.; Vermant, J. Complex Fluid-Fluid Interfaces: Rheology and Structure. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 519–543. [Google Scholar] [CrossRef]
- Vandebril, S.; Franck, A.; Fuller, G.G.; Moldenaers, P.; Vermant, J. A Double Wall-Ring Geometry for Interfacial Shear Rheometry. Rheol. Acta 2010, 49, 131–144. [Google Scholar] [CrossRef]
- Megone, W.; Kong, D.; Peng, L.; Gautrot, J.E. Extreme Reversal in Mechanical Anisotropy in Liquid-Liquid Interfaces Reinforced with Self-Assembled Protein Nanosheets. J. Colloid. Interface Sci. 2021, 594, 650–657. [Google Scholar] [CrossRef]
- Freer, E.M.; Yim, K.S.; Fuller, G.G.; Radke, C.J. Interfacial Rheology of Globular and Flexible Proteins at the Hexadecane/Water Interface: Comparison of Shear and Dilatation Deformation. J. Phys. Chem. B 2004, 108, 3835–3844. [Google Scholar] [CrossRef]
- Cao, D.; Xie, Y.; Song, J. DNA Hydrogels in the Perspective of Mechanical Properties. Macromol. Rapid Commun. 2022, 43, 2200281. [Google Scholar] [CrossRef]
- Bush, J.; Hu, C.H.; Veneziano, R. Mechanical Properties of DNA Hydrogels: Towards Highly Programmable Biomaterials. Appl. Sci. 2021, 11, 1885. [Google Scholar] [CrossRef]
- Zhang, N.H.; Chen, J.Z. Mechanical Properties of Double-Stranded DNA Biolayers Immobilized on Microcantilever under Axial Compression. J. Biomech. 2009, 42, 1483–1487. [Google Scholar] [CrossRef]
- Gore, J.; Bryant, Z.; Nöllmann, M.; Le, M.U.; Cozzarelli, N.R.; Bustamante, C. DNA Overwinds When Stretched. Nature 2006, 442, 836–839. [Google Scholar] [CrossRef]
- Kawamata, I.; Nomura, S.I.M.; Murata, S. Autonomous and Programmable Strand Generator Implemented as DNA and Enzymatic Chemical Reaction Cascade. New Gener. Comput. 2022, 40, 723–736. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, I.H.; Kim, D.; Zhang, B.T. Multiobjective Evolutionary Optimization of DNA Sequences for Reliable DNA Computing. IEEE Trans. Evol. Comput. 2005, 9, 143–158. [Google Scholar] [CrossRef]
- Tanaka, F.; Kameda, A.; Yamamoto, M.; Ohuchi, A. Design of Nucleic Acid Sequences for DNA Computing Based on a Thermodynamic Approach. Nucleic Acids Res. 2005, 33, 903–911. [Google Scholar] [CrossRef]
- Zadeh, J.N.; Steenberg, C.D.; Bois, J.S.; Wolfe, B.R.; Pierce, M.B.; Khan, A.R.; Dirks, R.M.; Pierce, N.A. NUPACK: Analysis and Design of Nucleic Acid Systems. J. Comput. Chem. 2011, 32, 170–173. [Google Scholar] [CrossRef]
- Henrich, O.; Gutiérrez Fosado, Y.A.; Curk, T.; Ouldridge, T.E. Coarse-Grained Simulation of DNA Using LAMMPS. Eur. Phys. J. E 2018, 41, 57. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Bohlin, J.; Matthies, M.; Poppleton, E.; Procyk, J.; Mallya, A.; Yan, H.; Šulc, P. Design and Simulation of DNA, RNA and Hybrid Protein–Nucleic Acid Nanostructures with OxView. Nat. Protoc. 2022, 17, 1762–1788. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Sim, A.Y.L.; Lipfert, J.; Herschlag, D.; Doniach, S. Salt Dependence of the Radius of Gyration and Flexibility of Single-Stranded DNA in Solution Probed by Small-Angle X-ray Scattering. Phys. Rev. E 2012, 86, 21901. [Google Scholar] [CrossRef]
- Palma, E.; Klapper, D.G.; Cho, M.J. Antibodies as Drug Carriers III: Design of Oligonucleotides with Enhanced Binding Affinity for Immunoglobulin G. Pharm. Res. 2005, 22, 122–127. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes-Salguero, K.; Gutiérrez Fosado, Y.A.; Megone, W.; Gautrot, J.E.; Palma, M. Programmed Self-Assembly of DNA Nanosheets with Discrete Single-Molecule Thickness and Interfacial Mechanics: Design, Simulation, and Characterization. Molecules 2023, 28, 3686. https://doi.org/10.3390/molecules28093686
Cervantes-Salguero K, Gutiérrez Fosado YA, Megone W, Gautrot JE, Palma M. Programmed Self-Assembly of DNA Nanosheets with Discrete Single-Molecule Thickness and Interfacial Mechanics: Design, Simulation, and Characterization. Molecules. 2023; 28(9):3686. https://doi.org/10.3390/molecules28093686
Chicago/Turabian StyleCervantes-Salguero, Keitel, Yair Augusto Gutiérrez Fosado, William Megone, Julien E. Gautrot, and Matteo Palma. 2023. "Programmed Self-Assembly of DNA Nanosheets with Discrete Single-Molecule Thickness and Interfacial Mechanics: Design, Simulation, and Characterization" Molecules 28, no. 9: 3686. https://doi.org/10.3390/molecules28093686





