The Development of Naringin for Use against Bone and Cartilage Disorders
Abstract
:1. Introduction
2. The Protective Activities of Naringin against Osteoporosis
2.1. Osteogenic Differentiation Induction
2.2. Osteoclast Formation Inhibition
2.3. The Underlying Mechanisms of Naringin in Protecting against Osteoporosis
2.4. Potential Applications of Naringin in Bone Tissue Engineering
3. The Protective Activities of Naringin against Intervertebral Disc Degeneration (IDD)
4. The Protective Activities of Naringin against Osteoarthritis (OA)
5. The Protective Activities of Naringin against Rheumatoid Arthritis (RA)
6. The Protective Activities of Naringin against Femoral Head (FH) Diseases
7. The Protective Activities of Naringin against Bone and Cartilage Tumors
8. The Protective Activities of Naringin against Tibial Dyschondroplasia
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials 2023, 13, 692. [Google Scholar] [CrossRef]
- Zan, P.; Wang, H.; Cai, Z.; Shen, J.; Sun, W. Revision surgeries for tumor endoprostheses around the knee joint: A mid-long-term follow-up of 20 cases. World J. Surg. Oncol. 2022, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Li, X.; Luo, B.; Ye, Q.; Wang, H.; Yang, L.; Zhu, X.; Han, L.; Zhang, R.; et al. A mechanistic review of chinese medicine polyphenols on bone formation and resorption. Front. Pharm. 2022, 13, 1017538. [Google Scholar] [CrossRef] [PubMed]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Health Mater. 2022, 12, e2202766. [Google Scholar] [CrossRef]
- Wollenhaupt, J.; Lee, E.B.; Curtis, J.R.; Silverfield, J.; Terry, K.; Soma, K.; Mojcik, C.; DeMasi, R.; Strengholt, S.; Kwok, K.; et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res. Ther. 2019, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fumery, M.; Singh, A.G.; Singh, N.; Prokop, L.J.; Dulai, P.S.; Sandborn, W.J.; Curtis, J.R. Comparative Risk of Cardiovascular Events With Biologic and Synthetic Disease-Modifying Antirheumatic Drugs in Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2020, 72, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Rzhepakovsky, I.; Anusha Siddiqui, S.; Avanesyan, S.; Benlidayi, M.; Dhingra, K.; Dolgalev, A.; Enukashvily, N.; Fritsch, T.; Heinz, V.; Kochergin, S.; et al. Anti-arthritic effect of chicken embryo tissue hydrolyzate against adjuvant arthritis in rats (X-ray microtomographic and histopathological analysis). Food Sci. Nutr. 2021, 9, 5648–5669. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021, 13, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Rehman, K.; Shahid, M.; Suhail, S.; Akash, M.S.H. Therapeutic potentials of genistein: New insights and perspectives. J. Food Biochem. 2022, 46, e14228. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, Z.; Amiri, M.S.; Mohammadzadeh, V.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Mashreghi, M.; Qayoomian, M.; Hashemzadeh, M.R.; Simal-Gandara, J.; Taghavizadeh Yazdi, M.E. Icariin: A Promising Natural Product in Biomedicine and Tissue Engineering. J. Funct. Biomater. 2023, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Garro, A.G.; Gaitán, A.; Murature, M.; Galiano, M.; Brignone, S.G.; Palma, S.D. Naringin: Nanotechnological Strategies for Potential Pharmaceutical Applications. Pharmaceutics 2023, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, X.; Xu, H.; Liu, G.; Wang, Y.; Sun, H.; Geng, F.; Zhang, N. Tissue Distribution of Total Flavonoids Extracts of Drynariae Rhizoma in Young and Old Rats by UPLC-MS/MS Determination. J. Anal. Methods Chem. 2022, 2022, 2447945. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Memariani, Z.; Abbas, S.Q.; Ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharm. Res. 2021, 171, 105264. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Dong, S.; Su, Z.H.; Zhang, H.W.; Peng, J.B.; Yu, C.Y.; Zou, Z.M. Comparative pharmacokinetics of naringin in rat after oral administration of chaihu-shu-gan-san aqueous extract and naringin alone. Metabolites 2013, 3, 867–880. [Google Scholar] [CrossRef]
- Zeng, X.; Yao, H.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Li, P.; Su, W. Tissue distribution of naringin and derived metabolites in rats after a single oral administration. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1136, 121846. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Su, W.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Peng, W.; Yao, H. Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Naringin in Aged Rats. Front. Pharm. 2019, 10, 34. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Guan, X.; Liu, B.; Wang, Y.; Xu, K.; Peng, W.; Su, W.; Zhang, K. Acute and 13 weeks subchronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.E.; Alder, K.D.; Morris, M.T.; Munger, A.M.; Lee, I.; Cahill, S.V.; Kwon, H.K.; Back, J.; Lee, F.Y. Re-appraising the potential of naringin for natural, novel orthopedic biotherapies. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720x20966135. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Song, J.; Yi, Y.; Li, J.; Zhang, T.; Shu, Y.; Wu, X. Controlled released naringin-loaded liposome/sucrose acetate isobutyrate hybrid depot for osteogenesis in vitro and in vivo. Front. Bioeng. Biotechnol. 2022, 10, 1097178. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- van den Blink, Q.U.; Garcez, K.; Henson, C.C.; Davidson, S.E.; Higham, C.E. Pharmacological interventions for the prevention of insufficiency fractures and avascular necrosis associated with pelvic radiotherapy in adults. Cochrane Database Syst. Rev. 2018, 4, Cd010604. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Crittenden, D.B.; Bolognese, M.A.; Genant, H.K.; Engelke, K.; Oliveri, B.; Brown, J.P.; Langdahl, B.L.; Yan, C.; Grauer, A.; et al. Greater Gains in Spine and Hip Strength for Romosozumab Compared With Teriparatide in Postmenopausal Women With Low Bone Mass. J. Bone Miner. Res. 2017, 32, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M. The why and how of sequential and combination therapy in osteoporosis. A review of the current evidence. Arch. Endocrinol. Metab. 2022, 66, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Loomba, R. Emerging role of statin therapy in the prevention and management of cirrhosis, portal hypertension, and HCC. Hepatology 2023. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Rabie, A.B. Effect of naringin on bone cells. J. Orthop. Res. 2006, 24, 2045–2050. [Google Scholar] [CrossRef]
- Chiba, H.; Kim, H.; Matsumoto, A.; Akiyama, S.; Ishimi, Y.; Suzuki, K.; Uehara, M. Hesperidin prevents androgen deficiency-induced bone loss in male mice. Phytother. Res. PTR 2014, 28, 289–295. [Google Scholar] [CrossRef]
- Habauzit, V.; Sacco, S.M.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Barron, D.; Pinaud, S.; Offord, E.; Horcajada, M.N. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011, 49, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.M.; Saint, C.; LeBlanc, P.J.; Ward, W.E. Maternal Consumption of Hesperidin and Naringin Flavanones Exerts Transient Effects to Tibia Bone Structure in Female CD-1 Offspring. Nutrients 2017, 9, 250. [Google Scholar] [CrossRef]
- Ge, X.; Zhou, G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am. J. Transl. Res. 2021, 13, 6330–6341. [Google Scholar] [PubMed]
- Zhang, P.; Dai, K.R.; Yan, S.G.; Yan, W.Q.; Zhang, C.; Chen, D.Q.; Xu, B.; Xu, Z.W. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur. J. Pharm. 2009, 607, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, J.; Fan, Q. Naringin promotes differentiation of bone marrow stem cells into osteoblasts by upregulating the expression levels of microRNA-20a and downregulating the expression levels of PPARγ. Mol. Med. Rep. 2015, 12, 4759–4765. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bao, B.; Wang, S.; Elango, J.; Wu, W. Fabrication of Chinese Traditional Medicines incorporated collagen biomaterials for human bone marrow mesenchymal stem cells. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 139, 111659. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, W.; Qin, Z.; Yu, H.; Yu, Z.; Zhong, M.; Sun, K.; Zhang, W. Effects of Naringin on Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells In Vitro and In Vivo. Stem Cells Int. 2015, 2015, 758706. [Google Scholar] [CrossRef] [PubMed]
- Suwittayarak, R.; Klincumhom, N.; Ngaokrajang, U.; Namangkalakul, W.; Ferreira, J.N.; Pavasant, P.; Osathanon, T. Shear Stress Enhances the Paracrine-Mediated Immunoregulatory Function of Human Periodontal Ligament Stem Cells via the ERK Signalling Pathway. Int. J. Mol. Sci. 2022, 23, 7119. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Xie, Y.; Chen, T.; Fu, B.; Cui, S.; Wang, Y.; Cai, G.; Chen, X. ERK1/2 signaling mediated naringin-induced osteogenic differentiation of immortalized human periodontal ligament stem cells. Biochem. Biophys. Res. Commun. 2017, 489, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, J.; Hao, Y.; Qi, Y.; Liang, J.; Yan, L.; Wang, W. Naringin Alleviates H2O2-Inhibited Osteogenic Differentiation of Human Adipose-Derived Stromal Cells via Wnt/β-Catenin Signaling. Evid.-Based Complement. Altern. Med. ECAM 2022, 2022, 3126094. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.G.; Wang, X.M.; Ma, L.F.; Zhang, Y.M. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem. Biol. Interact. 2015, 242, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Y.; Yang, S.T. Effects of naringin on the proliferation and osteogenic differentiation of human amniotic fluid-derived stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhu, Y.; Hu, G. Naringin promotes cellular chemokine synthesis and potentiates mesenchymal stromal cell migration via the Ras signaling pathway. Exp. Ther. Med. 2018, 16, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, C.; Li, J.; Zhu, Y.; Jia, Y.; Zhang, Y.; Zhang, X.; Li, W.; Cui, L.; Li, W.; et al. Naringin enhances osteogenic differentiation through the activation of ERK signaling in human bone marrow mesenchymal stem cells. Iran. J. Basic Med. Sci. 2017, 20, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Li, S.; Chen, H.; Wu, Y.; Qiu, Y. IL-37 alleviates alveolar bone resorption and inflammatory response through the NF-κB/NLRP3 signaling pathway in male mice with periodontitis. Arch. Oral Biol. 2023, 147, 105629. [Google Scholar] [CrossRef] [PubMed]
- Ang, E.S.; Yang, X.; Chen, H.; Liu, Q.; Zheng, M.H.; Xu, J. Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-κB and ERK activation. FEBS Lett. 2011, 585, 2755–2762. [Google Scholar] [CrossRef]
- Chen, L.L.; Lei, L.H.; Ding, P.H.; Tang, Q.; Wu, Y.M. Osteogenic effect of Drynariae rhizoma extracts and Naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Arch. Oral Biol. 2011, 56, 1655–1662. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, P.; Zhang, Y.; Sse, W.C. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 2007, 35, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, N.; Xu, W.; Zhang, Z.; Yang, Y.; Zhang, J.; Xu, H. Effect of flavonoids from Rhizoma Drynariae on osteoporosis rats and osteocytes. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 153, 113379. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.; Ma, J.; Ma, X.; Zhao, B.; Zhang, Y.; Tian, P.; Li, Y.; Han, Z. Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochem. Biophys. Res. Commun. 2014, 452, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, F.; Ma, X.; Ma, J.; Zhao, B.; Zhang, Y.; Li, Y.; Lv, J.; Meng, X. The Effects of Combined Treatment with Naringin and Treadmill Exercise on Osteoporosis in Ovariectomized Rats. Sci. Rep. 2015, 5, 13009. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Lv, F.; Ge, X.; Li, G. Naringin protects against bone loss in steroid-treated inflammatory bowel disease in a rat model. Arch. Biochem. Biophys. 2018, 650, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Whitaker, C.; Xu, Z.; Heggeness, M.; Yang, S.Y. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016, 16, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Chang, F.; Zhang, T.; Huang, X.; Yu, C.; Hu, Z.; Ji, M.; Duan, Y. Naringin Protects Against Interleukin 1β (IL-1β)-Induced Human Nucleus Pulposus Cells Degeneration via Downregulation Nuclear Factor kappa B (NF-κB) Pathway and p53 Expression. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9963–9972. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gao, S.; Guan, H.; Zhang, X.; Gao, Y.; Su, Y.; Song, Y.; Jiang, Y.; Li, N. Naringin protects human nucleus pulposus cells against TNF-α-induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxid Med. Cell Longev. 2022, 2022, 7655142. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.P.; Wang, F.; Ran, D.; Zhou, S.F.; Liu, Y.; Zhang, Z.; Huang, Z.N.; Wang, Z.Y.; Wang, J.C.; Feng, X.M.; et al. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect. Tissue Res. 2020, 61, 554–567. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Wang, W.; Zhang, H.; Chen, J.; Su, P.; Liu, L.; Li, W. Naringin Protects Against Cartilage Destruction in Osteoarthritis Through Repression of NF-κB Signaling Pathway. Inflammation 2016, 39, 385–392. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.F.; Sun, W.X. Effect of Naringin on Monosodium Iodoacetate-Induced Osteoarthritis Pain in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3746–3751. [Google Scholar] [CrossRef]
- Ye, C.; Chen, J.; Qu, Y.; Qi, H.; Wang, Q.; Yang, Z.; Wu, A.; Wang, F.; Li, P. Naringin in the repair of knee cartilage injury via the TGF-β/ALK5/Smad2/3 signal transduction pathway combined with an acellular dermal matrix. J. Orthop. Transl. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Aihaiti, Y.; Song Cai, Y.; Tuerhong, X.; Ni Yang, Y.; Ma, Y.; Shi Zheng, H.; Xu, K.; Xu, P. Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation. Front. Pharm. 2021, 12, 672054. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, Z.; Chen, B.; Fang, G.; Sun, X.; Li, F.; Xu, H.; Chen, Y.; Ding, W. Naringin protects against steroid-induced avascular necrosis of the femoral head through upregulation of PPARγ and activation of the Notch signaling pathway. Mol. Med. Rep. 2018, 17, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.J.; Zhang, W.H.; He, W.W.; Sun, L.; Ma, J.X.; Wang, D.; Ma, X.L. Naringin regulates bone metabolism in glucocorticoid-induced osteonecrosis of the femoral head via the Akt/Bad signal cascades. Chem. Biol. Interact. 2019, 304, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Chuang, Q.; Jiashi, W.; Bin, L.; Guangbin, W.; Xianglu, J. Naringin targets Zeb1 to suppress osteosarcoma cell proliferation and metastasis. Aging 2018, 10, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.W.; Chou, Y.E.; Yang, W.H.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Naringin suppress chondrosarcoma migration through inhibition vascular adhesion molecule-1 expression by modulating miR-126. Int. Immunopharmacol. 2014, 22, 107–114. [Google Scholar] [CrossRef]
- Jiang, X.; Li, A.; Wang, Y.; Iqbal, M.; Waqas, M.; Yang, H.; Li, Z.; Mehmood, K.; Qamar, H.; Li, J. Ameliorative effect of naringin against thiram-induced tibial dyschondroplasia in broiler chicken. Environ. Sci. Pollut Res. Int. 2020, 27, 11337–11348. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, W.; Zhang, X.; Zeng, B.; Qian, Y. Naringin increases osteoprotegerin expression in fibroblasts from periprosthetic membrane by the Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2020, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, X.; Wu, T.; Zhou, Z.; Gao, Y.; Wang, X.; Zhang, C.Q. Inhibiting wear particles-induced osteolysis with naringin. Int. Orthop. 2013, 37, 137–143. [Google Scholar] [CrossRef]
- Li, N.; Xu, Z.; Wooley, P.H.; Zhang, J.; Yang, S.Y. Therapeutic potentials of naringin on polymethylmethacrylate induced osteoclastogenesis and osteolysis, in vitro and in vivo assessments. Drug Des. Dev. Ther. 2014, 8, 1–11. [Google Scholar] [CrossRef]
- Yu, G.Y.; Zheng, G.Z.; Chang, B.; Hu, Q.X.; Lin, F.X.; Liu, D.Z.; Wu, C.C.; Du, S.X.; Li, X.D. Naringin Stimulates Osteogenic Differentiation of Rat Bone Marrow Stromal Cells via Activation of the Notch Signaling Pathway. Stem Cells Int. 2016, 2016, 7130653. [Google Scholar] [CrossRef]
- Sun, C.; Chen, X.; Yang, S.; Jin, C.; Ding, K.; Chen, C. LBP1C-2 from Lycium barbarum alleviated age-related bone loss by targeting BMPRIA/BMPRII/Noggin. Carbohydr. Polym. 2023, 310, 120725. [Google Scholar] [CrossRef]
- Dong, G.C.; Ma, T.Y.; Li, C.H.; Chi, C.Y.; Su, C.M.; Huang, C.L.; Wang, Y.H.; Lee, T.M. A study of Drynaria fortunei in modulation of BMP–2 signalling by bone tissue engineering. Turk. J. Med. Sci. 2020, 50, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Mang, T.; Kleinschmidt-Doerr, K.; Ploeger, F.; Schoenemann, A.; Lindemann, S.; Gigout, A. BMPR1A is necessary for chondrogenesis and osteogenesis, whereas BMPR1B prevents hypertrophic differentiation. J. Cell Sci. 2020, 133, jcs246934. [Google Scholar] [CrossRef]
- Wong, R.W.; Rabie, A.B. Effect of naringin collagen graft on bone formation. Biomaterials 2006, 27, 1824–1831. [Google Scholar] [CrossRef]
- Wu, J.B.; Fong, Y.C.; Tsai, H.Y.; Chen, Y.F.; Tsuzuki, M.; Tang, C.H. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharm. 2008, 588, 333–341. [Google Scholar] [CrossRef]
- Liu, K.; Ge, H.; Liu, C.; Jiang, Y.; Yu, Y.; Zhou, Z. Notch-RBPJ Pathway for the Differentiation of Bone Marrow Mesenchymal Stem Cells in Femoral Head Necrosis. Int. J. Mol. Sci. 2023, 24, 6295. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.G.; Adolpho, L.F.; Lopes, H.B.; Weffort, D.; Souza, A.T.P.; Oliveira, F.S.; Rosa, A.L.; Beloti, M.M. Effects of Modulation of the Hedgehog and Notch Signaling Pathways on Osteoblast Differentiation Induced by Titanium with Nanotopography. J. Funct. Biomater. 2023, 14, 79. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells. Biochem. Biophys. Res. Commun. 2014, 443, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yu, Y.J.; Li, J.Y.; Li, M.Y.; Xia, S.M.; Xue, K.; Wang, S.Y.; Yang, C. Activating Wnt/β-catenin signaling by autophagic degradation of APC contributes to the osteoblast differentiation effect of soy isoflavone on osteoporotic mesenchymal stem cells. Acta Pharm. Sin. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; Wang, F.; Dong, J.; Wang, D.; Sun, B.; Wang, B. Stimulation of Wnt/β-Catenin Signaling to Improve Bone Development by Naringin via Interacting with AMPK and Akt. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Gerbaix, M.; Vico, L.; Ferrari, S.L.; Bonnet, N. Periostin expression contributes to cortical bone loss during unloading. Bone 2015, 71, 94–100. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74. [Google Scholar] [CrossRef]
- Ma, X.; Lv, J.; Sun, X.; Ma, J.; Xing, G.; Wang, Y.; Sun, L.; Wang, J.; Li, F.; Li, Y.; et al. Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci. Rep. 2016, 6, 24562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Z.; Yang, R.; Zong, S.; Wei, X.; Wang, C.; Guo, L.; Sun, J.; Li, H.; Li, P. Inactivation of Ihh in Sp7-Expressing Cells Inhibits Osteoblast Proliferation, Differentiation, and Bone Formation, Resulting in a Dwarfism Phenotype with Severe Skeletal Dysplasia in Mice. Calcif. Tissue Int. 2022, 111, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.X.; Du, S.X.; Liu, D.Z.; Hu, Q.X.; Yu, G.Y.; Wu, C.C.; Zheng, G.Z.; Xie, D.; Li, X.D.; Chang, B. Naringin promotes osteogenic differentiation of bone marrow stromal cells by up-regulating Foxc2 expression via the IHH signaling pathway. Am. J. Transl. Res. 2016, 8, 5098–5107. [Google Scholar] [PubMed]
- Lv, J.; Sun, X.; Ma, J.; Ma, X.; Xing, G.; Wang, Y.; Sun, L.; Wang, J.; Li, F.; Li, Y. Involvement of periostin-sclerostin-Wnt/β-catenin signaling pathway in the prevention of neurectomy-induced bone loss by naringin. Biochem. Biophys. Res. Commun. 2015, 468, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, J.; Wang, X.; Luo, H.; Zhang, H.; Cao, D.; Chen, L.; Huang, N. Double directional adjusting estrogenic effect of naringin from Rhizoma drynariae (Gusuibu). J. Ethnopharmacol. 2011, 138, 451–457. [Google Scholar] [CrossRef]
- Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, M.S. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br. J. Pharm. 2010, 159, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Chen, K.Y.; Yang, J.D.; Liu, S.H.; Chen, R.M. Naringin Improves Osteoblast Mineralization and Bone Healing and Strength through Regulating Estrogen Receptor Alpha-Dependent Alkaline Phosphatase Gene Expression. J. Agric. Food Chem. 2021, 69, 13020–13033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, W.; Li, Y.; Zhu, Z.; Zhang, W. Effect of Naringin Treatment on Postmenopausal Osteoporosis in Ovariectomized Rats: A Meta-Analysis and Systematic Review. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 6016874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Zhou, L.; Chen, C.; Chen, G. Sequential release of vascular endothelial growth factor-A and bone morphogenetic protein-2 from osteogenic scaffolds assembled by PLGA microcapsules: A preliminary study in vitro. Int. J. Biol. Macromol. 2023, 232, 123330. [Google Scholar] [CrossRef]
- Shangguan, W.J.; Zhang, Y.H.; Li, Z.C.; Tang, L.M.; Shao, J.; Li, H. Naringin inhibits vascular endothelial cell apoptosis via endoplasmic reticulum stress- and mitochondrial-mediated pathways and promotes intraosseous angiogenesis in ovariectomized rats. Int. J. Mol. Med. 2017, 40, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhao, Z.; Ma, X.; Sun, X.; Ma, J.; Li, F.; Sun, L.; Lv, J. Naringin promotes fracture healing through stimulation of angiogenesis by regulating the VEGF/VEGFR-2 signaling pathway in osteoporotic rats. Chem. Biol. Interact. 2017, 261, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Venkatesha, V.A.; Reddy, T.K. Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Reddy, T.K. The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: A micronucleus study. Mutat. Res. 2002, 519, 37–48. [Google Scholar] [CrossRef]
- Rivoira, M.; Rodríguez, V.; Picotto, G.; Battaglino, R.; Tolosa de Talamoni, N. Naringin prevents bone loss in a rat model of type 1 Diabetes mellitus. Arch. Biochem. Biophys. 2018, 637, 56–63. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, P.; Zhang, C.; Zhu, Z. Promotion of bone formation by naringin in a titanium particle-induced diabetic murine calvarial osteolysis model. J. Orthop. Res. 2010, 28, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, Y.; Wu, Q.; Ji, Y.C.; Feng, Z.F.; Kang, F.W. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J. Cell Physiol. 2019, 234, 21182–21192. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, J.; Sun, L.; Yao, Z.; Liu, R.; Huang, J.; Liu, X. RANKL downregulates cell surface CXCR6 expression through JAK2/STAT3 signaling pathway during osteoclastogenesis. Biochem. Biophys. Res. Commun. 2012, 429, 156–162. [Google Scholar] [CrossRef]
- Wang, W.; Mao, J.; Chen, Y.; Zuo, J.; Chen, L.; Li, Y.; Gao, Y.; Lu, Q. Naringin promotes osteogenesis and ameliorates osteoporosis development by targeting JAK2/STAT3 signalling. Clin. Exp. Pharmacol. Physiol. 2022, 49, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, Y.; Wooley, P.H.; Xu, Z.; Yang, S.Y. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J. Orthop. Sci. 2013, 18, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, X.; Ma, P.; Peng, Z.; Cai, K. Composite coatings of Mg-MOF74 and Sr-substituted hydroxyapatite on titanium substrates for local antibacterial, anti-osteosarcoma and pro-osteogenesis applications. Mater. Lett. 2019, 241, 18–22. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, B.; Gong, Y.; Chen, R.; Yang, W.; Lin, C.; Chen, M.; Qin, L.; Jia, Y.; Cai, K. Functionalization of Ti substrate with pH-responsive naringin-ZnO nanoparticles for the reconstruction of large bony after osteosarcoma resection. J. Biomed. Mater. Res. Part A 2020, 108, 2190–2205. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Almassri, H.N.S.; Zhang, Q.; Ma, Y.; Zhang, D.; Chen, M.; Wu, X. Electrosprayed naringin-loaded microsphere/SAIB hybrid depots enhance bone formation in a mouse calvarial defect model. Drug Deliv. 2019, 26, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; You, D.; Zhuang, J.; Lin, S.; Dong, L.; Weng, S.; Zhang, B.; Cheng, K.; Weng, W.; Wang, H. Controlled Release of Naringin in Metal-Organic Framework-Loaded Mineralized Collagen Coating to Simultaneously Enhance Osseointegration and Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 19698–19705. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Zhang, X.; Tang, Q.; Fang, X.; Zhang, C.; Zhu, Z.; Hou, Y.; Lai, M. Microstructured titanium functionalized by naringin inserted multilayers for promoting osteogenesis and inhibiting osteoclastogenesis. J. Biomater. Sci. Polym. Ed. 2021, 32, 1865–1881. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, L.; Watts, D.C.; Qiu, H.; You, T.; Deng, F.; Wu, X. Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent. Mater. 2014, 30, 1263–1273. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, S.; Li, H.; Li, Q.; Wu, G.; Zhou, C. In vitro evaluation of electrospun PLGA/PLLA/PDLLA blend fibers loaded with naringin for guided bone regeneration. Dent. Mater. J. 2018, 37, 317–324. [Google Scholar] [CrossRef]
- Elkhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Almeida, R.; Gaspar, V.; Kahn, C.; Cleymand, F.; Arab-Tehrany, E.; Mano, J.F. Gelatin Methacryloyl (GelMA) Nanocomposite Hydrogels Embedding Bioactive Naringin Liposomes. Polymers 2020, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shen, G.; Shang, Q.; Zhang, Z.; Zhao, W.; Zhang, P.; Liang, D.; Ren, H.; Jiang, X. A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat. Int. J. Biol. Macromol. 2021, 193, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Ma, X.L.; Zhao, B.; Tian, P.; Ma, J.X.; Kang, J.Y.; Zhang, Y.; Guo, Y.; Sun, L. Naringin-inlaid silk fibroin/hydroxyapatite scaffold enhances human umbilical cord-derived mesenchymal stem cell-based bone regeneration. Cell Prolif. 2021, 54, e13043. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Ma, X.L.; Ma, J.X.; Kang, J.Y.; Zhang, Y.; Guo, Y. Sustained release of naringin from silk-fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Mater. Today Bio 2022, 13, 100206. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Q.; Xiong, Q.; Li, J.; Tang, C.; Zhang, Y.; Wang, D. Naringin Release from a Nano-Hydroxyapatite/Collagen Scaffold Promotes Osteogenesis and Bone Tissue Reconstruction. Polymers 2022, 14, 3260. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, F.; Lin, Z.; Cao, X.; Chen, D.; Chen, X. Local delivery of naringin in beta-cyclodextrin modified mesoporous bioactive glass promotes bone regeneration: From anti-inflammatory to synergistic osteogenesis and osteoclastogenesis. Biomater. Sci. 2022, 10, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lin, W.; Liang, C.; Zhang, D.; Yang, F.; Zhang, Y.; Zhang, X.; Feng, J.; Chen, C. Naringin rescued the TNF-α-induced inhibition of osteogenesis of bone marrow-derived mesenchymal stem cells by depressing the activation of NF-кB signaling pathway. Immunol. Res. 2015, 62, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, Q.; Ren, Q.; Luo, L.; Ji, G.; Zheng, T. Endoplasmic reticulum stress associates with the development of intervertebral disc degeneration. Front. Endocrinol. 2022, 13, 1094394. [Google Scholar] [CrossRef]
- Zhu, L.; Xie, Z.Y.; Jiang, Z.L.; Wang, X.H.; Shi, H.; Chen, L.; Wu, X.T. Unfolded protein response alleviates acid-induced premature senescence by promoting autophagy in nucleus pulposus cells. Cell Biol. Int. 2022, 46, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, X. Mesenchymal stem cell-derived exosomes are beneficial to suppressing inflammation and promoting autophagy in intervertebral disc degeneration. Folia Morphol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Liu, H.; Fu, C. Natural products can modulate inflammation in intervertebral disc degeneration. Front. Pharm. 2023, 14, 1150835. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Bao, S.; Zhang, C.; Zhang, J.; Lv, J.; Li, X.; Chudhary, M.; Ren, X.; Kong, L. Stimulation of AMPK Prevents Diabetes-Induced Photoreceptor Cell Degeneration. Oxid Med. Cell Longev. 2021, 2021, 5587340. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Lin, J.; Jin, H.; Wang, K.; Yan, Y.; Wang, J.; Wu, C.; Nisar, M.; Tian, N.; et al. Therapeutic Potential of Naringin for Intervertebral Disc Degeneration: Involvement of Autophagy Against Oxidative Stress-Induced Apoptosis in Nucleus Pulposus Cells. Am. J. Chin. Med. 2018, 46, 1561–1580. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shangguan, W.J.; Zhao, Z.J.; Zhou, F.C.; Liu, H.T.; Liang, Z.H.; Song, J.; Shao, J. Naringin Inhibits Apoptosis Induced by Cyclic Stretch in Rat Annular Cells and Partially Attenuates Disc Degeneration by Inhibiting the ROS/NF-κB Pathway. Oxid Med. Cell Longev. 2022, 2022, 6179444. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, L. The protective activity of genistein against bone and cartilage diseases. Front. Pharm. 2022, 13, 1016981. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, C.; Wu, H.; Li, C.; Li, S.; Luo, J.; Liu, T.; Ding, Y. Genetically Modified Mesenchymal Stromal Cells in Cartilage Regeneration. Stem Cells Dev. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Ye, C.; Chen, J.; Qu, Y.; Liu, H.; Yan, J.; Lu, Y.; Yang, Z.; Wang, F.; Li, P. Naringin and bone marrow mesenchymal stem cells repair articular cartilage defects in rabbit knees through the transforming growth factor-β superfamily signaling pathway. Exp. Ther. Med. 2020, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Recent advances in the opioid mu receptor based pharmacotherapy for rheumatoid arthritis. Expert Opin. Pharm. 2020, 21, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mohan, K.; Muzammil, S.; Alam, M.A.; Khayyam, K.U. Current Prospects in Rheumatoid Arthritis: Pathophysiology, Genetics, and Treatments. Recent Adv. Anti-Infect. Drug Discov. 2023. [Google Scholar] [CrossRef]
- Mohanty, S.; Sahoo, A.K.; Konkimalla, V.B.; Pal, A.; Si, S.C. Naringin in Combination with Isothiocyanates as Liposomal Formulations Potentiates the Anti-inflammatory Activity in Different Acute and Chronic Animal Models of Rheumatoid Arthritis. ACS Omega 2020, 5, 28319–28332. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, T.; Liao, J.; Gu, H.; Lin, X.; Jiang, Q.; Bulsara, M.K.; Zheng, M.; Zheng, Q. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: A prospective, double-blinded, randomized, controlled study. Stem Cell Res. Ther. 2014, 5, 115. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, K.; Hasegawa, Y.; Seki, T.; Takegami, Y.; Amano, T.; Ishiguro, N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod. Rheumatol. 2015, 25, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Park, S.; Song, J.H.; Jung, Y.Y.; Cho, M.R.; Rhyu, K.H. Prevalence of osteonecrosis of the femoral head: A nationwide epidemiologic analysis in Korea. J. Arthroplast. 2009, 24, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Yu, M.; Hu, K.; Wang, W.; Yang, L.; Wang, B.J.; Gao, X.H.; Guo, Y.M.; Xu, Y.Q.; Wei, Y.S.; et al. Prevalence of Nontraumatic Osteonecrosis of the Femoral Head and its Associated Risk Factors in the Chinese Population: Results from a Nationally Representative Survey. Chin. Med. J. 2015, 128, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, W.; Lv, X.; Wang, B.; Han, L.; Gong, S.; Wang, P.; Feng, Y. Advances in experimental models of osteonecrosis of the femoral head. J. Orthop. Transl. 2023, 39, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front. Pharm. 2021, 12, 639840. [Google Scholar] [CrossRef]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S.; et al. Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. Int. 2022, 29, 31025–31041. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, C.; Li, J.; Li, J.; Yu, R.; Cai, F.; Qu, G.; Yu, B.; Liu, L.; Zeng, D.; et al. Indirect bilirubin impairs invasion of osteosarcoma cells via inhibiting the PI3K/AKT/MMP-2 signaling pathway by suppressing intracellular ROS. J. Bone Oncol. 2023, 39, 100472. [Google Scholar] [CrossRef]
- Degnan, A.J.; Chung, C.Y.; Shah, A.J. Quantitative diffusion-weighted magnetic resonance imaging assessment of chemotherapy treatment response of pediatric osteosarcoma and Ewing sarcoma malignant bone tumors. Clin. Imaging 2018, 47, 9–13. [Google Scholar] [CrossRef]
- Hua, K.C.; Hu, Y.C. Treatment method and prognostic factors of chondrosarcoma: Based on Surveillance, Epidemiology, and End Results (SEER) database. Transl. Cancer Res. 2020, 9, 4250–4266. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Sun, H.; Jiang, H.; Wang, Q.; Gan, L.; Tian, Y.; Sun, J.; Wen, D.; Deng, J. Exogenous growth hormone functionally alleviates glucocorticoid-induced longitudinal bone growth retardation in male rats by activating the Ihh/PTHrP signaling pathway. Mol. Cell Endocrinol. 2022, 545, 111571. [Google Scholar] [CrossRef] [PubMed]
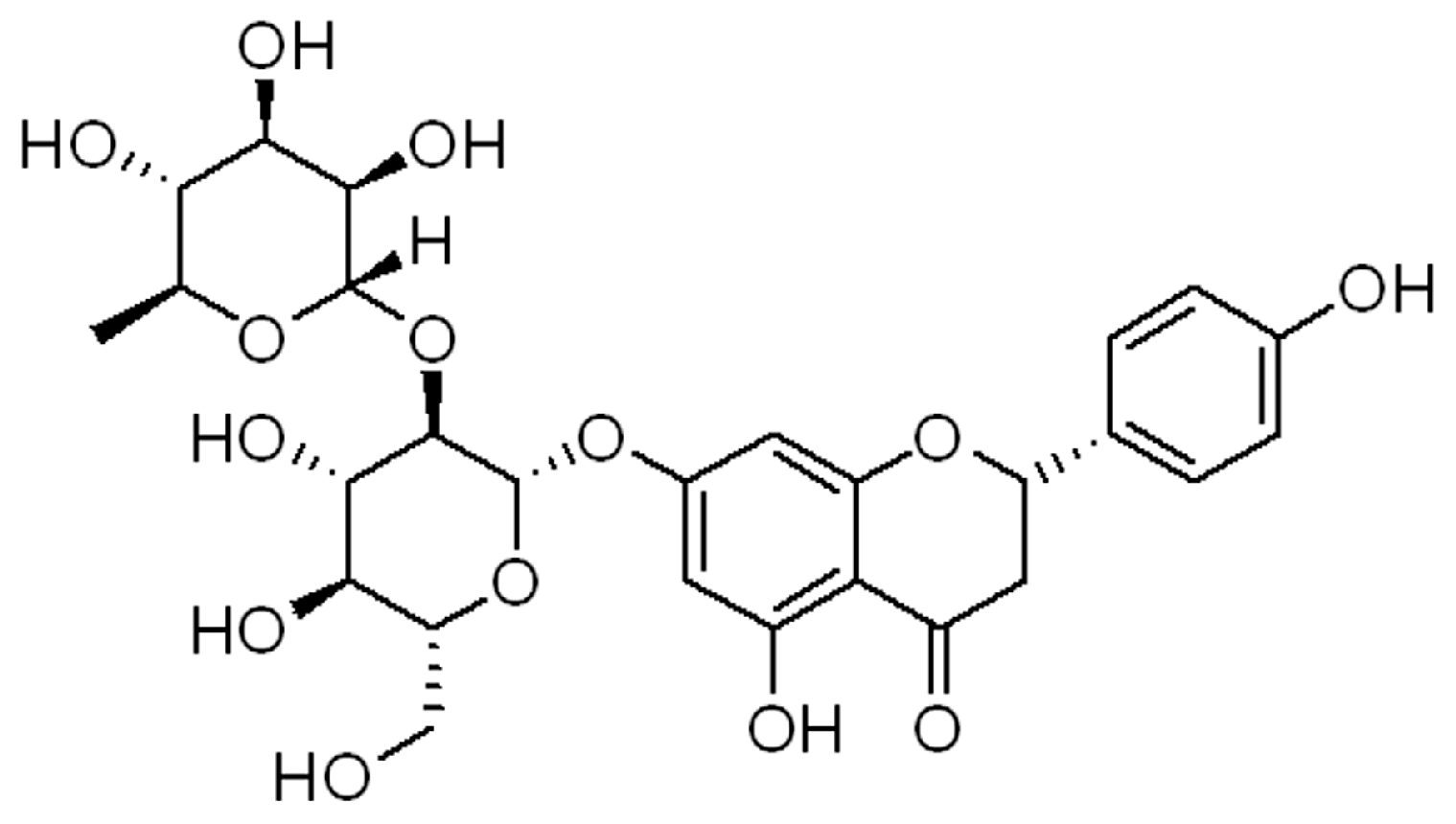


| Models/Cells | Doses/Concentrations | Biological Actions | Ref. |
|---|---|---|---|
| ReA-induced rat OP | 50 mg/kg | Increases BMD of femur shaft, increases BV/TV and Tb.Sp, increases ALP activity, decreases SOST, TRAP, and RANKL expression, and increases PTH1R expression. | [50] |
| IDG-SW3 cells | 50 μM | Decreases SOST and RANKL expression. | [50] |
| OVX-induced rat OP | 40, 100, 200 mg/kg | Improves BMD, bone indices, and pathological changes. Increases the average maximum fracture load. Increases the serum OC and decreases the serum CTX-1. | [51] |
| Osteoclasts | 20 ng/mL | Reduces the number of TRAP-positive osteoclasts; promotes cell apoptosis. | [51] |
| OVX-induced rat OP | 300 mg/kg + treadmill exercise | Increases bone indices, BMD, and mechanical strength; increases OCN expression and decreases CTX-1 expression. | [52] |
| GC-treated IBD rats | 100 and 200 mg/kg | Decreases the serum TNFα and increases the serum P1NP; improves the bone indices; decreases MDA, CAT, and SOD activity; increases ALP, OC, and RUNX-2 expression. | [53] |
| NP cells from patients with IDD | 20 μg/mL | Increases cell proliferation, increases BMP2, Sox6, and aggrecan expression, and decreases MMP-3 expression. | [54] |
| IL-1β-treated human NP cells | 0.4, 0.8, 1.2, and 1.6 μM | Decreases MMP-3, MMP-13, ADAMTS4, and ADMATS5 expression, increases collagen II and aggrecan production; attenuates p65 and IκBα phosphorylation; decreases p65 and p53 expression. | [55] |
| TNFα-treated NP cells | 10 μg/mL | Decreases COX-2, cleaved caspase-3, Bax, MMP-3, ADAMTS4, and p63 expression; enhances SOD, Bcl-2, collagen II, aggrecan, Sox9, LC3-II/I ratio, AMPK, and Sirt1 expression; improves mitochondrial functions. | [56] |
| H2O2-treated NP-derived MSCs | 10 μM | Inhibits apoptosis; improves mitochondrial functions; decreases Bax, caspase-3, and p53 expression; increases Bcl-2, PI3K, and AKT expression | [57] |
| TNFα-treated mouse chondrocytes | 5 μM | Decreases IL-1β, iNOS, COX-2, MMP-13, ADAMTS5, p-IκBα, and NF-κB2 expression. | [58] |
| ACLT-induced mouse OA | 100 mg/kg | Decreases IL-1β, iNOS, COX-2, MMP-13, ADAMTS5, p-IκBα, and NF-κB2 expression. | [58] |
| MIA-induced rat OA | 5 and 10 mg/kg | Decreases the serum levels of PGE2, IL-6, IL-1β, and TNFα; improves histopathological changes. | [59] |
| LPS-treated RAW 264.7 cells | 5 and 10 μg/mL | Decreases the production of PGE2, NO, IL-6, and TNFα. | [59] |
| Cartilage defects in New Zealand rabbits | 84 mg/kg + ADM | Improves the repair morphology of defect cartilages and enhances the expression of TGFβ2, TGFβ3, and SOX9. | [60] |
| TNFα-treated RA FLSs | 20, 40, 60, and 80 μg/mL | Decreases cell viability, increases apoptosis, downregulates the expression of IL-1, IL-6, IL-8, MMP-1, MMP-2, and MMP-13, and suppresses the MAPK/ERK and PI3K/AKT signaling pathways. | [61] |
| MPS-induced rat SANFH | 5, 10, and 20 mg/kg | Increases the serum OC levels, decreases the total cholesterol, the LDL/HDL ratio, and caspase-3 expression; promotes osteogenic differentiation by increasing the expression of PPARγ, Notch, β-catenin, and p-AKT. | [62] |
| Dex-treated MLO-Y4, MC3T3-E1, and RAW 264.7 cells | 100 μM | Attenuates cell apoptosis and decreases Bax and cleaved caspase-3 expression; increases Bcl-2 expression; promotes osteogenic differentiation and inhibits osteoclast formation by activating the PI3K/AKT signaling pathway. | [63] |
| MPS-induced rat GIONFH | 300 mg/kg | Improves histopathological changes and enhances the expression of OC and AKT | [63] |
| MG63 cells | 10 and 20 μmol/L | Decreases the expression of Cyclin D1, MMP2, and Bcl-2 and inhibits cell proliferation, migration, and invasion. | [64] |
| JJ012 and SW1353 cells | 3, 10, and 30 μM | Decreases VCAM-1 expression, increases miR-126 expression, and suppresses cell migration and invasion. | [65] |
| Thiram-induced TD broiler chickens | 30 mg/kg | Restores the tibia weight and length, inhibits the reduction in blood vessels, and increases the expression of Ihh and PTHrP. | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, J.; Deng, X.; Le, Y.; Lai, J.; Liao, X. The Development of Naringin for Use against Bone and Cartilage Disorders. Molecules 2023, 28, 3716. https://doi.org/10.3390/molecules28093716
Gan J, Deng X, Le Y, Lai J, Liao X. The Development of Naringin for Use against Bone and Cartilage Disorders. Molecules. 2023; 28(9):3716. https://doi.org/10.3390/molecules28093716
Chicago/Turabian StyleGan, Juwen, Xiaolan Deng, Yonghong Le, Jun Lai, and Xiaofei Liao. 2023. "The Development of Naringin for Use against Bone and Cartilage Disorders" Molecules 28, no. 9: 3716. https://doi.org/10.3390/molecules28093716
APA StyleGan, J., Deng, X., Le, Y., Lai, J., & Liao, X. (2023). The Development of Naringin for Use against Bone and Cartilage Disorders. Molecules, 28(9), 3716. https://doi.org/10.3390/molecules28093716





