Dry Water as a Promoter for Gas Hydrate Formation: A Review
Abstract
1. Introduction
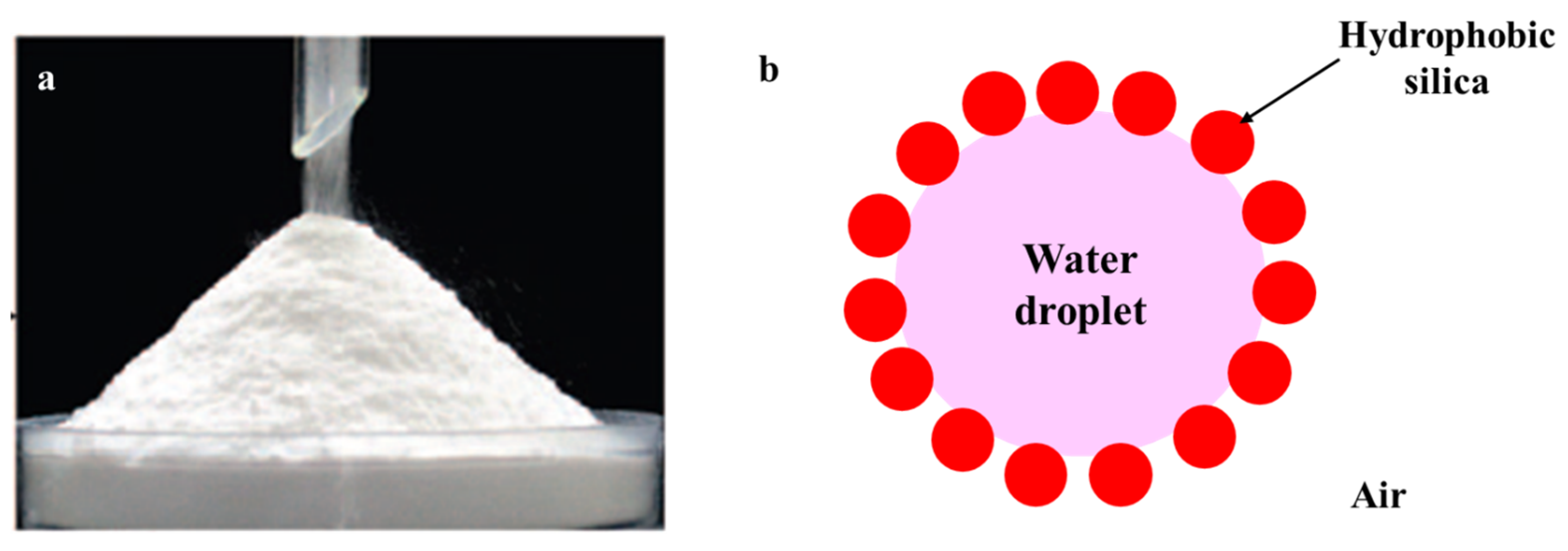
2. Formation of Stable Dry Water
2.1. Preparation Procedure of Dry Water
2.2. The Effect of Nanoparticle Size
2.3. The Effect of Nanoparticle Shape
2.4. The Effect of Particle Hydrophobicity
2.5. The Effect of Experimental Conditions
2.6. The Effect of the Aqueous Phase
2.7. Lifetime of the Dry Water
2.8. The Effect of External Stresses on the Stability of Dry Water
2.9. Size Distribution of Dry Water Droplets
2.10. Alternative Material to Nanosilica
3. The Effect of Dry Water on Gas Hydrate Formation
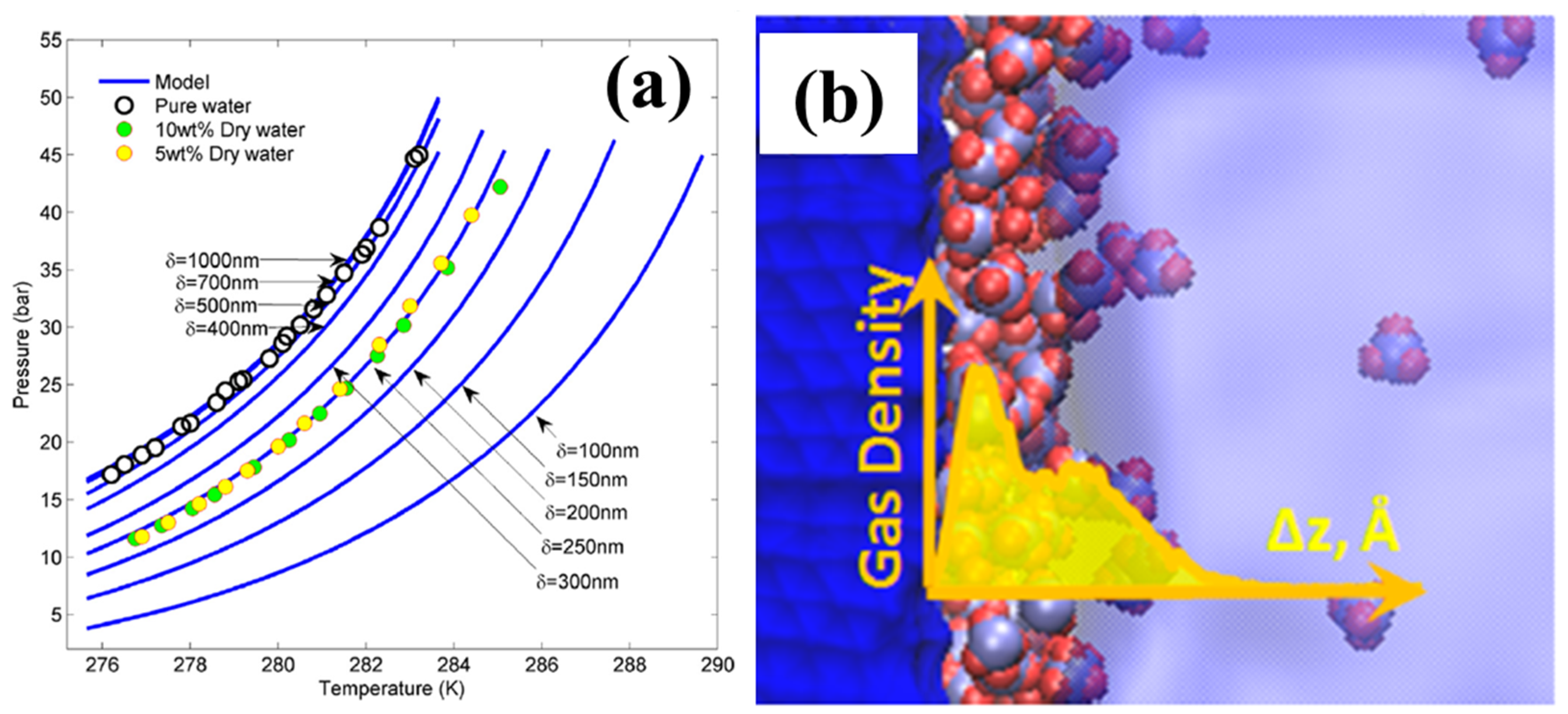
3.1. Thermodynamic Effect
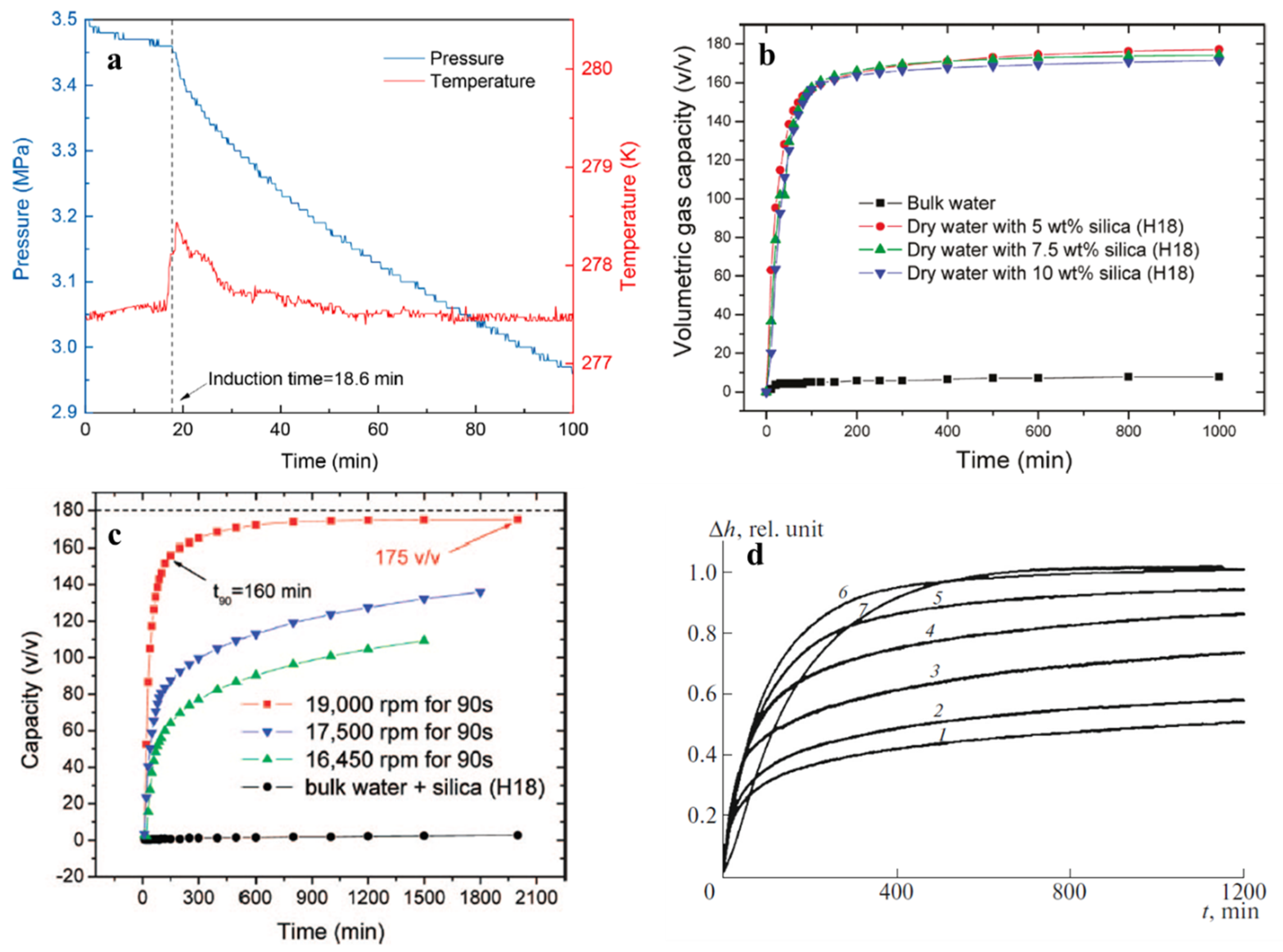
3.2. Kinetic Effect
3.3. Enhancement of the Gas Storage
3.4. Synergies with Other Chemicals
3.5. Comparison to Other Hydrate Promoters
4. The Effect of Dry Water on the Self-Preservation Effect
5. The Reusability of the Dry Water
6. Future Research Directions
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zavahir, S.; Ben Yahia, H.; Schneider, J.; Han, D.; Krupa, I.; Altamash, T.; Atilhan, M.; Amhamed, A.; Kasak, P.J.M. Fluorescent Zn (II)-Based Metal-Organic Framework: Interaction with Organic Solvents and CO2 and Methane Capture. Molecules 2022, 27, 3845. [Google Scholar] [CrossRef] [PubMed]
- Boufares, A.; Provost, E.; Dalmazzone, D.; Osswald, V.; Clain, P.; Delahaye, A.; Fournaison, L. Kinetic study of CO2 hydrates crystallization: Characterization using FTIR/ATR spectroscopy and contribution modeling of equilibrium/non-equilibrium phase-behavior. Chem. Eng. Sci. 2018, 192, 371–379. [Google Scholar] [CrossRef]
- Ke, W.; Svartaas, T.M.; Chen, D. Engineering. A review of gas hydrate nucleation theories and growth models. J. Nat. Gas Sci. Eng. 2019, 61, 169–196. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, X.; Wang, F. Methane hydrate production using a novel spiral-agitated reactor: Promotion of hydrate formation kinetics. AIChE J. 2022, 68, e17423. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.; Kumar, R.J.I.; Research, E.C. Role of surfactants in promoting gas hydrate formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Okutani, K.; Kuwabara, Y.; Mori, Y.H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: An experimental study using methane and sodium alkyl sulfates. Chem. Eng. Sci. 2008, 63, 183–194. [Google Scholar] [CrossRef]
- Ganji, H.; Manteghian, M.; Omidkhah, M.; Mofrad, H.R. Effect of different surfactants on methane hydrate formation rate, stability and storage capacity. Fuel 2007, 86, 434–441. [Google Scholar] [CrossRef]
- Lin, W.; Chen, G.-J.; Sun, C.-Y.; Guo, X.-Q.; Wu, Z.-K.; Liang, M.-Y.; Chen, L.-T.; Yang, L.-Y. Effect of surfactant on the formation and dissociation kinetic behavior of methane hydrate. Chem. Eng. Sci. 2004, 59, 4449–4455. [Google Scholar] [CrossRef]
- Zhong, Y.; Rogers, R. Surfactant effects on gas hydrate formation. Chem. Eng. Sci. 2000, 55, 4175–4187. [Google Scholar] [CrossRef]
- Karaaslan, U.; Parlaktuna, M.J.E. Surfactants as hydrate promoters? Energy Fuels 2000, 14, 1103–1107. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Jamaluddin, A.; Dholabhai, P.; Bishnoi, P. Effect of surfactants on hydrate formation kinetics. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, Louisiana, March 1993; OnePetro: New Orleans, Louisiana, 1993. [Google Scholar]
- Zhang, J.; Lee, S.; Lee, J.W. Kinetics of methane hydrate formation from SDS solution. Ind. Eng. Chem. Res. 2007, 46, 6353–6359. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, J.-W. Kinetic behaviors of CO2 hydrates in porous media and effect of kinetic promoter on the formation kinetics. Chem. Eng. Sci. 2010, 65, 1840–1845. [Google Scholar] [CrossRef]
- Majid, A.A.; Worley, J.; Koh, C.A. Thermodynamic and kinetic promoters for gas hydrate technological applications. Energy Fuels 2021, 35, 19288–19301. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Linga, P. Amino acids as kinetic promoters for gas hydrate applications: A mini review. Energy Fuels 2021, 35, 7553–7571. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Hong, Q.W.; Linga, P. Morphology study of methane hydrate formation and dissociation in the presence of amino acid. Cryst. Growth Des. 2016, 16, 5932–5945. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Prasad, P.S.; Sai Kiran, B. Clathrate hydrates of greenhouse gases in the presence of natural amino acids: Storage, transportation and separation applications. Sci. Rep. 2018, 8, 8560. [Google Scholar] [CrossRef]
- Li, R.; Sun, Z.; Song, J. Enhancement of hydrate formation with amino acids as promoters. J. Mol. Liq. 2021, 344, 117880. [Google Scholar] [CrossRef]
- Qin, Y.; Shang, L.; Lv, Z.; Liu, Z.; He, J.; Li, X.; Binama, M.; Yang, L.; Wang, D. Rapid formation of methane hydrate in environment-friendly leucine-based complex systems. Energy 2022, 254, 124214. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Kim, K.-S.; Kang, S.-P. Kinetic promotion and inhibition of methane hydrate formation by morpholinium ionic liquids with chloride and tetrafluoroborate anions. Energy Fuels 2016, 30, 3879–3885. [Google Scholar] [CrossRef]
- Gupta, P.; Mondal, S.; Gardas, R.L.; Sangwai, J.S. Investigation on the Effect of Ionic Liquids and Quaternary Ammonium Salts on the Kinetics of Methane Hydrate. Ind. Eng. Chem. Res. 2023. [Google Scholar] [CrossRef]
- Tariq, M.; Connor, E.; Thompson, J.; Khraisheh, M.; Atilhan, M.; Rooney, D. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly. RSC Adv. 2016, 6, 23827–23836. [Google Scholar] [CrossRef]
- Zare, M.; Haghtalab, A.; Ahmadi, A.N.; Nazari, K.; Mehdizadeh, A. Effect of imidazolium based ionic liquids and ethylene glycol monoethyl ether solutions on the kinetic of methane hydrate formation. J. Mol. Liq. 2015, 204, 236–242. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Chen, H.; Tan, Z.; Chen, C.; Sun, L. Methane hydrates with a high capacity and a high formation rate promoted by biosurfactants. Chem. Commun. 2012, 48, 11638–11640. [Google Scholar] [CrossRef]
- Mofrad, H.R.; Ganji, H.; Nazari, K.; Kameli, M.; Rod, A.R.; Kakavand, M. Rapid formation of dry natural gas hydrate with high capacity and low decomposition rate using a new effective promoter. J. Pet. Sci. Eng. 2016, 147, 756–759. [Google Scholar] [CrossRef]
- Yi, J.; Zhong, D.-L.; Yan, J.; Lu, Y.-Y. Impacts of the surfactant sulfonated lignin on hydrate based CO2 capture from a CO2/CH4 gas mixture. Energy 2019, 171, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Chen, F.-L.; Yu, S.-J.; Wang, F. Biopromoters for gas hydrate formation: A mini review of current status. Front. Chem. 2020, 8, 514. [Google Scholar] [CrossRef]
- Rogers, R.E.; Kothapalli, C.; Lee, M.S.; Woolsey, J. Catalysis of gas hydrates by biosurfactants in seawater-saturated sand/clay. Can. J. Chem. Eng 2003, 81, 973–980. [Google Scholar] [CrossRef]
- Arora, A.; Cameotra, S.S.; Kumar, R.; Balomajumder, C.; Singh, A.K.; Santhakumari, B.; Kumar, P.; Laik, S. Biosurfactant as a promoter of methane hydrate formation: Thermodynamic and kinetic studies. Sci. Rep. 2016, 6, 20893. [Google Scholar] [CrossRef]
- Lee, W.; Kang, D.W.; Ahn, Y.-H.; Lee, J. Rapid formation of hydrogen-enriched hydrocarbon gas hydrates under static conditions. ACS Sustain. Chem. Eng. 2021, 9, 8414–8424. [Google Scholar] [CrossRef]
- Baek, S.; Lee, W.; Min, J.; Ahn, Y.-H.; Kang, D.W.; Lee, J. Hydrate seeding effect on the metastability of CH4 hydrate. Korean J. Chem. Eng. 2020, 37, 341–349. [Google Scholar] [CrossRef]
- Lee, W.; Kang, D.W.; Ahn, Y.-H.; Lee, J.W. Blended hydrate seed and liquid promoter for the acceleration of hydrogen hydrate formation. Renew. Sustain. Energy Rev. 2023, 177, 113217. [Google Scholar] [CrossRef]
- Kakavandi, A.; Akbari, M. Experimental investigation of thermal conductivity of nanofluids containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751. [Google Scholar] [CrossRef]
- Aliabadi, M.; Rasoolzadeh, A.; Esmaeilzadeh, F.; Alamdari, A. Engineering. Experimental study of using CuO nanoparticles as a methane hydrate promoter. J. Nat. Gas Sci. Eng. 2015, 27, 1518–1522. [Google Scholar] [CrossRef]
- Ahuja, A.; Iqbal, A.; Iqbal, M.; Lee, J.W.; Morris, J.F. Rheology of hydrate-forming emulsions stabilized by surfactant and hydrophobic silica nanoparticles. Energy Fuels 2018, 32, 5877–5884. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Methane hydrate growth promoted by microporous zeolitic imidazolate frameworks ZIF-8 and ZIF-67 for enhanced methane storage. ACS Sustain. Chem. Eng. 2021, 9, 9001–9010. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, M.; Khoo, B.C.; Linga, P. Size effect of porous media on methane hydrate formation and dissociation in an excess gas environment. Ind. Eng. Chem. Res. 2016, 55, 7981–7991. [Google Scholar] [CrossRef]
- Benmesbah, F.D.; Clain, P.; Fandino, O.; Osswald, V.; Fournaison, L.; Dicharry, C.; Ruffine, L.; Delahaye, A. Calorimetric study of carbon dioxide (CO2) hydrate formation and dissociation processes in porous media. Chem. Eng. Sci. 2022, 264, 118108. [Google Scholar] [CrossRef]
- Benmesbah, F.D.; Ruffine, L.; Clain, P.; Osswald, V.; Fandino, O.; Fournaison, L.; Delahaye, A. Methane hydrate formation and dissociation in sand media: Effect of water saturation, gas flowrate and particle size. Energies 2020, 13, 5200. [Google Scholar] [CrossRef]
- Smith, D.H.; Wilder, J.W.; Seshadri, K. Methane hydrate equilibria in silica gels with broad pore-size distributions. AIChE J. 2002, 48, 393–400. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, W.; Ahn, Y.-H. Superabsorbent polymer for improved CO2 hydrate formation under a quiescent system. J. CO2 Util. 2022, 61, 102005. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, W.; Ahn, Y.-H.; Lee, J.W. Confined tetrahydrofuran in a superabsorbent polymer for sustainable methane storage in clathrate hydrates. Chem. Eng. J. 2021, 411, 128512. [Google Scholar] [CrossRef]
- Su, F.; Bray, C.L.; Carter, B.O.; Overend, G.; Cropper, C.; Iggo, J.A.; Khimyak, Y.Z.; Fogg, A.M.; Cooper, A.I. Reversible hydrogen storage in hydrogel clathrate hydrates. Adv. Mater. 2009, 21, 2382–2386. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Impact of Modified Silica Beads on Methane Hydrate Formation in a Fixed-Bed Reactor. Ind. Eng. Chem. Res. 2019, 58, 16687–16695. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Roy, S.; Kumar, R. Methane hydrate formation in a test sediment of sand and clay at various levels of water saturation. Can. J. Chem. 2015, 93, 874–881. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Fauth, F.; Such-Basanez, I.; Martinez-Escandell, M.; Silvestre-Albero, J. Methane hydrate formation in the confined nanospace of activated carbons in seawater environment. Microporous Mesoporous Mater. 2018, 255, 220–225. [Google Scholar] [CrossRef]
- Cuadrado-Collados, C.; Majid, A.A.; Martinez-Escandell, M.; Daemen, L.L.; Missyul, A.; Koh, C.; Silvestre-Albero, J. Freezing/melting of water in the confined nanospace of carbon materials: Effect of an external stimulus. Carbon 2020, 158, 346–355. [Google Scholar] [CrossRef]
- Em, Y.; Stoporev, A.; Semenov, A.; Glotov, A.; Smirnova, E.; Villevald, G.; Vinokurov, V.; Manakov, A.; Lvov, Y. Methane hydrate formation in halloysite clay nanotubes. ACS Sustain. Chem. Eng. 2020, 8, 7860–7868. [Google Scholar] [CrossRef]
- Pasieka, J.; Coulombe, S.; Servio, P. Investigating the effects of hydrophobic and hydrophilic multi-wall carbon nanotubes on methane hydrate growth kinetics. Chem. Eng. Sci. 2013, 104, 998–1002. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.; Lin, P.; Sun, L.; Cooper, A.I. Gas storage in renewable bioclathrates. Energy Environ. Sci. 2013, 6, 105–107. [Google Scholar] [CrossRef]
- Stoporev, A.S.; Semenov, A.P.; Medvedev, V.I.; Kidyarov, B.I.; Manakov, A.Y.; Vinokurov, V.A. Nucleation of gas hydrates in multiphase systems with several types of interfaces. J. Therm. Anal. Calorim. 2018, 134, 783–795. [Google Scholar] [CrossRef]
- Li, H.; Wang, L. Hydrophobized particles can accelerate nucleation of clathrate hydrates. Fuel 2015, 140, 440–445. [Google Scholar] [CrossRef]
- Wang, L.; Dou, M.; Wang, Y.; Xu, Y.; Li, Y.; Chen, Y.; Li, L. A Review of the Effect of Porous Media on Gas Hydrate Formation. ACS Omega 2022, 7, 33666–33679. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Bhat, A.; Mahmood, S.M.; Akbari, S.; Murshid, G. The effects of salt, particle and pore size on the process of carbon dioxide hydrate formation: A critical review. AIP Conf. Proc. 2016, 1787, 060001. [Google Scholar]
- Siangsai, A.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S.; Linga, P. Investigation on the roles of activated carbon particle sizes on methane hydrate formation and dissociation. Chem. Eng. Sci. 2015, 126, 383–389. [Google Scholar] [CrossRef]
- Saleh, K.; Forny, L.; Guigon, P.; Pezron, I. Dry water: From physico-chemical aspects to process-related parameters. Chem. Eng. Res. Des. 2011, 89, 537–544. [Google Scholar] [CrossRef]
- Wang, W.; Bray, C.L.; Adams, D.J.; Cooper, A.I. Methane Storage in Dry Water Gas Hydrates. JACS Commun. 2008, 130, 11608–11609. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.O.; Wang, W.; Adams, D.J.; Cooper, A.I. Gas storage in “dry water” and “dry gel” clathrates. Langmuir 2010, 26, 3186–3193. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Stanwix, P.; Aman, Z.; Johns, M.; May, E.; Wang, L. Raman spectroscopic studies of clathrate hydrate formation in the presence of hydrophobized particles. J. Phys. Chem. A 2016, 120, 417–424. [Google Scholar] [CrossRef]
- Farhang, F.; Nguyen, A.V.; Sewell, K.B. Fundamental Investigation of the Effects of Hydrophobic Fumed Silica on the Formation of Carbon Dioxide Gas Hydrates. Energy Fuels 2014, 28, 7025–7037. [Google Scholar] [CrossRef]
- Rong, X.; Yang, H.; Zhao, N.J.L. Rationally turning the interface activity of mesoporous silicas for preparing Pickering foam and “dry water”. Langmuir 2017, 33, 9025–9033. [Google Scholar] [CrossRef] [PubMed]
- Forny, L.; Pezron, I.; Saleh, K.; Guigon, P.; Komunjer, L. Storing water in powder form by self-assembling hydrophobic silica nanoparticles. Powder Technol. 2007, 171, 15–24. [Google Scholar] [CrossRef]
- Farhang, F.; Nguyen, T.D.; Nguyen, A.V. Non-destructive high-resolution X-ray micro computed tomography for quantifying dry water particles. Adv. Powder Technol. 2014, 25, 1195–1204. [Google Scholar] [CrossRef]
- Drachuk, A.O.; Melnikov, V.P.; Molokitina, N.S.; Nesterov, A.N.; Podenko, L.S.; Reshetnikov, A.M.; Manakov, A.Y. Dissociation behavior of “dry water” C3H8 hydrate below ice point: Effect of phase state of unreacted residual water on a mechanism of gas hydrates dissociation. J. Energy Chem. 2015, 24, 309–314. [Google Scholar] [CrossRef]
- Podenko, L.; Nesterov, A.; Drachuk, A.; Molokitina, N.; Reshetnikov, A. Formation of propane hydrates in Frozed dry water. Russ. J. Appl. Chem. 2013, 86, 1509–1514. [Google Scholar] [CrossRef]
- Zou, Y.; Li, K.; Yuan, B.; Chen, X.; Fan, A.; Sun, Y.; Shang, S.; Chen, G.; Huang, C.; Dai, H.; et al. Inspiration from a thermosensitive biomass gel: A novel method to improving the stability of core-shell “dry water” fire extinguishing agent. Powder Technol. 2019, 356, 383–390. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Du, Z.; Xu, F.; Li, S.; Zhang, J. New-type gel dry-water extinguishants and its effectiveness. J. Clean. Prod. 2017, 166, 590–600. [Google Scholar] [CrossRef]
- Ding, A.; Yang, L.; Fan, S.; Lou, X. Reversible methane storage in porous hydrogel supported clathrates. Chem. Eng. Sci. 2013, 96, 124–130. [Google Scholar] [CrossRef]
- Shi, B.-H.; Fan, S.-S.; Lou, X. Application of the shrinking-core model to the kinetics of repeated formation of methane hydrates in a system of mixed dry-water and porous hydrogel particulates. Chem. Eng. Sci. 2014, 109, 315–325. [Google Scholar] [CrossRef]
- Yang, L.; Cui, G.; Liu, D.; Fan, S.; Xie, Y.; Chen, J. Rapid and repeatable methane storage in clathrate hydrates using gel-supported surfactant dry solution. Chem. Eng. Sci. 2016, 146, 10–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Wu, Q.; Zhang, B. Effect of dry water on methane separation and recovery from coal mine gas based on hydrate. RSC Adv. 2018, 8, 27171–27180. [Google Scholar] [CrossRef]
- Binks, B.P.; Johnson, A.J.; Rodrigues, J.A. Inversion of ‘dry water’to aqueous foam on addition of surfactant. Soft Matter 2010, 6, 126–135. [Google Scholar] [CrossRef]
- Binks, B.P.; Duncumb, B.; Murakami, R. Effect of pH and salt concentration on the phase inversion of particle-stabilized foams. Langmuir 2007, 23, 9143–9146. [Google Scholar] [CrossRef] [PubMed]
- Podenko, L.; Drachuk, A.; Molokitina, N.; Nesterov, A. Effect of silica nanoparticles on dry water gas hydrate formation and self-preservation efficiency. Russ. J. Phys. Chem. A 2018, 92, 255–261. [Google Scholar] [CrossRef]
- Carter, B.O.; Weaver, J.V.; Wang, W.; Spiller, D.G.; Adams, D.J.; Cooper, A.I. Microencapsulation using an oil-in-water-in-air ‘dry water emulsion’. Chem. Commun. 2011, 47, 8253–8255. [Google Scholar] [CrossRef]
- Carter, B.O.; Adams, D.J.; Cooper, A.I. Pausing a stir: Heterogeneous catalysis in “dry water”. Green Chem. 2010, 12, 783–785. [Google Scholar] [CrossRef]
- Fan, S.; Yang, L.; Wang, Y.; Lang, X.; Wen, Y.; Lou, X. Rapid and high capacity methane storage in clathrate hydrates using surfactant dry solution. Chem. Eng. Sci. 2014, 106, 53–59. [Google Scholar] [CrossRef]
- Park, J.; Shin, K.; Kim, J.; Lee, H.; Seo, Y.; Maeda, N.; Tian, W.; Wood, C.D. Effect of Hydrate Shell Formation on the Stability of Dry Water. J. Phys. Chem. C 2015, 119, 1690–1699. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Yoon, R.-H.; Seol, Y. Use of Hydrophobic Particles as Kinetic Promoters for Gas Hydrate Formation. J. Chem. Eng. Data 2014, 60, 383–388. [Google Scholar] [CrossRef]
- Hu, G.; Ye, Y.; Liu, C.; Meng, Q.; Zhang, J.; Diao, S. Direct measurement of formation and dissociation rate and storage capacity of dry water methane hydrates. Fuel Process. Technol. 2011, 92, 1617–1622. [Google Scholar] [CrossRef]
- Al-Wabel, M.; Elfaki, J.; Usman, A.; Hussain, Q.; Ok, Y.S. Performance of dry water-and porous carbon-based sorbents for carbon dioxide capture. Environ. Res. 2019, 174, 69–79. [Google Scholar] [CrossRef]
- Han, Z.; Gong, L.; Du, Z.; Duan, H. A novel environmental-friendly gel dry-water extinguishant containing additives with efficient combustion suppression efficiency. Fire Technol. 2020, 56, 2365–2385. [Google Scholar] [CrossRef]
- Tianwei, Z.; Cunwei, Z.; Hao, L.; Zhiyue, H. Experimental investigation of novel dry liquids with aqueous potassium Solution@ Nano-SiO2 for the suppression of liquid fuel fires: Preparation, application, and stability. Fire Saf. J. 2020, 115, 103144. [Google Scholar] [CrossRef]
- Forny, L.; Saleh, K.; Denoyel, R.; Pezron, I. Contact angle assessment of hydrophobic silica nanoparticles related to the mechanisms of dry water formation. Langmuir 2010, 26, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Maham, Y.; Masliyah, J.H.; Gray, M.R.; Mather, A.E. Measurement of contact angles for fumed silica nanospheres using enthalpy of immersion data. J. Colloid Interface Sci. 2000, 228, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, D.A.; Maham, Y.; Chuang, K.T. Calculation of contact angle for hydrophobic powders using heat of immersion data. J. Phys. Chem. 1996, 100, 6626–6630. [Google Scholar] [CrossRef]
- Etzler, F.M. Characterization of surface free energies and surface chemistry of solids. Contact Angle Wettability Adhes. 2003, 3, 219–264. [Google Scholar]
- Binks, B.P.; Murakami, R. Phase inversion of particle-stabilized materials from foams to dry water. Nat. Mater. 2006, 5, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, A.; Yuan, B.; Sun, Y.; Zhang, Y.; Niu, Y. Renewable biomass gel reinforced core-shell dry water material as novel fire extinguishing agent. J. Loss Prev. Process. Ind. 2019, 59, 14–22. [Google Scholar] [CrossRef]
- Azizian, S.; Fujii, S.; Kasahara, M.; Butt, H.-J.; Kappl, M. Effect of particle morphology on mechanical properties of liquid marbles. Adv. Powder Technol. 2019, 30, 330–335. [Google Scholar] [CrossRef]
- Lankes, H.; Sommer, K.; Weinreich, B. Liquid absorption capacity of carriers in the food technology. Powder Technol. 2003, 134, 201–209. [Google Scholar] [CrossRef]
- Hasenzahl, S.; Gray, A.; Walzer, E.; Braunagel, A. Dry water for the skin. SÖFW J. 2005, 131, 2–8. [Google Scholar]
- Bonnaud, P.A.; Ji, Q.; Van Vliet, K.J. Effects of elevated temperature on the structure and properties of calcium–silicate–hydrate gels: The role of confined water. Soft Matter 2013, 9, 6418–6429. [Google Scholar] [CrossRef]
- Dieter, S.; Franz-Theo, S.; Helmut, B. Predominantly Aqueous Compositions in a Fluffy Powdery form Approximating Powdered Solids Behavior and Process for Forming Same. Google Patents (Patent number: 3393155), 16 July 1968. [Google Scholar]
- Mel’nikov, V.P.; Podenko, L.S.; Nesterov, A.N.; Drachuk, A.O.; Molokitina, N.S.; Reshetnikov, A.M. Self-preservation of methane hydrates produced in “dry water”. Dokl. Chem. 2016, 466, 53–56. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Manderfeld, E.; Kleinberg, M.N.; Rosenhahn, A.; Arnusch, C.J. Superhydrophobic candle soot as a low fouling stable coating on water treatment membrane feed spacers. ACS Appl. Bio Mater. 2021, 4, 4191–4200. [Google Scholar] [CrossRef]
- Tohidi, B.; Burgass, R.; Danesh, A.; Østergaard, K.; Todd, A. Improving the accuracy of gas hydrate dissociation point measurements. Ann. N. Y. Acad. Sci. 2000, 912, 924–931. [Google Scholar] [CrossRef]
- Zebardast, S.; Haghtalab, A. Thermodynamic modeling and measurement of CO2 clathrate equilibrium conditions with a hydrophobic surface—An application in dry water hydrate. Chem. Eng. Sci. 2022, 251, 117486. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V. Hydrophobic effect on gas hydrate formation in the presence of additives. Energy Fuels 2017, 31, 10311–10323. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V.; Steel, K.M.; Dang, L.X.; Galib, M. Interfacial Gas Enrichment at Hydrophobic Surfaces and the Origin of Promotion of Gas Hydrate Formation by Hydrophobic Solid Particles. J. Phys. Chem. C 2017, 121, 3830–3840. [Google Scholar] [CrossRef]
- Hu, G.W.; Ye, Y.G.; Li, C.F.; Liu, C.L.; Meng, Q.G. Study on reuse of dry water to store methane in a hydrate form. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2012; pp. 3200–3203. [Google Scholar]
- Lang, X.; Fan, S.; Wang, Y. Intensification of methane and hydrogen storage in clathrate hydrate and future prospect. J. Nat. Gas Chem. 2010, 19, 203–209. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Wang, B.; Lou, X.; Lipiński, W. Experimental and numerical analysis of CO2 and CH4 hydrate formation kinetics in microparticles: A comparative study based on shrinking core model. Chem. Eng. J. 2022, 446, 137247. [Google Scholar] [CrossRef]
- Naeiji, P.; Varaminian, F. Effect of Sodium Dodecyl Sulphate on Gas Hydrate Formation Kinetics of Methane and Ethane Mixtures. Gas Process. J. 2017, 5, 65–74. [Google Scholar]
- Zhang, X.; Maeda, N. Nucleation curves of ice in the presence of nucleation promoters. Chem. Eng. Sci. 2022, 262, 118017. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sloan, E.D., Jr. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.A.; Koh, C.A. Self-preservation phenomenon in gas hydrates and its application for energy storage. In Intra-and Intermolecular Interactions between Non-Covalently Bonded Species; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–285. [Google Scholar]
- Melnikov, V.; Nesterov, A.; Podenko, L.; Reshetnikov, A.; Shalamov, V. NMR evidence of supercooled water formation during gas hydrate dissociation below the melting point of ice. Chem. Eng. Sci. 2012, 71, 573–577. [Google Scholar] [CrossRef]
- Madygulov, M.S.; Nesterov, A.N.; Reshetnikov, A.M.; Vlasov, V.A.; Zavodovsky, A.G. Study of gas hydrate metastability and its decay for hydrate samples containing unreacted supercooled liquid water below the ice melting point using pulse NMR. Chem. Eng. Sci. 2015, 137, 287–292. [Google Scholar] [CrossRef]
- Melnikov, V.; Podenko, L.; Nesterov, A.; Drachuk, A.; Molokitina, N.; Reshetnikov, A. Dissociation of gas hydrates produced from methane and “dry water” at temperatures below 273 K. In Doklady Physical Chemistry; Pleiades Publishing: New York, NY, USA, 2015; pp. 49–52. [Google Scholar]
- Yang, L.; Lan, X.; Liu, D.; Cui, G.; Dou, B.; Wang, J. Multi-cycle methane hydrate formation in micro droplets of gelatinous dry solution. Chem. Eng. J. 2019, 374, 802–810. [Google Scholar] [CrossRef]
- Golkhou, F.; Haghtalab, A. Kinetic and thermodynamic study of CO2 storage in reversible gellan gum supported dry water clathrates. J. Taiwan Inst. Chem. Eng. 2020, 115, 79–95. [Google Scholar] [CrossRef]
- Shi, B.-H.; Yang, L.; Fan, S.-S.; Lou, X. An investigation on repeated methane hydrates formation in porous hydrogel particles. Fuel 2017, 194, 395–405. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.-T.; Chen, C.; Zhang, G.-D.; Chao, K.; Lin, Y.; Wang, F. Surfactant-based promotion to gas hydrate formation for energy storage. J. Mater. Chem. A 2019, 7, 21634–21661. [Google Scholar] [CrossRef]




| Pickering Agent Name | Pickering Agent Size | Solid-to-Water Ratio | Mixing Speed and Time | Dry Water Droplet Size | Reference |
|---|---|---|---|---|---|
| Aerosil R812S nanosilica | 7 nm | 4:96 | >12,000 rpm, 30 s | d50 = 112 μm | [63] |
| Aerosil R972 nanosilica | 16 nm | 10:90 | 18,000 rpm, 10 s | d50 = 131 μm | [63] |
| Wacker H18 nanosilica | 5–30 nm | 2–6 wt% | 14,000 rpm, 180 s | d50 = 151–191μm * | [64] |
| Aerosil R202 nanosilica | 14 nm | 5–10 wt% | 18,700 rpm, 60 s | d50 = 4 μm | [65,66] |
| Aerosil R812S nanosilica | 7 nm | 9:100 | 5000 rpm, 300 s | 50 μm < d50 < 100 μm | [67] |
| Mesoporous Silica Particles | 14 nm | 5 wt% | 1800 rpm, 180 s | 30 μm–100 μm | [62] |
| HB-630 nanosilica | 5–15 nm | 5 wt% | 22,000 rpm, 30 s | N/A | [68] |
| HB-630 nanosilica | 5–15 nm | 1:17 | 18,000 rpm, 45 s | 15 μm | [69,70] |
| Wacker H18 nanosilica | 7–35 nm | 7.5 wt% ** | 18,000 rpm, 60 s | 25–50 μm | [71] |
| HB-630 nanosilica | 5–15 nm | 5 wt% | 19,000 rpm, 90 s | N/A | [72] |
| Wacker H18 nanosilica | 20 nm | 4 wt% | 25,000 rpm, 30 s | several hundred μm *** | [73] |
| Wacker H18 nanosilica | 20–30 nm | 2 wt% | 25,000 rpm, 30 s | 50–several hundred μm | [74] |
| Aerosil R202 nanosilica | 14 nm | 2–15 wt% | 19,000 rpm, 90 s | 6–16 μm * | [75] |
| Wacker H18 nanosilica | 20 nm | 5 wt% | 19,000 rpm, 90 s | <20 μm | [58] |
| Wacker H18 nanosilica | 5–30 nm | 10 wt% | 16,450 rpm, 60 s | N/A | [76] |
| Wacker H18 nanosilica | 5–30 nm | 5 wt% | 14,000 rpm, 180 s | 100–5500 μm | [61] |
| Wacker H18 nanosilica | 5–30 nm | 5 wt% | 19,000 rpm, 90 s | 52 ± 14 μm | [59] |
| Wacker H18 nanosilica | 5–30 nm | 5 wt% | 37,000 rpm, 90 s | 26 ± 17 μm | [77] |
| HB-630 nanosilica | 5–15 nm | 2.5–10 wt% | 18,000 rpm, 30 s | 1–120 μm * | [78] |
| Wacker H18 nanosilica | 5–30 nm | 5 wt% | 19,000 rpm, 60 s | 13 μm | [79] |
| Teflon particle | 1 and 12 μm | 15 wt% | 14,100 rpm, 180 s | N/A | [80] |
| Wacker H18 nanosilica | 5–30 nm | 5 wt% | 12,000, 17,000, 22,000 rpm, 90 s | N/A | [81] |
| Aerosil R812 | 7 nm | 20 wt% | 14,000 rpm, 10 s | N/A | [82] |
| HB-630 nanosilica | 5–15 nm | 5–8 wt% | 17,000 rpm, 25 s | 100 μm < d50 < 300 μm * | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Maeda, N. Dry Water as a Promoter for Gas Hydrate Formation: A Review. Molecules 2023, 28, 3731. https://doi.org/10.3390/molecules28093731
Wei Y, Maeda N. Dry Water as a Promoter for Gas Hydrate Formation: A Review. Molecules. 2023; 28(9):3731. https://doi.org/10.3390/molecules28093731
Chicago/Turabian StyleWei, Yu, and Nobuo Maeda. 2023. "Dry Water as a Promoter for Gas Hydrate Formation: A Review" Molecules 28, no. 9: 3731. https://doi.org/10.3390/molecules28093731
APA StyleWei, Y., & Maeda, N. (2023). Dry Water as a Promoter for Gas Hydrate Formation: A Review. Molecules, 28(9), 3731. https://doi.org/10.3390/molecules28093731







