Glucosylation of Isoeugenol and Monoterpenes in Corynebacterium glutamicum by YdhE from Bacillus lichenformis
Abstract
1. Introduction
2. Results and Discussion
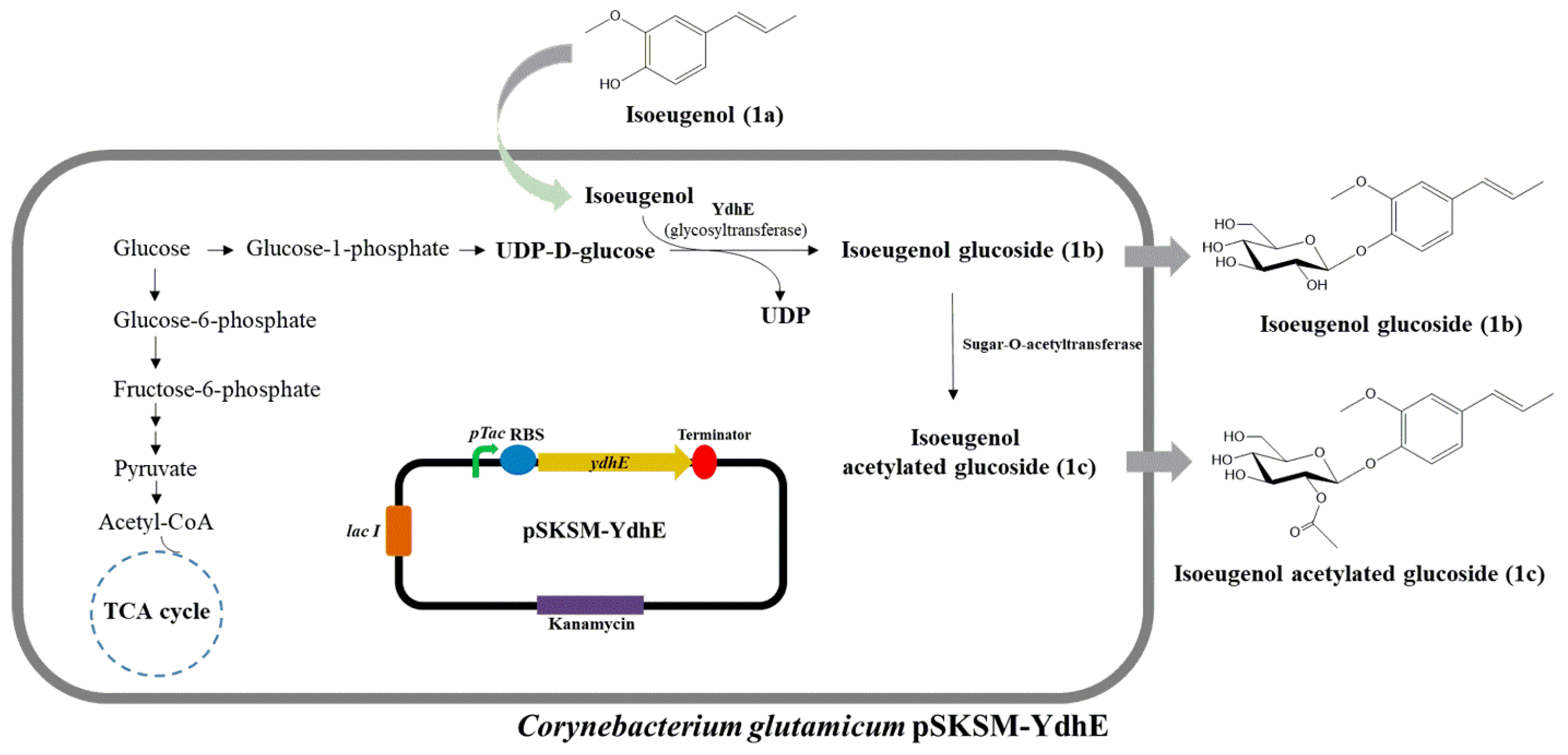
2.1. Biotransformation of Isoeugenol in C. glutamicum Expressing CO-YdhE
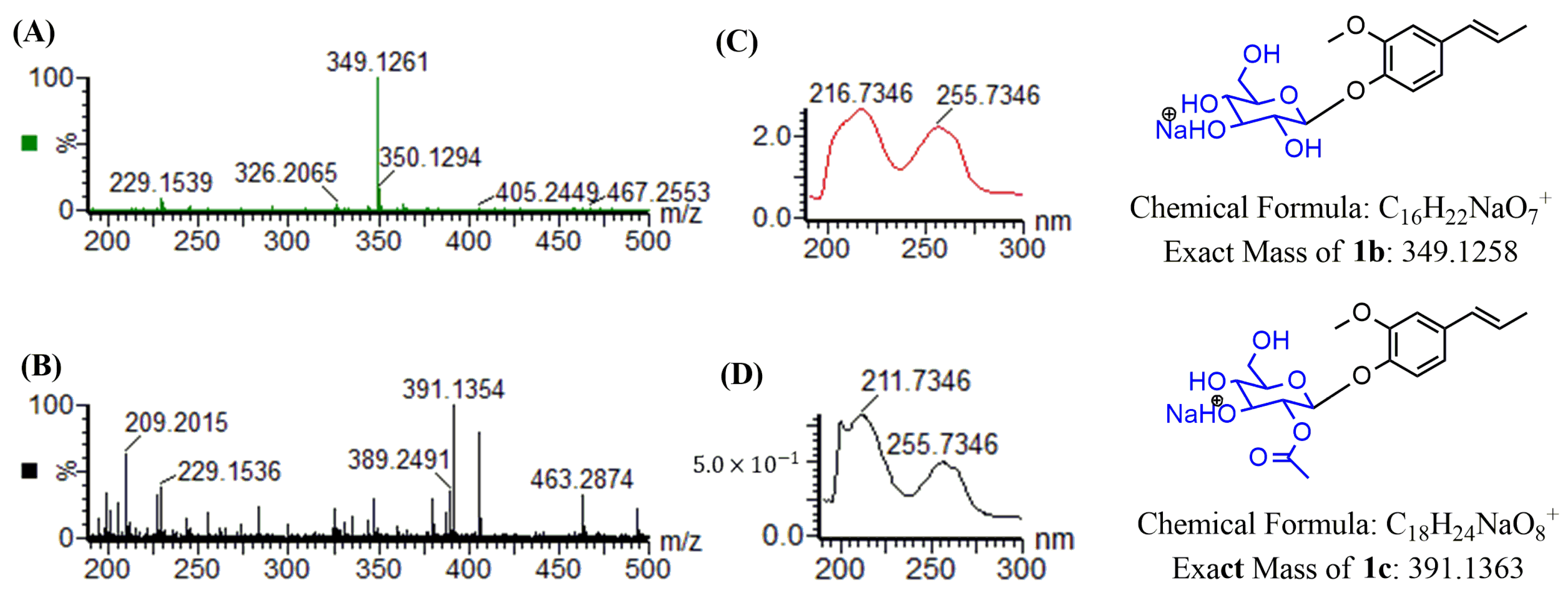
2.2. Structure Determination of Two Isoeugenol Derivatives 1b and 1c
2.3. Production of Isoeugenol Glucoside
2.4. Water Solubility Test
2.5. Modification of Monoterpenes by C. glutamicum pSKSM-YdhE
3. Methods and Materials
3.1. General Procedures
3.2. Generation of Recombinant Strains
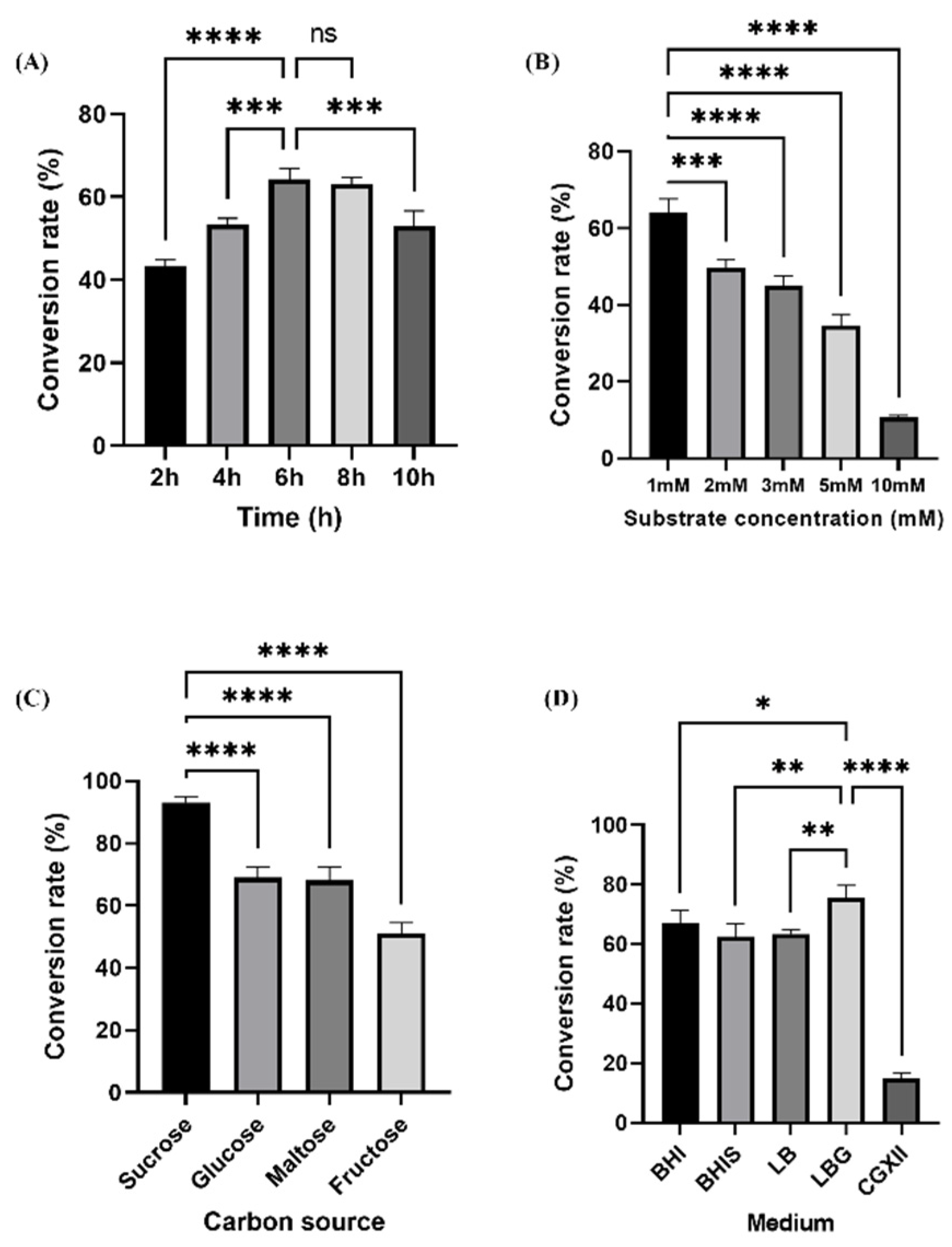
3.3. Determination of Optimal Culture Conditions
3.4. In Vivo Glucosylation of Monoterpenes
3.5. Water Solubility Test
3.6. Fermentation and Analytical Procedures
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Sgobba, E.; Blöbaum, L.; Wendisch, V.F. Production of Food and Feed Additives from Non-Food-Competing Feedstocks: Valorizing N-Acetylmuramic Acid for Amino Acid and Carotenoid Fermentation with Corynebacterium glutamicum. Front. Microbiol. 2018, 9, 2046. [Google Scholar] [CrossRef]
- Kinoshita, S.; Udaka, S.; Shimono, M. Studies on the Amino Acid Fermentation. Part 1. Production of L-Glutamic Acid by Various Microorganisms. J. Gen. Appl. Microbiol. 1957, 3, 193–205. [Google Scholar] [CrossRef]
- Ikeda, M.; Nakagawa, S. The Corynebacterium glutamicum Genome: Features and Impacts on Biotechnological Processes. Appl. Microbiol. Biotechnol. 2003, 62, 99–109. [Google Scholar] [CrossRef]
- Bao, Q.; Liu, G.; Wu, J.; Yang, H. Codon Usage Patterns in Corynebacterium glutamicum: Mutational Bias, Natural Selection and Amino Acid Conservation. Comp. Funct. Genom. 2010, 2010, 343569. [Google Scholar] [CrossRef]
- Yang, J.; Yang, S. Comparative Analysis of Corynebacterium glutamicum Genomes: A New Perspective for the Industrial Production of Amino Acids. BMC Genom. 2017, 18, 940. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Becker, J.; Tsuge, Y.; Kawaguchi, H.; Kondo, A.; Marienhagen, J.; Bott, M.; Wendisch, V.F.; Wittmann, C. Advances in Metabolic Engineering of Corynebacterium glutamicum to Produce High-Value Active Ingredients for Food, Feed, Human Health, and Well-Being. Essays Biochem. 2021, 65, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Makishah, N.H.; Sun, X.; Wen, Z.; Jiang, Y.; Yang, S. Advances and Perspectives for Genome Editing Tools of Corynebacterium glutamicum. Front. Microbiol. 2021, 12, 654058. [Google Scholar] [CrossRef] [PubMed]
- Cankar, K.; Henke, N.A.; Wendisch, V.F. Functional Food Additives/Ingredients Production by Engineered Corynebacterium glutamicum. Syst. Microbiol. Biomanuf. 2022, 3, 110–121. [Google Scholar] [CrossRef]
- Kogure, T.; Inui, M. Recent Advances in Metabolic Engineering of Corynebacterium glutamicum for Bioproduction of Value-Added Aromatic Chemicals and Natural Products. Appl. Microbiol. Biotechnol. 2018, 102, 8685–8705. [Google Scholar] [CrossRef]
- Becker, J.; Rohles, C.M.; Wittmann, C. Metabolically Engineered Corynebacterium glutamicum for Bio-Based Production of Chemicals, Fuels, Materials, and Healthcare Products. Metab. Eng. 2018, 50, 122–141. [Google Scholar] [CrossRef]
- Chin, Y.W.; Park, J.B.; Park, Y.C.; Kim, K.H.; Seo, J.H. Metabolic Engineering of Corynebacterium glutamicum to Produce GDP-l-Fucose from Glucose and Mannose. Bioprocess. Biosyst. Eng. 2013, 36, 749–756. [Google Scholar] [CrossRef]
- Woo, H.M.; Park, J.B. Recent Progress in Development of Synthetic Biology Platforms and Metabolic Engineering of Corynebacterium glutamicum. J. Biotechnol. 2014, 180, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.E.; Peters-Wendisch, P.; Netzer, R.; Stafnes, M.; Brautaset, T.; Wendisch, V.F. Production and Glucosylation of C50 and C40 Carotenoids by Metabolically Engineered Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2014, 98, 1223–1235. [Google Scholar] [CrossRef]
- Gauttam, R.; Desiderato, C.K.; Radoš, D.; Link, H.; Seibold, G.M.; Eikmanns, B.J. Metabolic Engineering of Corynebacterium glutamicum for Production of UDP-N-Acetylglucosamine. Front. Bioeng. Biotechnol. 2021, 9, 748510. [Google Scholar] [CrossRef]
- Amoah, O.J.; Nguyen, H.T.; Sohng, J.K. N-Glucosylation in Corynebacterium glutamicum with YdhE from Bacillus lichenformis. Molecules 2022, 27, 3405. [Google Scholar] [CrossRef] [PubMed]
- Prell, C.; Vonderbank, S.A.; Meyer, F.; Pérez-García, F.; Wendisch, V.F. Metabolic Engineering Corynebacterium glutamicum for de Novo Production of 3-Hydroxycadaverine. Curr. Res. Biotechnol. 2022, 4, 32–46. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Piotrowska, R.; Foss, M.; Meyer, R.L. Isoeugenol Has a Non-Disruptive Detergent-like Mechanism of Action. Front. Microbiol. 2015, 6, 754. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Falleh, H.; ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.E.; Marta, E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; et al. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Silva, L.P.; Ferreira, O.; Schröder, B.; Coutinho, J.A.P.; Pinho, S.P. Terpenes Solubility in Water and Their Environmental Distribution. J. Mol. Liq. 2017, 241, 996–1002. [Google Scholar] [CrossRef]
- Razak, M.A.I.A.; Hamid, H.A.; Othman, R.N.I.R.; Moktar, S.A.M.; Miskon, A. Improved Drug Delivery System for Cancer Treatment by D-Glucose Conjugation with Eugenol From Natural Product. Curr. Drug. Deliv. 2020, 18, 312–322. [Google Scholar] [CrossRef]
- Resende, D.B.; de Abreu Martins, H.H.; de Souza, T.B.; Carvalho, D.T.; Piccoli, R.H.; Schwan, R.F.; Dias, D.R. Synthesis and in Vitro Evaluation of Peracetyl and Deacetyl Glycosides of Eugenol, Isoeugenol and Dihydroeugenol Acting against Food-Contaminating Bacteria. Food Chem. 2017, 237, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, P.; Pandey, R.P.; Thapa, S.B.; Kang, M.K.; Kim, C.J.; Sohng, J.K. Biocatalytic Synthesis of Non-Natural Monoterpene O-Glycosides Exhibiting Superior Antibacterial and Antinematodal Properties. ACS Omega 2019, 4, 9367–9375. [Google Scholar] [CrossRef]
- Weymouth-Wilson, A.C. The Role of Carbohydrates in Biologically Active Natural Products. Nat. Prod. Rep. 1997, 14, 99–110. [Google Scholar] [CrossRef]
- Kurosu, J.; Sato, T.; Yoshida, K.; Tsugane, T.; Shimura, S.; Kirimura, K.; Kino, K.; Usami, S. Enzymatic Synthesis of α-Arbutin by α-Anomer-Selective Glucosylation of Hydroquinone Using Lyophilized Cells of Xanthomonas Campestris WU-9701. J. Biosci. Bioeng. 2002, 93, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki, D.; Toshiyuki, S.; Kohtaro, K.; Kuniki, K.; Shoji, U. Enzymatic Synthesis of L-Menthyl α-Maltoside and l-Menthyl α-Maltooligosides from l-Menthyl α-Glucoside by Cyclodextrin Glucanotransferase. J. Biosci. Bioeng. 2002, 94, 119–123. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; de Souza, M.D. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Yue, X.J.; Tang, Y.J.; Wu, C.; Li, Y.Z. Effects of Glycosylation on the Bioactivity of Rapamycin. Appl. Microbiol. Biotechnol. 2020, 104, 9125–9134. [Google Scholar] [CrossRef] [PubMed]
- Takeyoshi, M.; Iida, K.; Suzuki, K.; Yamazaki, S. Skin Sensitization Potency of Isoeugenol and Its Dimers Evaluated by a Non-Radioisotopic Modification of the Local Lymph Node Assay and Guinea Pig Maximization Test. J. Appl. Toxicol. 2008, 28, 530–534. [Google Scholar] [CrossRef]
- Bassanetti, I.; Carcelli, M.; Buschini, A.; Montalbano, S.; Leonardi, G.; Pelagatti, P.; Tosi, G.; Massi, P.; Fiorentini, L.; Rogolino, D. Investigation of Antibacterial Activity of New Classes of Essential Oils Derivatives. Food Control 2017, 73, 606–612. [Google Scholar] [CrossRef]
- Rothe, H.; Schepky, A.; Hewitt, N.; Cubberley, R.; Duplan, H.; Eilstein, J.; Gerstel, D.; Grégoire, S.; Jacques-Jamin, C.; Klaric, M. Solubility of Cosmetics Ingredients in 6 Different Solvents and Applicability to Skin Bioavailability Assays. Toxicol. Lett. 2015, 238, S326. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.; Cui, H.; Lin, L. Encompassment of Isoeugenol in 2-Hydroxypropyl-β-Cyclodextrin Using Ultrasonication: Characterization, Antioxidant and Antibacterial Activities. J. Mol. Liq. 2019, 296, 111777. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.; Gan, L.; Liu, W.; Liang, R.; Liu, C.; Niu, J.; Cao, Y.; Liu, Z.; et al. Storage Stability and Antibacterial Activity of Eugenol Nanoliposomes Prepared by an Ethanol Injection–Dynamic High-Pressure Microfluidization Method. J. Food Prot. 2015, 78, 22–30. [Google Scholar] [CrossRef]
- Kim, B.; Park, H.; Na, D.; Lee, S.Y. Metabolic Engineering of Escherichia coli for the Production of Phenol from Glucose. Biotechnol. J. 2014, 9, 621–629. [Google Scholar] [CrossRef]
- Zha, J.; Zang, Y.; Mattozzi, M.; Plassmeier, J.; Gupta, M.; Wu, X.; Clarkson, S.; Koffas, M.A.G. Metabolic Engineering of Corynebacterium glutamicum for Anthocyanin Production. Microb. Cell Fact 2018, 17, 143. [Google Scholar] [CrossRef]
- Blombach, B.; Seibold, G.M. Carbohydrate Metabolism in Corynebacterium glutamicum and Applications for the Metabolic Engineering of L-Lysine Production Strains. Appl. Microbiol. Biotechnol. 2010, 86, 1313–1322. [Google Scholar] [CrossRef]
- Zahoor, A.; Lindner, S.N.; Wendisch, V.F. Metabolic Engineering of Corynebacterium glutamicum Aimed at Alternative Carbon Sources and New Products. Comput. Struct. Biotechnol. J. 2012, 3, e201210004. [Google Scholar] [CrossRef] [PubMed]
- Keilhauer, C.; Eggeling, L.; Sahm, H. Isoleucine Synthesis in Corynebacterium glutamicum: Molecular Analysis of the IlvB-IlvN-IlvC Operon. J. Bacterial. 1993, 175, 5595–5603. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for Fermentation Medium Optimization: An in-Depth Review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Zieringer, J.; Haas, T.; Nieß, A.; Blombach, B.; Takors, R. Physiological Response of Corynebacterium glutamicum to Increasingly Nutrient-Rich Growth Conditions. Front. Microbiol. 2018, 9, 2058. [Google Scholar] [CrossRef]
- Ramp, P.; Lehnert, A.; Matamouros, S.; Wirtz, A.; Baumgart, M.; Bott, M. Metabolic Engineering of Corynebacterium glutamicum for Production of Scyllo-Inositol, a Drug Candidate against Alzheimer’s Disease. Metab. Eng. 2021, 67, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, Y.; Gong, A.D. Development of a Defined Medium for Corynebacterium glutamicum Using Urea as Nitrogen Source. 3 Biotech 2021, 11, 405. [Google Scholar] [CrossRef]
- Thapa, S.B.; Pandey, R.P.; Bashyal, P.; Yamaguchi, T.; Sohng, J.K. Cascade Biocatalysis Systems for Bioactive Naringenin Glucosides and Quercetin Rhamnoside Production from Sucrose. Appl. Microbiol. Biotechnol. 2019, 103, 7953–7969. [Google Scholar] [CrossRef]
- Hao, X.; Xie, J.; Li, Y.; Chen, W. Acetylated Pelargonidin-3-O-Glucoside Exhibits Promising Thermostability, Lipophilicity, and Protectivity against Oxidative Damage by Activating the Nrf2/ARE Pathway. Food Funct. 2022, 13, 2618–2630. [Google Scholar] [CrossRef]

| No | Isoeugenol-1-O-β-d-glucoside (1b) (DMSO-d6) | Isoeugenol-1-O-β-d-(2″-acetyl)-glucoside (1c) (DMSO-d6) | ||
|---|---|---|---|---|
| 13C | 1H (Multiplicity, J) | 13C | 1H (Multiplicity, J) | |
| 1′ | 100.50 | 4.87 (1H, d, J = 7.2 H) | 100.27 | 4.91 (1H, d, J = 7.0 Hz) |
| 2′ | 73.67 | 3.31–3.24 (1H, m) | 73.60 | 3.28 (1H, q, J = 7.1 Hz) |
| 3′ | 77.29 | 3.31–3.24 (1H, m) | 74.04 | 3.28 (1H, q, J = 7.1 Hz) |
| 4′ | 70.12 | 3.31–3.24 (1H, m) | 70.29 | 3.18 (1H, m) |
| 5′ | 77.45 | 3.16 (1H, t, J = 8.9 Hz) | 77.01 | 3.57 (1H, ddd) |
| 6′ | 61.12 | 3.45 (1H, d, J = 12.3 Hz) | 63.84 | 4.07 (1H, dd, J = 11.9, 6.8 Hz) |
| 3.67 (1H, d, J = 11.5 Hz) | 4.25 (1H, dd, J = 11.9, 2.1 Hz) | |||
| 1″ | 170.70 | |||
| 2″ | 21.12 | 1.99 (3H,s) | ||
| 1 | 132.07 | 132.26 | ||
| 2 | 130.99 | 130.96 | ||
| 3 | 118.89 | 7.00 (1H, d, J = 8.4 Hz) | 118.75 | 7.02 (1H, d, J = 2.0 Hz) |
| 4 | 124.16 | 124.30 | ||
| 5 | 109.98 | 6.84 (1H, d, J = 2.1Hz) | 110.02 | 6.96 (1H,d, J = 2.1Hz) |
| 6 | 115.74 | 7.00 (1H, d, J = 8.4 Hz) | 115.75 | 6.85 (1H, dd, J = 8.4, 2.0 Hz) |
| 7 | 149.44 | 6.44 (1H, dd, J = 16.1, 1.9 Hz) | 149.46 | 6.34 (1H, dd, J = 15.9, 2.0 Hz) |
| 8 | 146.10 | 6.19 (1H, dq, J = 15.9, 6.6 Hz) | 145.83 | 6.19 (1H, dq, J = 15.8, 6.6 Hz) |
| 9 | 18.64 | 1.82 (3H, d, J = 6.5 Hz) | 18.63 | 1.82 (3H, d, J = 6.5Hz) |
| 10 | 56.06 | 3.77 (3H, s) | 56.05 | 3.77 (3H, s) |
| Name | Conversion Rate (%) | Total (%) | |
|---|---|---|---|
| Glucoside | Acetylated Glucoside | ||
| Isoeugenol | 58.5% | 24% | 82.5% |
| Eugenol | 25% | 12% | 37% |
| Thymol | 52% | - | 52% |
| Carvacrol | 27% | - | 27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.Y.; Amoah, O.J.; Nguyen, H.T.; Sohng, J.K. Glucosylation of Isoeugenol and Monoterpenes in Corynebacterium glutamicum by YdhE from Bacillus lichenformis. Molecules 2023, 28, 3789. https://doi.org/10.3390/molecules28093789
Ma SY, Amoah OJ, Nguyen HT, Sohng JK. Glucosylation of Isoeugenol and Monoterpenes in Corynebacterium glutamicum by YdhE from Bacillus lichenformis. Molecules. 2023; 28(9):3789. https://doi.org/10.3390/molecules28093789
Chicago/Turabian StyleMa, Su Yeong, Obed Jackson Amoah, Hue Thi Nguyen, and Jae Kyung Sohng. 2023. "Glucosylation of Isoeugenol and Monoterpenes in Corynebacterium glutamicum by YdhE from Bacillus lichenformis" Molecules 28, no. 9: 3789. https://doi.org/10.3390/molecules28093789
APA StyleMa, S. Y., Amoah, O. J., Nguyen, H. T., & Sohng, J. K. (2023). Glucosylation of Isoeugenol and Monoterpenes in Corynebacterium glutamicum by YdhE from Bacillus lichenformis. Molecules, 28(9), 3789. https://doi.org/10.3390/molecules28093789







