Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers
Abstract
:1. Introduction
2. Results
2.1. Essential Elements
2.2. Potentially Toxic Elements
2.3. Black vs. Green Tea
2.4. Multivariate Analysis
2.5. Estimation of Hazard Quotient (HQ) and Hazard Index (HI)
3. Discussion
3.1. Essential Elements in Tea Leaves
| Garden or Market Location, Country | Type | Co | Cu | Fe | Mn | Se | V | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|
| European Gardens | Black | 0.2 ± 0.2 (0.03–0.58) | 16 ± 3 (12.2–19.8) | 75 ± 18 (48.9–101) | 345 ± 181 (150–628) | 0.11 ± 0.02 (0.089–0.14) | <0.03 | 30 ± 9 (21.9–46.4) | This study |
| European Gardens | Green | 0.07 ± 0.05 (0.01–0.14) | 16 ± 3 (12.9–20.3) | 78 ± 21 (50.1–99.2) | 295 ± 262 (7.8–709) | 0.12 ± 0.02 0.098–0.12) | 0.3 ± 0.6 (<0.03–1.46) | 28 ± 6 (23.7–39.7) | This study |
| Sylhet and Moulvibazar district, Bangladesh | Black | (0.006–10.8) | [12] | ||||||
| India | Black | 14.56 ± 6.85 (0.033–52.26) | [13] | ||||||
| Sri Lanka | Black | 11.29 ± 5.906 (0.033–64–24) | [13] | ||||||
| Cairo, Egypt | Black | <LOQ | 17.3 ± 1.7 | 213.3 ± 47.1 | 809.0 ± 288.3 | 26.7 ± 3.5 | [14] | ||
| Cairo, Egypt | Green | <LOQ | 18.5 ± 4.52 | 218.5 ± 18.62 | 991.8 ± 66.2 | 31.86 ± 6.72 | [14] | ||
| Cairo, Egypt | Herbal | <LOQ | 15.6 ± 1.63 | 373.5 ± 198 | 52.3 ± 2.03 | 24.7 ± 9.4 | [14] | ||
| Local market, China | Green | 0.29 ± 0.13 (0.11–0.58) | 17.04 ± 4.69 (8.42–31.48) | [23] | |||||
| Anhui, China | 8 varieties | 24.21 ± 5.22 (17.23–40.01) | 1293.71 ± 696.25 (581.16–2844.22) | [16] | |||||
| Gilan, Iran | Black | 49.39 | 188.1 | 608.3 | 24.10 | [17] | |||
| India | Black | 23.21 | 146.9 | 496.3 | 27.26 | [17] | |||
| Ceylon | Black | 38.16 | 168.14 | 488.8 | 28.31 | [17] | |||
| Hsinchu market, Taiwan | Green | 0.7 (0.4–1.2) | 0.4 (0.2–0.9) | 0.6 (0.4–1.2) | 0.3 (0.1–0.6) | 6.3 (4.8–9.7) | [18] | ||
| Hsinchu market, Taiwan | Oolong | 0.3 (0.09–0.6) | 0.9 (0.7–1.5) | 0.9 (0.7–1.3) | 0.09 (0.005–0.1) | 3.4 (2.5–4.6) | [18] | ||
| Hsinchu market, Taiwan | Black | 0.2 (0.06–0.4) | 0.3 (0.05–0.7) | 0.9 (0.7–1.2) | 0.06 (0.001–0.1) | 1.2 (0.9–1.5) | [18] | ||
| Wushwush, Ethiopia | Green | (0.03–2.84) | (<LOD–19.15) | (26.03–100.43) | (501–1281) | (57.9–330.5) | [19] | ||
| Guizhou, China | 10.70 ± 3.42 (6.17–16.25) | 1940 ± 1120 (536–4610) | 13.5 ± 2.6 (9.1–20) | [20] | |||||
| Yunnan, China | Puerh | 11.7 ± 1.7 (9.4–13.8) | 12.1 ± 3.3 (7.7–17.5) | [22] | |||||
| Local market, Brazil | Lemongrass | 0.08 ± 0.028 | [41] | ||||||
| Not specified | Black | 1.58 ± 0.10 | [39] | ||||||
| China | Different varieties | (0.49–0.55) | [40] |
3.2. Potentially Toxic Elements in Tea Leaves
3.3. Source and Accumulation Mechanisms
3.4. Benefits and Risk Assessment
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Treatment
4.3. Chemical Analysis
4.4. Statistical Analysis
4.5. Health Hazard Estimation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wight, W. Tea Classification Revised. Curr. Sci. 1962, 31, 298–299. [Google Scholar]
- FAO. International Tea Market: Market Situation, Prospects and Emerging Issues; FAO: Rome, Italy, 2022. [Google Scholar]
- Chung, F.-L.; Schwartz, J.; Herzog, C.R.; Yang, Y.-M. Tea and Cancer Prevention: Studies in Animals and Humans. J. Nutr. 2003, 133, S3268–S3274. [Google Scholar] [CrossRef]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of Tea Components with Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Karak, T.; Bhagat, R.M. Trace Elements in Tea Leaves, Made Tea and Tea Infusion: A Review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Pilgrim, T.S.; Watling, R.J.; Grice, K. Application of Trace Element and Stable Isotope Signatures to Determine the Provenance of Tea (Camellia sinensis) Samples. Food Chem. 2010, 118, 921–926. [Google Scholar] [CrossRef]
- Woerndle, G.E.; Tsz-Ki Tsui, M.; Sebestyen, S.D.; Blum, J.D.; Nie, X.; Kolka, R.K. New Insights on Ecosystem Mercury Cycling Revealed by Stable Isotopes of Mercury in Water Flowing from a Headwater Peatland Catchment. Environ. Sci. Technol. 2018, 52, 1854–1861. [Google Scholar] [CrossRef]
- Muller, M.; de Beer, D.; Truzzi, C.; Annibaldi, A.; Carloni, P.; Girolametti, F.; Damiani, E.; Joubert, E. Cold Brewing of Rooibos Tea Affects Its Sensory Profile and Physicochemical Properties Compared to Regular Hot, and Boiled Brewing. LWT 2020, 132, 109919. [Google Scholar] [CrossRef]
- Barman, T.; Barooah, A.K.; Goswami, B.C.; Sharma, N.; Panja, S.; Khare, P.; Karak, T. Contents of Chromium and Arsenic in Tea (Camellia sinensis L.): Extent of Transfer into Tea Infusion and Health Consequence. Biol. Trace Elem. Res. 2020, 196, 318–329. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L364, 5–24.
- Rashid, M.H.; Fardous, Z.; Chowdhury, M.A.Z.; Alam, M.K.; Bari, M.L.; Moniruzzaman, M.; Gan, S.H. Determination of Heavy Metals in the Soils of Tea Plantations and in Fresh and Processed Tea Leaves: An Evaluation of Six Digestion Methods. Chem. Cent. J. 2016, 10, 7. [Google Scholar] [CrossRef]
- Pourramezani, F.; Akrami Mohajeri, F.; Salmani, M.H.; Dehghani Tafti, A.; Khalili Sadrabad, E. Evaluation of Heavy Metal Concentration in Imported Black Tea in Iran and Consumer Risk Assessments. Food Sci. Nutr. 2019, 7, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Ghuniem, M.M. Assessment of the Potential Health Risk of Heavy Metal Exposure from the Consumption of Herbal, Black and Green Tea. Biomed. J. Sci. Tech. Res. 2019, 16, 11810–11817. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Zhang, N.; Su, D.; Zeer, L.; Du, H.; Hu, K. Collaborative Assessment and Health Risk of Heavy Metals in Soils and Tea Leaves in the Southwest Region of China. Int. J. Environ. Res. Public Health 2021, 18, 10151. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhu, X.; Hou, R.; Ge, G.; Hua, R.; Wan, X.; Cai, H. Aluminum and Heavy Metal Accumulation in Tea Leaves: An Interplay of Environmental and Plant Factors and an Assessment of Exposure Risks to Consumers. J. Food Sci. 2018, 83, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Salahinejad, M.; Aflaki, F. Toxic and Essential Mineral Elements Content of Black Tea Leaves and Their Tea Infusions Consumed in Iran. Biol. Trace Elem. Res. 2010, 134, 109–117. [Google Scholar] [CrossRef]
- Shen, F.-M.; Chen, H.-W. Element Composition of Tea Leaves and Tea Infusions and Its Impact on Health. Bull. Environ. Contam. Toxicol. 2008, 80, 300–304. [Google Scholar] [CrossRef]
- Yemane, M.; Chandravanshi, B.; Wondimu, T. Levels of Essential and Non-Essential Metals in Leaves of the Tea Plant (Camellia sinensis L.) and Soil of Wushwush Farms, Ethiopia. Food Chem. 2007, 107, 1236–1243. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Chen, R.; Peng, Y.; Wen, X.; Gao, L. Accumulation of Heavy Metals in Tea Leaves and Potential Health Risk Assessment: A Case Study from Puan County, Guizhou Province, China. Int. J. Environ. Res. Public Health 2018, 15, 133. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Li, Z.; Wang, Y.; Yang, Y.; Liu, M.; Li, D.; Sun, G.; Zeng, B. Concentrations, Leachability, and Health Risks of Mercury in Green Tea from Major Production Areas in China. Ecotoxicol. Environ. Saf. 2022, 232, 113279. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, L.; Zhang, H.; Chen, J. Exposure and Risk Assessment for Aluminium and Heavy Metals in Puerh Tea. Sci. Total Environ. 2010, 408, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, Q.-L.; Achal, V.; Liu, Y. A Comparison of the Potential Health Risk of Aluminum and Heavy Metals in Tea Leaves and Tea Infusion of Commercially Available Green Tea in Jiangxi, China. Environ. Monit. Assess. 2015, 187, 228. [Google Scholar] [CrossRef]
- Podwika, W.; Kleszcz, K.; Krośniak, M.; Zagrodzki, P. Copper, Manganese, Zinc, and Cadmium in Tea Leaves of Different Types and Origin. Biol. Trace Elem. Res. 2018, 183, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bobková, A.; Demianová, A.; Belej, Ľ.; Harangozo, Ľ.; Bobko, M.; Jurčaga, L.; Poláková, K.; Božiková, M.; Bilčík, M.; Árvay, J. Detection of Changes in Total Antioxidant Capacity, the Content of Polyphenols, Caffeine, and Heavy Metals of Teas in Relation to Their Origin and Fermentation. Foods 2021, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-Y.; Zhao, F.-J.; Shi, Y.-Z.; Ma, L.-F.; Ruan, J.-Y. Scale and Causes of Lead Contamination in Chinese Tea. Environ. Pollut. 2006, 139, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Metallic Mineral Elements and Heavy Metals in Medicinal Plants. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 39–53. [Google Scholar]
- Aschner, J.L.; Aschner, M. Nutritional Aspects of Manganese Homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing Acts. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Briat, J.-F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New Insights into Ferritin Synthesis and Function Highlight a Link between Iron Homeostasis and Oxidative Stress in Plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef]
- Hajiboland, R.; Barceló, J.; Poschenrieder, C.; Tolrà, R. Amelioration of Iron Toxicity: A Mechanism for Aluminum-Induced Growth Stimulation in Tea Plants. J. Inorg. Biochem. 2013, 128, 183–187. [Google Scholar] [CrossRef]
- Deshpande, J.; Joshi, M.; Giri, P. Zinc: The Trace Element of Major Importance in Human Nutrition and Health. Int. J. Med. Sci. Public Health 2013, 2, 1. [Google Scholar] [CrossRef]
- Tsonev, T.; Cebola Lidon, F.J. Zinc in Plants—An Overview. Emir. J. Food Agric. (EJFA) 2012, 24, 322–333. [Google Scholar]
- Zhang, W.; Liu, D.; Liu, Y.; Chen, X.; Zou, C. Overuse of Phosphorus Fertilizer Reduces the Grain and Flour Protein Contents and Zinc Bioavailability of Winter Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2017, 65, 1473–1482. [Google Scholar] [CrossRef]
- Brun, L.A.; Maillet, J.; Hinsinger, P.; Pépin, M. Evaluation of Copper Availability to Plants in Copper-Contaminated Vineyard Soils. Environ. Pollut. 2001, 111, 293–302. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium Compounds in Medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.K.; Tuzen, M.; Gul Kazi, T.; Soylak, M. Graphite Furnace Atomic Absorption Spectrometric Detection of Vanadium in Water and Food Samples after Solid Phase Extraction on Multiwalled Carbon Nanotubes. Talanta 2013, 116, 205–209. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Zhang, Z. Relationship between Multi-Element Composition in Tea Leaves and in Provenance Soils for Geographical Traceability. Food Control 2017, 76, 82–87. [Google Scholar] [CrossRef]
- Schunk, P.F.T.; Kalil, I.C.; Pimentel-Schmitt, E.F.; Lenz, D.; de Andrade, T.U.; Ribeiro, J.S.; Endringer, D.C. ICP-OES and Micronucleus Test to Evaluate Heavy Metal Contamination in Commercially Available Brazilian Herbal Teas. Biol. Trace Elem. Res. 2016, 172, 258–265. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Barceloux, D. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- European Parliament and the Council Commission Regulation (EU) No 420/2011 29 April 2011 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Communities 2011, L111, 3–6.
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.W.; Zheng, S.J.; He, Y.F.; Zhou, G.; Zhou, Z.X. Lead Contamination in Tea Garden Soils and Factors Affecting Its Bioavailability. Chemosphere 2005, 59, 1151–1159. [Google Scholar] [CrossRef]
- Natesan, S.; Ranganathan, V. Content of Various Elements in Different Parts of the Tea Plant and in Infusions of Black Tea from Southern India. J. Sci. Food Agric. 1990, 51, 125–139. [Google Scholar] [CrossRef]
- Kabelitz, L. Heavy Metals in Herbal Drugs. Eur. J. Herb. Med. 1998, 4, 25–29. [Google Scholar]
- Gasser, U.; Klier, B.; Kuhn, A.V.; Steinhoff, B. Current Findings on the Heavy Metal Content in Herbal Drugs. Pharmeuropa 2009, 1, 37–49. [Google Scholar]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- de Meeûs, C.; Eduljee, G.H.; Hutton, M. Assessment and Management of Risks Arising from Exposure to Cadmium in Fertilisers. I. Sci. Total Environ. 2002, 291, 167–187. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Hettick, B.E.; Cañas-Carrell, J.E.; French, A.D.; Klein, D.M. Arsenic: A Review of the Element’s Toxicity, Plant Interactions, and Potential Methods of Remediation. J. Agric. Food Chem. 2015, 63, 7097–7107. [Google Scholar] [CrossRef] [PubMed]
- Karak, T.; Abollino, O.; Bhattacharyya, P.; Das, K.K.; Paul, R.K. Fractionation and Speciation of Arsenic in Three Tea Gardens Soil Profiles and Distribution of As in Different Parts of Tea Plant (Camellia sinensis L.). Chemosphere 2011, 85, 948–960. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, Translocation, and Transformation of Silver Nanoparticles in Plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Bragança, V.L.C.; Melnikov, P.; Zanoni, L.Z. Trace Elements in Different Brands of Yerba Mate Tea. Biol. Trace Elem. Res. 2011, 144, 1197–1204. [Google Scholar] [CrossRef]
- Tomljenovic, L. Aluminum and Alzheimer’s Disease: After a Century of Controversy, Is There a Plausible Link? J. Alzheimer’s Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef]
- Hayacibara, M.F.; Queiroz, C.S.; Tabchoury, C.P.M.; Cury, J.A. Fluoride and Aluminum in Teas and Tea-Based Beverages. Rev. Saude Publica 2004, 38, 100–105. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium in Tea—Concentrations, Speciation and Bioavailability. Coord. Chem. Rev. 2002, 228, 385–395. [Google Scholar] [CrossRef]
- Hideaki, M.; Hirasawa, E.; Morimura, S.; Takahashi, E. Localization of Aluminium in Tea Leaves. Plant Cell Physiol. 1976, 17, 627–631. [Google Scholar] [CrossRef]
- Kośla, T.; Lasocka, I.; Skibniewska, E.M.; Kołnierzak, M.; Skibniewski, M. Trivalent Chromium (CrIII) as a Trace Element Essential for Animals and Humans. Med. Weter. 2018, 74, 6035-2018. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Montes, M.; de la Rosa, G.; Corral-Diaz, B. Bioaccumulation of Cadmium, Chromium and Copper by Convolvulus Arvensis L.: Impact on Plant Growth and Uptake of Nutritional Elements. Bioresour. Technol. 2004, 92, 229–235. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A Review of Its Sources and Environmental Toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; Whether Toxic or Essential for Plants and Environment—A Review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, Y.; Zhang, Y.; Shi, Y.; Hu, G.; Zhu, J.; Rogers, K.M. Geographical Traceability of Chinese Green Tea Using Stable Isotope and Multi-element Chemometrics. Rapid Commun. Mass Spectrom. 2019, 33, 778–788. [Google Scholar] [CrossRef]
- Taylor, G.J.; Blarney, F.P.C.; Edwards, D.G. Antagonistic and Synergistic Interactions between Aluminum and Manganese on Growth of Vigna Unguiculata at Low Ionic Strength. Physiol. Plant 1998, 104, 183–194. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Z.H.; Wang, Z.H. Heavy Metal Uptake by Bryophytes and Vascular Plants in a Manganese Carbonate Slag Field, China. Plant Biol. 2022, 24, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.M.; Mukhiya, Y.K.; Singh, V.P. Mercury, Manganese Interaction Studies on Barley Germination and Phyto Toxicity. Indian J. Plant Physiol. 1987, 30, 13–19. [Google Scholar]
- Imadi, S.R.; Waseem, S.; Kazi, A.G.; Azooz, M.M.; Ahmad, P. Aluminum Toxicity in Plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. [Google Scholar]
- Tang, T.; Miller, D.M. Growth and Tissue Composition of Rice Grown in Soil Treated with Inorganic Copper, Nickel, and Arsenic. Commun. Soil Sci. Plant Anal. 1991, 22, 2037–2045. [Google Scholar] [CrossRef]
- Li, F.; Jing, M.; Ma, F.; Wang, W.; Li, M. Comparison and Risk Assessment of Macroelements and Trace Metals in Commercial Teas from Different Regions of China. Biol. Trace Elem. Res. 2023, 201, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council. Commission Regulation (EU) No 1006/2011 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs. Off. J. Eur. Communities 2015, L161, 14–16. [Google Scholar]
- Karimi, G.; Hasanzadeh, M.K.; Nili, A.; Khashayarmanesh, Z.; Samiei, Z.; Nazari, F.; Teimuri, M. Concentrations and Health Risk of Heavy Metals in Tea Samples Marketed in Iran. Pharmacology 2008, 3, 164–174. [Google Scholar]
- Wong, M.H.; Zhang, Z.Q.; Wong, J.W.C.; Lan, C.Y. Trace Metal Contents (Al, Cu and Zn) of Tea: Tea and Soil from Two Tea Plantations, and Tea Products from Different Provinces of China. Environ. Geochem. Health 1998, 20, 87–94. [Google Scholar] [CrossRef]
- Street, R.A. Heavy Metals in Medicinal Plant Products—An African Perspective. S. Afr. J. Bot. 2012, 82, 67–74. [Google Scholar] [CrossRef]
- NY/T 288-2012; Green Food: Tea. China Standards Press, MAPRC: Beijing, China, 2012.
- NY 659-2003; The Limit of Chromium, Cadmium, Mercury, Arsenic and Fuoride in Tea. China Standards Press, MAPRC: Beijing, China, 2003.
- Gazette of India PFA (Prevention of Food Adulteration). The Prevention of Food Adulteration Rules. 1955. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.indiacode.nic.in/bitstream/123456789/15399/1/the_prevention_of_food_adulteration_act%2C_1954.pdf (accessed on 5 April 2023).
- USDA (United States Department of Agriculture). GAIN Report Number: VM3070 Providing the Vietnamese Technical Regulations on Mycotoxin and Heavy Metals MRLs in Foods; USDA United States Department of Agriculture: Washington, DC, USA, 2013.
- WHO (World Health Organization). Quality Control Methods for Medicinal Plant Materials; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Trevisanato, S.I.; Kim, Y.I. Tea and Health. Nutr. Rev. 2009, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Karim, M.R.; Marzec, Z.; Miyataka, H.; Himeno, S.; Asakawa, Y. Dietary Intake of Metals by the Young Adult Population of Eastern Poland: Results from a Market Basket Study. J. Trace Elem. Med. Biol. 2016, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Girolametti, F.; Annibaldi, A.; Carnevali, O.; Pignalosa, P.; Illuminati, S.; Truzzi, C. Potential Toxic Elements (PTEs) in Wild and Farmed Atlantic Bluefin Tuna (Thunnus Thynnus) from Mediterranean Sea: Risks and Benefits for Human Consumption. Food Control 2021, 125, 108012. [Google Scholar] [CrossRef]
- Truzzi, C.; Annibaldi, A.; Girolametti, F.; Giovannini, L.; Riolo, P.; Ruschioni, S.; Olivotto, I.; Illuminati, S. A Chemically Safe Way to Produce Insect Biomass for Possible Application in Feed and Food Production. Int. J. Environ. Res. Public Health 2020, 17, 2121. [Google Scholar] [CrossRef]
- Truzzi, C.; Illuminati, S.; Girolametti, F.; Antonucci, M.; Scarponi, G.; Ruschioni, S.; Riolo, P.; Annibaldi, A. Influence of Feeding Substrates on the Presence of Toxic Metals (Cd, Pb, Ni, as, Hg) in Larvae of Tenebrio Molitor: Risk Assessment for Human Consumption. Int. J. Environ. Res. Public Health 2019, 16, 4815. [Google Scholar] [CrossRef] [PubMed]
- Girolametti, F.; Panfili, M.; Colella, S.; Frapiccini, E.; Annibaldi, A.; Illuminati, S.; Marini, M.; Truzzi, C. Mercury Levels in Merluccius Merluccius Muscle Tissue in the Central Mediterranean Sea: Seasonal Variation and Human Health Risk. Mar. Pollut. Bull. 2022, 176, 113461. [Google Scholar] [CrossRef] [PubMed]
- Girolametti, F.; Frapiccini, E.; Annibaldi, A.; Illuminati, S.; Panfili, M.; Marini, M.; Santojanni, A.; Truzzi, C. Total Mercury (THg) Content in Red Mullet (Mullus Barbatus) from Adriatic Sea (Central Mediterranean Sea): Relation to Biological Parameters, Sampling Area and Human Health Risk Assessment. Appl. Sci. 2022, 12, 10083. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology Step 5 Note for Guidance on Validation of Analytical Procedures: Text And Methodology (CPMP/ICH/381/95) Approval by CPMP November 1994 Date for Coming into Operation; European Medicines Agency: Amsterdam, The Netherland, 1995. [Google Scholar]
- Roveta, C.; Pica, D.; Calcinai, B.; Girolametti, F.; Truzzi, C.; Illuminati, S.; Annibaldi, A.; Puce, S. Hg Levels in Marine Porifera of Montecristo and Giglio Islands (Tuscan Archipelago, Italy). Appl. Sci. 2020, 10, 4342. [Google Scholar] [CrossRef]
- Roveta, C.; Annibaldi, A.; Calcinai, B.; Girolametti, F.; Illuminati, S.; Pulido Mantas, T.; Truzzi, C.; Puce, S. Distribution of Mercury inside the Mediterranean Sponge Chondrosia Reniformis: A Study Case from the Tuscan Archipelago National Park (Tyrrhenian Sea). J. Sea Res. 2022, 184, 102206. [Google Scholar] [CrossRef]
- Wold, S. Cross-Validatory Estimation of the Number of Components in Factor and Principal Components Models. Technometrics 1978, 20, 397–405. [Google Scholar] [CrossRef]
- Wood, M.D.; Beresford, N.A.; Copplestone, D. Limit of Detection Values in Data Analysis: Do They Matter? Radioprotection 2011, 46, S85–S90. [Google Scholar] [CrossRef]
- EFSA Food Consumption Statistics for FoodEx2; 2022. Available online: https://www.efsa.europa.eu/en/supporting/pub/en-7900 (accessed on 5 April 2023).

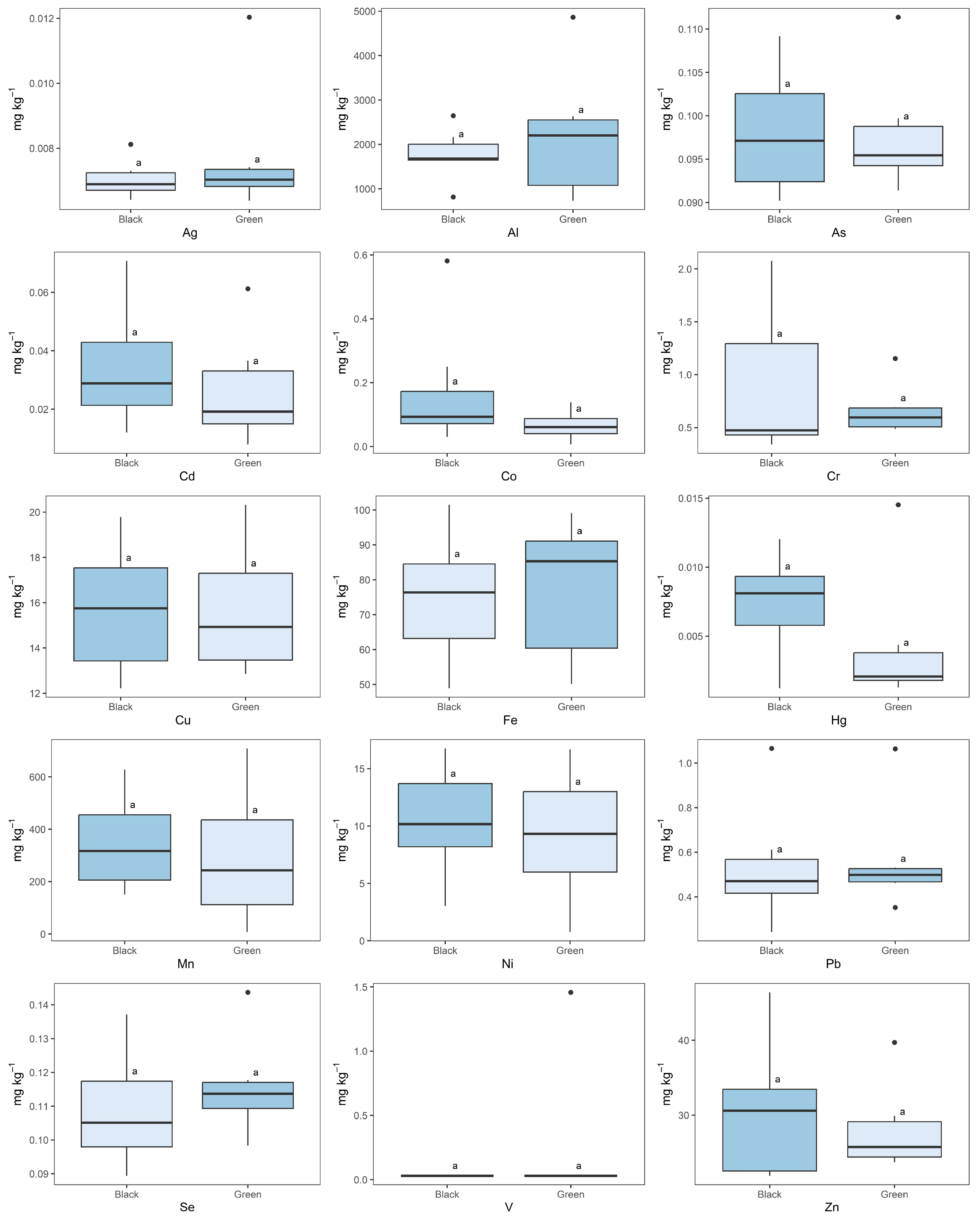
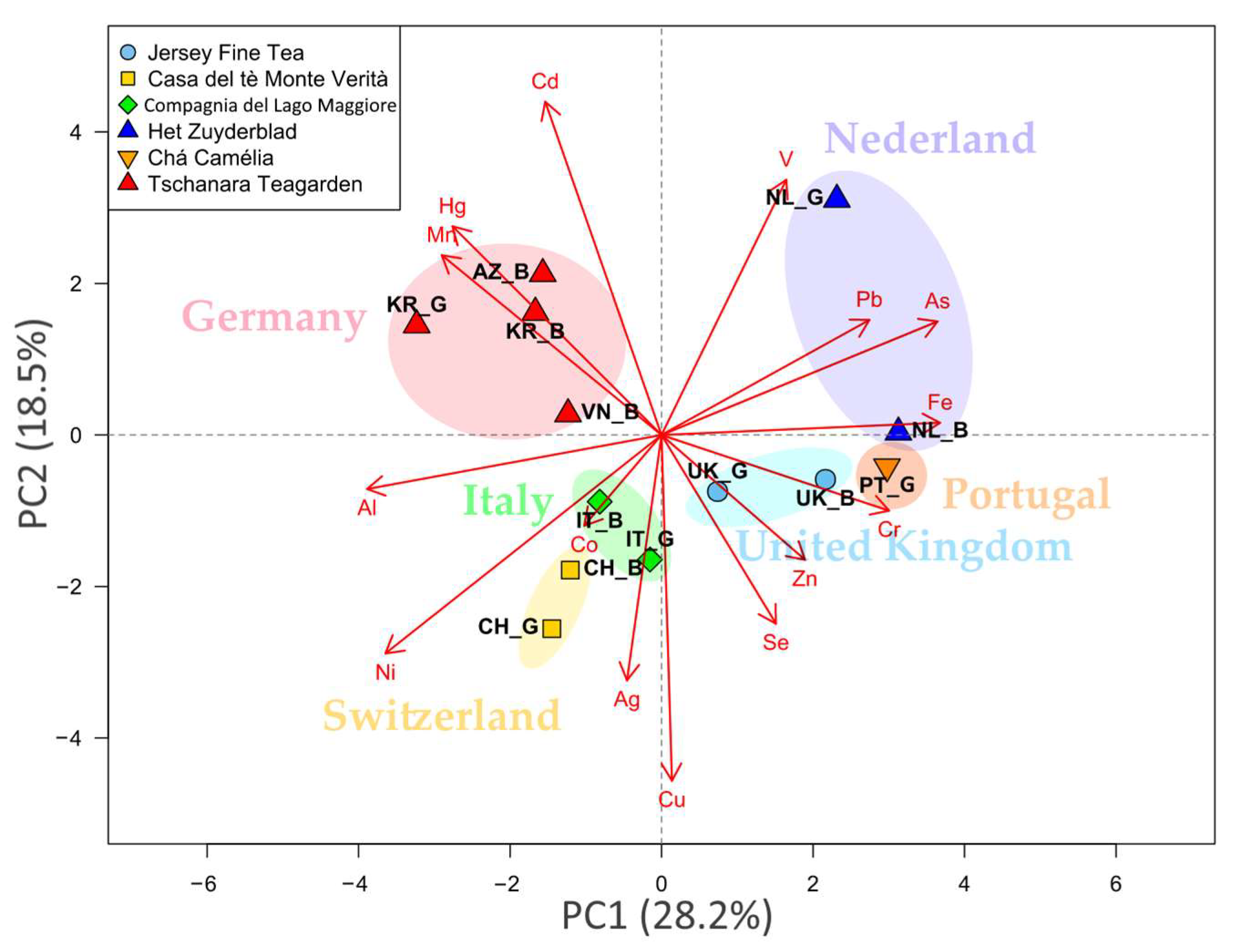
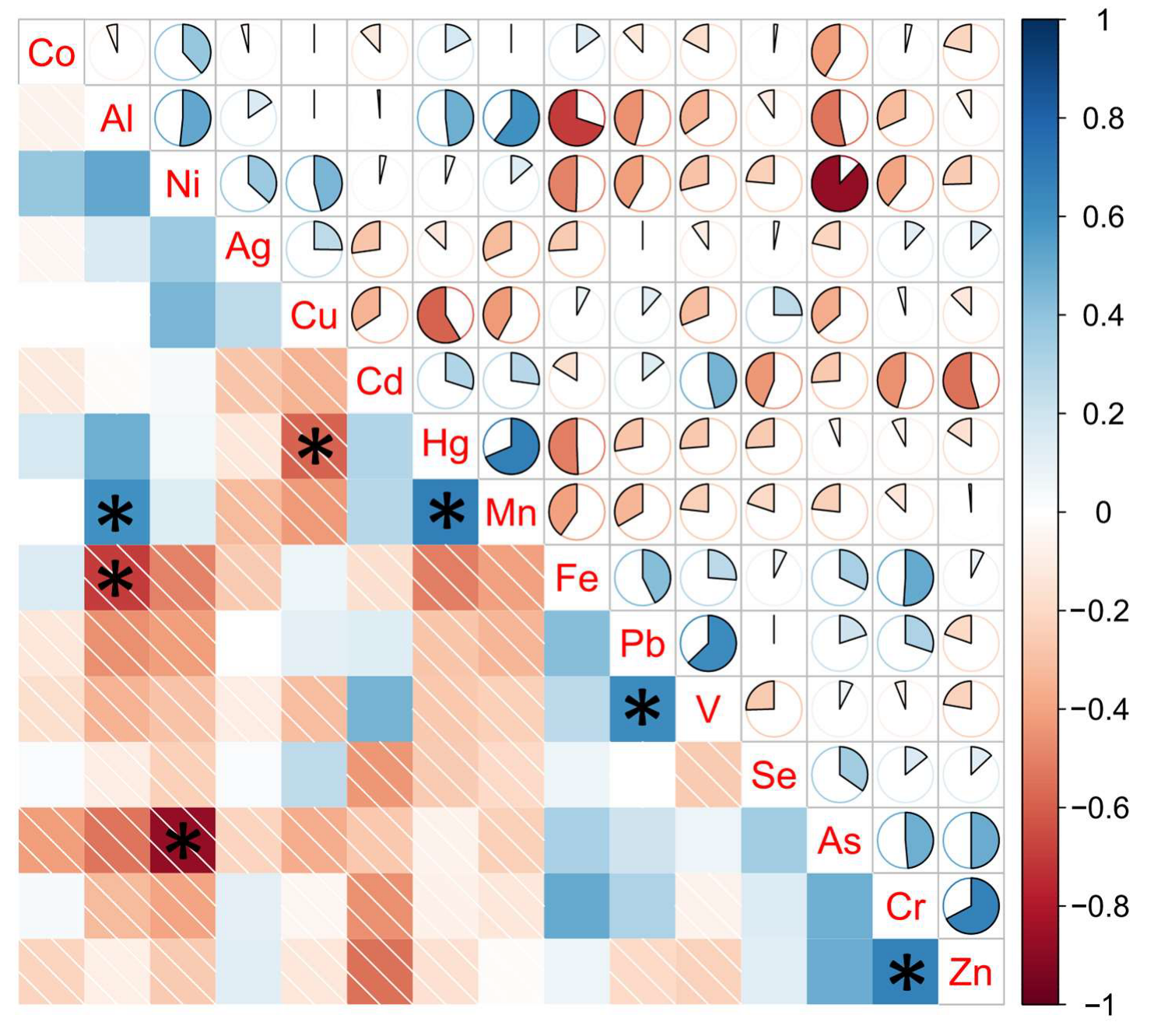
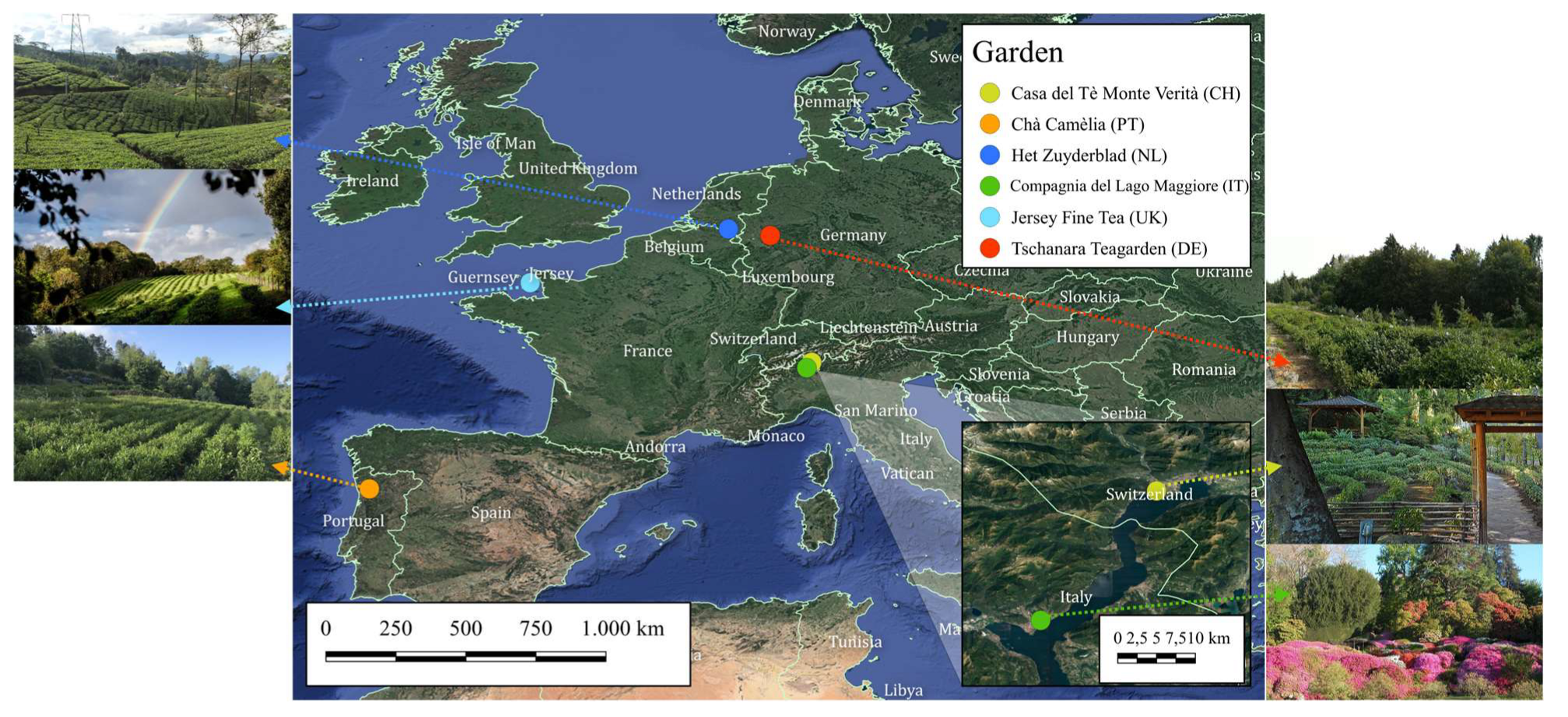
| Garden (Country) | Type | Co | Cu | Fe | Mn | Se | V | Zn |
|---|---|---|---|---|---|---|---|---|
| Jersey Fine Tea (The United Kingdom) | black | 0.09 ± 0.01 e | 13.5 ± 0.3 b | 77 ± 3 c,d,e | 317 ± 7 f | 0.127 ± 0.005 a,b | <0.06 | 46 ± 1 h |
| green | 0.14 ± 0.002 f | 14.8 ± 0.4 c | 99 ± 4 g | 471 ± 5 h | 0.11 ± 0.02 a,b | <0.06 | 39.7 ± 0.5 g | |
| all | 0.12 ± 0.03 | 14.2 ± 0.9 | 88 ± 16 | 394 ± 108 | 0.12 ± 0.01 | <0.06 | 43 ± 5 | |
| Casa del Tè Monte Verità (Switzerland) | black | 0.09 ± 0.01 e | 19.8 ± 0.3 g | 58 ± 3 a,b | 150 ± 6 c | 0.10 ± 0.02 a,b | <0.06 | 33.7 ± 0.3 f |
| green | 0.095 ± 0.005 e | 18.0 ± 0.1 e,f | 53 ± 4 a | 97 ± 4 b | 0.11 ± 0.01 a,b | <0.06 | 26.8 ± 0.2 d | |
| all | 0.095 ± 0.0001 | 19 ± 1 | 55 ± 4 | 124 ± 38 | 0.11 ± 0.007 | <0.06 | 30 ± 5 | |
| Compagnia del Lago Maggiore (Italy) | black | 0.58 ± 0.02 h | 15.8 ± 0.7 c,d | 92 ± 3 fg | 226 ± 4 e | 0.104 ± 0.007 a,b | <0.06 | 22.6 ± 0.2 a,b |
| green | 0.007 ± 0.001 a | 20.3 ± 0.7 g | 83 ± 2 d,e,f | 330 ± 3 f | 0.12 ± 0.01 a,b | <0.06 | 24.7 ± 0.1 c | |
| all | 0.3 ± 0.4 | 18 ± 3 | 88 ± 6 | 278 ± 73 | 0.111 ± 0.01 | <0.06 | 24 ± 1 | |
| Het Zuyderblad (The Netherlands) | black | 0.031 ± 0.001 b | 18.2 ± 0.5 f | 101 ± 2 g | 185 ± 8 d | 0.107 ± 0.009 a,b | <0.06 | 30.6 ± 0.1 e |
| green | 0.035 ± 0.001 b,c | 12.9 ± 0.2 a,b | 92 ± 2 f,g | 157 ± 5 c | 0.098 ± 0.006 a,b | 1.5 ± 0.2 | 23.7 ± 0.1 b,c | |
| all | 0.033 ± 0.003 | 16 ± 4 | 97 ± 6 | 171 ± 20 | 0.103 ± 0.006 | 1 ± 1 | 27 ± 5 | |
| Chá Camélia (Portugal) | green | 0.065 ± 0.003 d | 15.1 ± 0.5 c | 87 ± 2 e,f | 8 ± 1 a | 0.14 ± 0.02 b | <0.06 | 29.9 ± 0.2 e |
| Tschanara Teagarden (Germany) | green | 0.057 ± 0.006 c,d | 13 ± 0.3 a,b | 50 ± 2 a | 709 ± 9 m | 0.11 ± 0.03 a,b | <0.06 | 24.3 ± 0.1 c |
| black (Vietnam cultivar) | 0.250 ± 0.008 g | 16.9 ± 0.3 d,e | 68 ± 2 b,c | 535 ± 9 i | 0.14 ± 0.02 b | <0.06 | 21.9 ± 0.2 a | |
| black (Azores cultivar) | 0.053 ± 0.005 b,c,d | 13.3 ± 0.5 a,b | 76 ± 9 c,d | 375 ± 2 g | 0.089 ± 0.006 a | <0.06 | 22 ± 1 a,b | |
| black (Korea cultivar) | 0.090 ± 0.002 e | 12.2 ± 0.3 a | 49 ± 1 a | 628 ± 5 l | 0.09 ± 0.02 a | <0.06 | 33.2 ± 0.3 f | |
| all | 0.11 ± 0.09 | 14 ± 2 | 61 ± 14 | 562 ± 143 | 0.11 ± 0.02 | <0.06 | 25 ± 5 |
| Garden (Country) | Type | Ag | Al | As | Cd | Cr | Hg | Ni | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Jersey Fine Tea (The United Kingdom) | black | 0.0081 ± 0.0001 c | 1648 ± 49 b | 0.11 ± 0.01 a | 0.012 ± 0.002 a,b | 2.08 ± 0.01 i | 0.0081 ± 0.0009 d,e | 6.4 ± 0.2 d | 0.47 ± 0.03 b,c,d |
| green | 0.0074 ± 0.0001 b,c | 2105 ± 157 b,c,d | 0.10 ± 0.01 a | 0.016 ± 0.001 b,c | 1.150 ± 0.005 g | 0.0013 ± 0.0001 a | 8.9 ± 0.2 e | 0.35 ± 0.03 a,b | |
| all | 0.0078 ± 0.0005 | 1877 ± 324 | 0.103 ± 0.009 | 0.014 ± 0.003 | 1.6 ± 0.7 | 0.005 ± 0.005 | 8 ± 2 | 0.41 ± 0.08 | |
| Casa del Tè Monte Verità (Switzerland) | black | 0.0069 ± 0.0001 a,b | 2645 ± 63 d | 0.091 ± 0.007 a | 0.029 ± 0.002 f,g | 0.43 ± 0.009 b | 0.0012 ± 0.0001 a | 16.8 ± 0.5 h | 0.41 ± 0.03 b,c |
| green | 0.0120 ± 0.0004 d | 2636 ± 41 c,d | 0.09 ± 0.01 a | 0.0224 ± 0.0001 d,e | 0.500 ± 0.007 c,d | 0.0044 ± 0.0007 b | 16.7 ± 0.2 h | 0.53 ± 0.05 c,d | |
| all | 0.009 ± 0.004 | 2641 ± 6 | 0.0914 ± 0.0001 | 0.026 ± 0.005 | 0.46 ± 0.05 | 0.003 ± 0.002 | 16.72 ± 0.06 | 0.47 ± 0.08 | |
| Compagnia del Lago Maggiore (Italy) | black | 0.0072 ± 0.0008 a,b | 1646 ± 64 b | 0.09 ± 0.01 a | 0.0183 ± 0.0006 c,d | 0.95 ± 0.01 f | 0.0084 ± 0.0008 d,e | 15 ± 1 g | 0.52 ± 0.02 c,d |
| green | 0.0072 ± 0.0002 a,b | 2303 ± 75 b,c,d | 0.09 ± 0.02 a | 0.015 ± 0.001 b,c | 0.670 ± 0.009 e | 0.0017 ± 0.0002 a | 14.1 ± 0.3 g | 0.51 ± 0.03 c,d | |
| all | 0.00717 ± 0.00002 | 1975 ± 464 | 0.093 ± 0.003 | 0.016 ± 0.003 | 0.8 ± 0.2 | 0.005 ± 0.005 | 14.6 ± 0.8 | 0.519 ± 0.008 | |
| Het Zuyderblad (The Netherlands) | black | 0.0073 ± 0.0001 b,c | 815 ± 30 a | 0.104 ± 0.003 a | 0.024 ± 0.001 e,f | 1.64 ± 0.03 h | 0.0047 ± 0.0008 b,c | 3.04 ± 0.06 b | 1.1 ± 0.1 e |
| green | 0.0069 ± 0.0001 a,b | 735 ± 5 a | 0.100 ± 0.004 a | 0.061 ± 0.002 l | 0.69 ± 0.01 e | 0.0021 ± 0.0002 a | 5.02 ± 0.07 c | 1.06 ± 0.07 e | |
| all | 0.0071 ± 0.0003 | 775 ± 57 | 0.102 ± 0.003 | 0.04 ± 0.03 | 1.2 ± 0.7 | 0.003 ± 0.002 | 4 ± 1 | 1.064 ± 0.002 | |
| Chá Camélia (Portugal) | green | 0.0064 ± 0.0001 a | 733 ± 8 a | 0.11 ± 0.03 a | 0.008 ± 0.0003 a | 0.520 ± 0.001 d | 0.0021 ± 0.0002 a | 0.77 ± 0.02 a | 0.48 ± 0.02 b,c,d |
| Tschanara Teagarden (Germany) | green | 0.0068 ± 0.0001 a,b | 4865 ± 933 e | 0.09 ± 0.01 a | 0.037 ± 0.002 h | 0.49 ± 0.03 c,d | 0.0145 ± 0.0003 g | 9.8 ± 0.2 e | 0.46 ± 0.03 b,c,d |
| black (Vietnam cultivar) | 0.0064 ± 0.0001 a | 1843 ± 46 b,c | 0.09 ± 0.01 a | 0.054 ± 0.002 i | 0.430 ± 0.006 b | 0.0068 ± 0.0003 c,d | 12.2 ± 0.1 f | 0.61 ± 0.04 d | |
| black (Azores cultivar) | 0.0066 ± 0.0003 a,b | 1680 ± 158 b | 0.10 ± 0.01 a | 0.071 ± 0.004 m | 0.340 ± 0.005 a | 0.010 ± 0.001 e,f | 10.2 ± 0.1 e | 0.24 ± 0.02 a | |
| black (Korea cultivar) | 0.0068 ± 0.0002 a,b | 2167 ± 83 b,c,d | 0.10 ± 0.02 a | 0.032 ± 0.001 g,h | 0.47 ± 0.01 c | 0.012 ± 0.002 f | 9.9 ± 0.3 e | 0.42 ± 0.03 b,c | |
| all | 0.0067 ± 0.0002 | 2639 ± 1498 | 0.096 ± 0.003 | 0.05 ± 0.02 | 0.43 ± 0.07 | 0.011 ± 0.003 | 11 ± 1 | 0.4 ± 0.2 |
| Element | Jersey Fine Tea (United Kingdom) | Casa del Tè Monte Verità (Switzerland) | Compagnia del Lago Maggiore (Italy) | Het Zuyderblad (The Netherlands) | Chá Camèlia (Portugal) | Tschanara Teagarden (Germany) | All Tea Gardens |
|---|---|---|---|---|---|---|---|
| HQ | |||||||
| Ag | 7.4 × 10−6 | 9.1 × 10−6 | 6.9 × 10−6 | 6.8 × 10−6 | 6.1 × 10−6 | 6.4 × 10−6 | 7.1 × 10−6 |
| Al | 4.5 × 10−2 | 6.3 × 10−2 | 4.7 × 10−2 | 1.9 × 10−2 | 1.8 × 10−2 | 6.3 × 10−2 | 4.8 × 10−2 |
| As | 2.3 × 10−4 | 2.0 × 10−4 | 2.1 × 10−4 | 2.3 × 10−4 | 2.5 × 10−4 | 2.2 × 10−4 | 2.2 × 10−4 |
| Cd | 6.7 × 10−5 | 1.2 × 10−4 | 7.9 × 10−5 | 2.0 × 10−4 | 3.8 × 10−5 | 2.3 × 10−4 | 1.5 × 10−4 |
| Co | 1.8 × 10−5 | 1.5 × 10−5 | 4.7 × 10−5 | 5.2 × 10−6 | 1.0 × 10−5 | 1.8 × 10−5 | 2.0 × 10−5 |
| Cr | 2.6 × 10−3 | 7.4 × 10−4 | 1.3 × 10−3 | 1.9 × 10−3 | 8.4 × 10−4 | 6.9 × 10−4 | 1.3 × 10−3 |
| Cu | 1.4 × 10−4 | 1.8 × 10−4 | 1.7 × 10−4 | 1.5 × 10−4 | 1.4 × 10−4 | 1.3 × 10−4 | 1.5 × 10−4 |
| Fe | 4.2 × 10−4 | 2.7 × 10−4 | 4.2 × 10−4 | 4.6 × 10−4 | 4.2 × 10−4 | 2.9 × 10−4 | 3.6 × 10−4 |
| Hg | 3.9 × 10−5 | 2.3 × 10−5 | 4.3 × 10−5 | 2.9 × 10−5 | 1.7 × 10−5 | 9.1 × 10−5 | 5.0 × 10−5 |
| Mn | 2.6 × 10−2 | 8.3 × 10−3 | 1.9 × 10−2 | 1,1 × 10−2 | 5.2 × 10−4 | 3.8 × 10−2 | 2.2 × 10−2 |
| Ni | 1.8 × 10−3 | 4.0 × 10−3 | 3.5 × 10−3 | 9.7 × 10−4 | 1.9 × 10−4 | 2.5 × 10−3 | 2.4 × 10−3 |
| Pb | 5.5 × 10−4 | 6.3 × 10−4 | 7.0 × 10−4 | 1.4 × 10−3 | 6.5 × 10−4 | 5.8 × 10−4 | 7.4 × 10−4 |
| Se | 3.8 × 10−5 | 3.5 × 10−5 | 3.5 × 10−5 | 3.3 × 10−5 | 4.6 × 10−5 | 3.4 × 10−5 | 3.6 × 10−5 |
| V | 5.6 × 10−6 | 5.6 × 10−6 | 5.6 × 10−6 | 1.4 × 10−4 | 5.6 × 10−6 | 5.6 × 10−6 | 2.6 × 10−5 |
| Zn | 6.9 × 10−4 | 4.8 × 10−4 | 3.8 × 10−4 | 4.3 × 10−4 | 4.8 × 10−4 | 4.1 × 10−4 | 4.7 × 10−4 |
| HI | |||||||
| All | 7.8 × 10−2 | 7.8 × 10−2 | 7.3 × 10−2 | 3.6 × 10−2 | 2.1 × 10−2 | 1.1 × 10−1 | 7.5 × 10−2 |
| Garden or Market Location, Country | Type | Ag | Al | As | Cd | Cr | Hg | Ni | Pb | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| European Gardens | Black | 0.007 ± 0.0006 (0.006–0.008) | 1778 ± 560 (815–2645) | 0.098 ± 0.007 (0.09–0.11) | 0.03 ± 0.02 (0.012–0.071) | 0.9 ± 0.7 (0.34–2.1) | 0.007 ± 0.004 (0.001–0.012) | 11 ± 5 (3.04–16.8) | 0.5 ± 0.3 (0.24–1.07) | This study |
| European Gardens | Green | 0.008 ± 0.002 (0.006–0.012) | 2230 ± 1524 (735–4865) | 0.098 ± 0.007 (0.09–0.11) | 0.03 ± 0.02 (0.008–0.061) | 0.7 ± 0.3 (0.49–1.15) | 0.004 ± 0.005 (0.001–0.015) | 9 ± 6 (0.77–16.7) | 0.6 ± 0.3 (0.35–1.6) | This study |
| Sylhet and Moulvibazar district, Bangladesh | Black | (0.003–1.9) | (0.05–0.16) | (0.003–10.73) | (0.003–1.03) | [12] | ||||
| India | Black | 0.067 ± 0.036 (0.007–0.14) | 0.02 ± 0.013 (0.0002–0.052) | 0.01 ± 0.0049 (0.0016–0.019) | 0.21 ± 0.169 (0.001–0.73) | [13] | ||||
| Sri Lanka | Black | 0.057 ± 0.0323 (0.0083–0.13) | 0.017 ± 0.019 (0.0002–0.084) | 0.0076 ± 0.0046 (0.0001–0.0019) | 0.14 ± 0.109 (0.01–0.6) | [13] | ||||
| Cairo, Egypt | Black | 0.0 ± 0.0 | 6.1 ± 3.5 | 6.5 ± 1.4 | 0.4 ± 0.2 | [14] | ||||
| Cairo, Egypt | Green | 0.09 ± 0.03 | n.d. | 5.73 ± 2.2 | 1.23 ± 0.5 | [14] | ||||
| Cairo, Egypt | Herbal | 0.1 ± 0.06 | 0.5 ± 0.53 | 1.35 ± 0.38 | 0.15 ± 0.16 | [14] | ||||
| Local market, China | Green | 487.57 ± 234.46 (227.41–911.67) | 0.055 ± 0.020 (0.025–0.11) | 1.63 ± 0.67 (0.28–1.63) | 7.71 ± 2.91 (2.71–13.57) | 0.92 ± 0.42 (0.12–2.24) | [23] | |||
| Southwest of China, China | Different varieties | (0.001–2.42) | (0.005–0.620) | (0.13–49.0) | (0.04–2.90) | [15] | ||||
| Anhui, China | 8 varieties | 1836.77 ± 829.68 (742.81–4128.00) | 0.01 ± 0.01 (n.d.–0.03) | 1.07 ± 0.54 (0.23–2.59) | [16] | |||||
| Gilan, Iran | Black | 1143 | <0.76 | <1.54 | 10.03 | 1.91 | [17] | |||
| India | Black | 891.2 | <0.68 | <1.56 | 4.88 | 1.34 | [17] | |||
| Ceylon | Black | 968.2 | <0.55 | <1.21 | 5.09 | 1.71 | [17] | |||
| Hsinchu market, Taiwan | Green | n.d. | n.d. | 0.1 (n.d.–0.5) | 0.01 (n.d.–0.2) | [18] | ||||
| Hsinchu market, Taiwan | Oolong | 0.005 (n.d.–0.01) | 0.005 (n.d.–0.02) | 5.2 (3.9–6.2) | 0.4 (n.d.–1.2) | [18] | ||||
| Hsinchu market, Taiwan | Black | 0.01 (n.d.–0.05) | 0.07 (n.d.–0.1) | 7.92 (5.5–9.3) | 2.01 (n.d.–6.5) | [18] | ||||
| Wushwush, Ethiopia | Green | <LOD | <LOD | [19] | ||||||
| Guizhou, China | 7323 ± 1752 (4300–10400) | 0.291 ± 0.067 (0.189–0.453) | 0.061 ± 0.012 (0.040–0.087) | 1.47 ± 0.70 (0.69–2.91) | 0.063 ± 0.015 (0.043–0.089) | 9.44 ± 3.55 (3.43–14.20) | 0.931 ± 0.196 (0.560–1.265) | [20] | ||
| China | Green | 0.0063 ± 0.0064 (0.0018–0.103) | [21] | |||||||
| Yunnan, China | Puerh | 1538 ± 341 (1080–2020) | 0.049 ± 0.013 (0.0024–0.0066 | 0.003 ± 0.004 (n.d.–0.01) | 0.005 ± 0.005 (n.d.–0.13) | 0.94 ± 1.08 (0.31–3.42) | [22] |
| Country or Institution | Foodstuff | As | Cd | Cr | Cu | Hg | Ni | Pb | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Europe | Leaf vegetable | 0.2 | 0.1 | [11,44] | |||||
| Europe | Rice | 0.2 | [73] | ||||||
| Europe | Fish | 0.5 | [11,44] | ||||||
| Australia | Tea | 150 | [74] | ||||||
| Australia | Tea | 50 | [75] | ||||||
| Canada | Raw herbal materials | 0.3 | 2 | 0.2 | 10 | [76] | |||
| China (NY/T 288–2012) | Tea | 30 | 5 | [77] | |||||
| China (NY 659–2003) | Tea | 2 | 1 | 5 | 0.3 | [78] | |||
| Germany | Tea | 40 | [75] | ||||||
| Germany | Product of plant origin | 0.2 | 0.1 | 5 | [49] | ||||
| India | Tea | 150 | 5 | 10 | [79] | ||||
| Iran | Not reported | 0.15 | 150 | 0.2 | 1 | [74] | |||
| Japan | Tea | 100 | [74] | ||||||
| Singapore | Finished herbalproducts | 150 | 0.5 | [76] | |||||
| Thailand | Tea | 2 | 10 | [74] | |||||
| UK | Tea | 150 | [74] | ||||||
| USA | Tea | [74] | |||||||
| Vietnam | Tea and tea products | 1.0 | 0.5 | 2.0 | [80] | ||||
| World Health Organization (WHO), 1998 | Not reported | 0.3 | 10 | [81] | |||||
| Measured value | Tea | 0.098 ± 0.7 | 0.03 ± 0.2 | 0.8 ± 0.5 | 16 ± 3 | 0.006 ± 0.004 | 10 ± 5 | 0.6 ± 0.2 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolametti, F.; Annibaldi, A.; Illuminati, S.; Damiani, E.; Carloni, P.; Truzzi, C. Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers. Molecules 2023, 28, 3802. https://doi.org/10.3390/molecules28093802
Girolametti F, Annibaldi A, Illuminati S, Damiani E, Carloni P, Truzzi C. Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers. Molecules. 2023; 28(9):3802. https://doi.org/10.3390/molecules28093802
Chicago/Turabian StyleGirolametti, Federico, Anna Annibaldi, Silvia Illuminati, Elisabetta Damiani, Patricia Carloni, and Cristina Truzzi. 2023. "Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers" Molecules 28, no. 9: 3802. https://doi.org/10.3390/molecules28093802
APA StyleGirolametti, F., Annibaldi, A., Illuminati, S., Damiani, E., Carloni, P., & Truzzi, C. (2023). Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia sinensis) Leaves: Risk Assessment for Consumers. Molecules, 28(9), 3802. https://doi.org/10.3390/molecules28093802











