Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study
Abstract
:1. Introduction
1.1. Role of the Pellicle at the Tooth Surface
1.2. Olive Oil and Its Perspectives in Preventive Dentistry
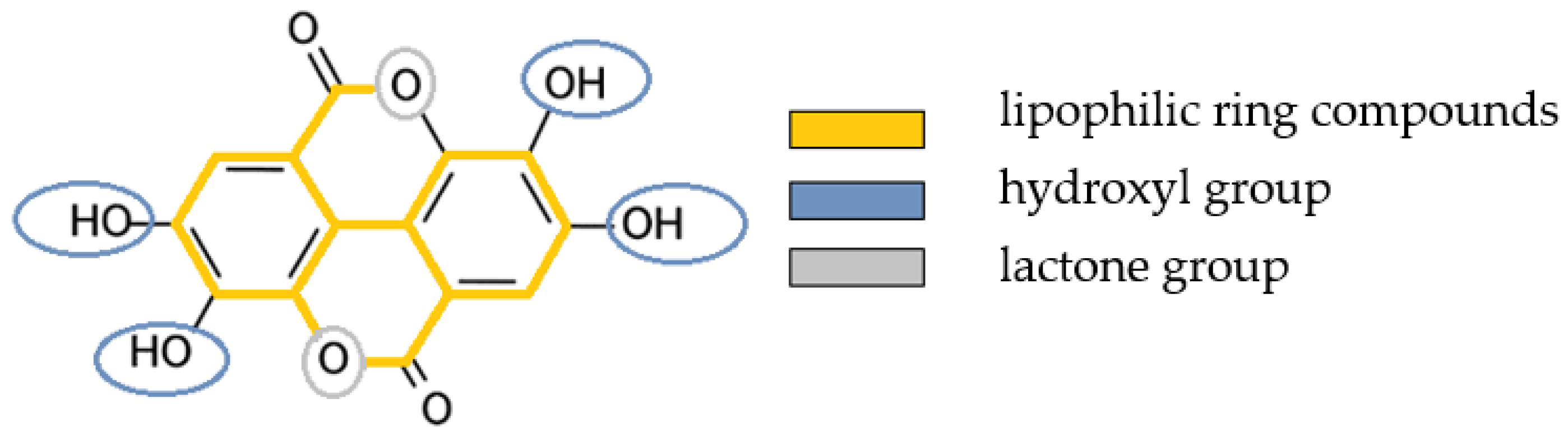
1.3. Olive Oil as a Transport Medium of Bioactive Plant Compounds
2. Results
2.1. Initial Bacterial Adhesion
2.1.1. DAPI/Glucan
2.1.2. BacLight
2.2. Calcium and Phosphate Release
2.3. Transmission Electron Microscopy
3. Discussion
4. Material and Methods
4.1. Subjects
4.2. Specimens
4.3. Preparation of EA in Water/in Oil
4.4. Pellicle Formation
4.5. Initial Bacterial Colonization and Glucan Formation
4.5.1. DAPI/Glucan
- Score 0: no glucans detectable;
- Score 1: single glucan structures exhibiting cloudy formations;
- Score 2: distinct glucan structures exhibiting cloudy structures surrounding the bacteria;
- Score 3: distinct glucan structures surrounding at least 50% of the bacteria;
- Score 4: distinct glucan structures surrounding almost all bacteria.
4.5.2. BacLight
4.6. Calcium and Phosphate Release
4.7. Transmission Electron Microscopy
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride Revolution and Dental Caries: Evolution of Policies for Global Use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.C.; Higgins, J.P.T.; Logan, S.; Sheiham, A. Systematic Review of Controlled Trials on the Effectiveness of Fluoride Gels for the Prevention of Dental Caries in Children. J. Dent. Educ. 2003, 67, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Appelbe, P.; Marinho, V.C.C.; Shi, X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2010, 1, CD007868. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Burke, F.; Allen, P.F. Incidence, Prevalence and Global Distribution of Root Caries. Monogr. Oral Sci. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Frencken, J. Caries Epidemiology and Its Challenges. Monogr. Oral Sci. 2018, 27, 11–23. [Google Scholar] [CrossRef]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2021, 101, 392–399. [Google Scholar] [CrossRef]
- Moore, C.; McLister, C.; Cardwell, C.; O’Neill, C.; Donnelly, M.; McKenna, G. Dental caries following radiotherapy for head and neck cancer: A systematic review. Oral Oncol. 2020, 100, 104484. [Google Scholar] [CrossRef]
- Hannig, M.; Joiner, A. The structure, function and properties of the acquired pellicle. Monogr. Oral Sci. 2006, 19, 29–64. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Kensche, A.; Carpenter, G. The mucosal pellicle—An underestimated factor in oral physiology. Arch. Oral Biol. 2017, 80, 144–152. [Google Scholar] [CrossRef]
- Siqueira, W.L.; Custodio, W.; McDonald, E.E. New Insights into the Composition and Functions of the Acquired Enamel Pellicle. J. Dent. Res. 2012, 91, 1110–1118. [Google Scholar] [CrossRef]
- Flemming, J.; Hannig, C.; Hannig, M. Caries Management—The Role of Surface Interactions in De- and Remineralization-Processes. J. Clin. Med. 2022, 11, 7044. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. The Pellicle and Erosion. In Erosive Tooth Wear: From Diagnosis to Therapy, 2nd ed.; Monogrphs in Oral Science; Karger: Basel, Switzerland, 2014; Volume 25, pp. 206–214. [Google Scholar] [CrossRef]
- Vukosavljevic, D.; Custodio, W.; Buzalaf, M.A.; Hara, A.T.; Siqueira, W.L. Acquired pellicle as a modulator for dental erosion. Arch. Oral Biol. 2014, 59, 631–638. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Modulation of Streptococcus mutans Adherence to Hydroxyapatite by Engineered Salivary Peptides. Microorganisms 2022, 10, 223. [Google Scholar] [CrossRef]
- Kirsch, J.; Hannig, M.; Winkel, P.; Basche, S.; Leis, B.; Putz, N.; Kensche, A.; Hannig, C. Influence of pure fluorides and stannous ions on the initial bacterial colonization in situ. Sci. Rep. 2019, 9, 18499. [Google Scholar] [CrossRef]
- Kensche, A.; Buschbeck, E.; König, B.; Koch, M.; Kirsch, J.; Hannig, C.; Hannig, M. Effect of fluoride mouthrinses and stannous ions on the erosion protective properties of the in situ pellicle. Sci. Rep. 2019, 9, 5336. [Google Scholar] [CrossRef]
- Kensche, A.; Kirsch, J.; Mintert, S.; Enders, F.; Pötschke, S.; Basche, S.; König, B.; Hannig, C.; Hannig, M. Impact of customary fluoride rinsing solutions on the pellicle’s protective properties and bioadhesion in situ. Sci. Rep. 2017, 7, 16584. [Google Scholar] [CrossRef]
- Hannig, C.; Gaeding, A.; Basche, S.; Richter, G.; Helbig, R.; Hannig, M. Effect of conventional mouthrinses on initial bioadhesion to enamel and dentin in situ. Caries Res. 2012, 47, 150–161. [Google Scholar] [CrossRef]
- Hertel, S.; Graffy, L.; Pötschke, S.; Basche, S.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Inula viscosa on the pellicle’s protective properties and initial bioadhesion in-situ. Arch. Oral Biol. 2016, 71, 87–96. [Google Scholar] [CrossRef]
- Kirsch, J.; Jung, A.; Hille, K.; König, B.; Hannig, C.; Kölling-Speer, I.; Speer, K.; Hannig, M. Effect of fragaria vesca, hamamelis and tormentil on the initial bacterial colonization in situ. Arch. Oral Biol. 2020, 118, 104853. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.-T.; Hannig, M.; Pötschke, S.; Höhne, F.; Hannig, C. Application of Plant Extracts for the Prevention of Dental Erosion: An In situ/In vitro Study. Caries Res. 2015, 49, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Sorg, J.; Spitzmüller, B.; Hannig, M.; Al-Ahmad, A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J. Dent. 2009, 37, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Pötschke, S.; Basche, S.; Delius, J.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Tannic Acid on the Protective Properties of the in situ Formed Pellicle. Caries Res. 2016, 51, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Rawat, M.; Singh, D.; Saraf, S. Lipid carriers: A versatile delivery vehicle for proteins and peptides. Yakugaku Zasshi 2008, 128, 269–280. [Google Scholar] [CrossRef]
- Pauwels, E.K.J. The Protective Effect of the Mediterranean Diet: Focus on Cancer and Cardiovascular Risk. Med. Princ. Pr. 2011, 20, 103–111. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Lassen, B.; Holmberg, K.; Brink, C.; Carlen, A.; Olsson, J. Binding of Salivary Proteins and Oral Bacteria to Hydrophobic and Hydrophilic Surfaces In-Vivo and In-Vitro. Colloid Polym. Sci. 1994, 272, 1143–1150. [Google Scholar] [CrossRef]
- Olsson, J.; van der Heijde, Y.; Holmberg, K. Plaque formation in vivo and bacterial attachment in vitro on permanently hydrophobic and hydrophilic surfaces. Caries Res. 1992, 26, 428–433. [Google Scholar] [CrossRef]
- Hannig, C.; Kirsch, J.; Al-Ahmad, A.; Kensche, A.; Hannig, M.; Kümmerer, K. Do edible oils reduce bacterial colonization of enamel in situ? Clin. Oral Investig. 2012, 17, 649–658. [Google Scholar] [CrossRef]
- Hannig, C.; Wagenschwanz, C.; Pötschke, S.; Kümmerer, K.; Kensche, A.; Hoth-Hannig, W.; Hannig, M. Effect of Safflower Oil on the Protective Properties of the in situ Formed Salivary Pellicle. Caries Res. 2012, 46, 496–506. [Google Scholar] [CrossRef]
- Peckys, D.; De Jonge, N.; Hannig, M. Oil droplet formation on pellicle covered tooth surfaces studied with environmental scanning electron microscopy. J. Microsc. 2019, 274, 158–167. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley Blackwell: Oxford, UK, 2018; Volume 1–2, pp. 115–123. [Google Scholar]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Nutrition—Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2019, 151, 104584. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Schestakow, A.; Hannig, M. Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ. Biomolecules 2020, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The Polyphenolic Composition of Cistus incanus Herbal Tea and Its Antibacterial and Anti-adherent Activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Hoth-Hannig, W.; Deng, S.; Jin, X.; Fu, B.; Hannig, M. The effect of polyphenol-containing solutions on in situ biofilm formation on enamel and dentin. J. Dent. 2020, 102, 103482. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C.; Kishan, B.; Chandra, R.; Karnati, M. Anti-arthritic activity of root bark of Oroxylum indicum (L.) vent against adjuvant-induced arthritis. Pharmacogn. Res. 2013, 5, 121–128. [Google Scholar] [CrossRef]
- Zaveri, M.; Khandhar, A.; Jain, S. Quantification of Baicalein, Chrysin, Biochanin-A and Ellagic Acid in Root Bark of Oroxylum indicum by RPHPLC with UV Detection. Eurasian J. Anal. Chem. 2008, 3, 245–257. [Google Scholar]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Amakura, Y.; Okada, M.; Tsuji, S.; Tonogai, Y. High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J. Chromatogr. A 2000, 896, 87–93. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Williner, M.R.; Pirovani, M.E.; Güemes, D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar] [CrossRef]
- Fikry, E.M.; Gad, A.; Eid, A.H.; Arab, H.H. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharmacother. 2018, 110, 878–886. [Google Scholar] [CrossRef]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative Analysis and In vitro Anti-Inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar] [CrossRef]
- Baek, B.; Lee, S.H.; Kim, K.; Lim, H.-W.; Lim, C.-J. Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J. Physiol. Pharmacol. 2016, 20, 269–277. [Google Scholar] [CrossRef]
- Karimi, A.; Moradi, M.-T.; Rabiei, M.; Alidadi, S. In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antivir. Chem. Chemother. 2020, 28, 1–6. [Google Scholar] [CrossRef]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.-J.; Ahn, Y.-J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef]
- Sampaio, A.D.G.; Gontijo, A.V.L.; Araujo, H.M.; Koga-Ito, C.Y. In Vivo Efficacy of Ellagic Acid against Candida albicans in a Drosophila melanogaster Infection Model. Antimicrob. Agents Chemother. 2018, 62, 16–18. [Google Scholar] [CrossRef]
- Loo, W.T.Y.; Jin, L.J.; Cheung, M.N.B.; Chow, L.W.C. Evaluation of Ellagic acid on the activities of oral bacteria with the use of adenosine triphosphate (ATP) bioluminescence assay. Afr. J. Biotechnol. 2010, 9, 3938–3943. [Google Scholar]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Hirt, H.M.; M’pia, B.; Lindsey, K. Natural Medicine in the Tropics; Anamed International e.V.: Winnenden, Germany, 2008. [Google Scholar]
- Avachat, A.M.; Patel, V.G. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm. J. 2014, 23, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Kensche, A.; Reich, M.; Kümmerer, K.; Hannig, M.; Hannig, C. Lipids in preventive dentistry. Clin. Oral Investig. 2012, 17, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Kensche, A.; Dürasch, A.; König, B.; Henle, T.; Hannig, C.; Hannig, M. Characterization of the in situ pellicle ultrastructure formed under the influence of bovine milk and milk protein isolates. Arch. Oral Biol. 2019, 104, 133–140. [Google Scholar] [CrossRef]
- Kensche, A.; Reich, M.; Hannig, C.; Kümmerer, K.; Hannig, M. Modification of the Lipid Profile of the Initial Oral Biofilm In Situ Using Linseed Oil as Mouthwash. Nutrients 2021, 13, 989. [Google Scholar] [CrossRef]
- Hannig, M.; Hess, N.J.; Hoth-Hannig, W.; De Vrese, M. Influence of salivary pellicle formation time on enamel demineralization—An in situ pilot study. Clin. Oral Investig. 2003, 7, 158–161. [Google Scholar] [CrossRef]
- Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive Applications of Polyphenols in Dentistry—A Review. Int. J. Mol. Sci. 2021, 22, 4892. [Google Scholar] [CrossRef]
- Nayak, A.; Carpenter, G.H. A physiological model of tea-induced astringency. Physiol. Behav. 2008, 95, 290–294. [Google Scholar] [CrossRef]
- Joiner, A.; Muller, D.; Elofsson, U.M.; Arnebrant, T. Ellipsometry analysis of the in vitro adsorption of tea polyphenols onto salivary pellicles. Eur. J. Oral Sci. 2004, 112, 510–515. [Google Scholar] [CrossRef]
- de Souza, E.S.C.M.; da Silva Ventura, T.M.; de Pau, L.; la Silva, C.; de Lima Leite, A.; Buzalaf, M.A.R. Effect of gels containing chlorhexidine or epigallocatechin-3-gallate on the protein composition of the acquired enamel pellicle. Arch. Oral Biol. 2017, 82, 92–98. [Google Scholar] [CrossRef]
- Kandra, L.; Gyémánt, G.; Zajácz, A.; Batta, G. Inhibitory effects of tannin on human salivary alpha-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef]
- Jean-Gilles, D.; Li, L.Y.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O.; Seeram, N.P. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem. Interact. 2013, 205, 90–99. [Google Scholar] [CrossRef]
- Yanagida, A.; Kanda, T.; Tanabe, M.; Matsudaira, F.; Cordeiro, J.G.O. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J. Agric. Food Chem. 2000, 48, 5666–5671. [Google Scholar] [CrossRef]
- Lei, M.; Jiang, F.C.; Cai, J.; Hu, S.; Zhou, R.; Liu, G.; Wang, Y.H.; Wang, H.B.; He, J.R.; Xiong, X.G. Facile microencapsulation of olive oil in porous starch granules: Fabrication, characterization, and oxidative stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef]
- Rehage, M.; Delius, J.; Hofmann, T.; Hannig, M. Oral astringent stimuli alter the enamel pellicle’s ultrastructure as revealed by electron microscopy. J. Dent. 2017, 63, 21–29. [Google Scholar] [CrossRef]
- Buchalla, W.; Attin, T.; Roth, P.; Hellwig, E. Influence of olive oil emulsions on dentin demineralization in vitro. Caries Res. 2003, 37, 100–107. [Google Scholar] [CrossRef]
- Wiegand, A.; Gutsche, M.; Attin, T. Effect of olive oil and an olive-oil-containing fluoridated mouthrinse on enamel and dentin erosion in vitro. Acta Odontol. Scand. 2007, 65, 357–361. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmüller, B.; Hannig, M. Characterisation of lysozyme activity in the in situ pellicle using a fluorimetric assay. Clin. Oral Investig. 2008, 13, 15–21. [Google Scholar] [CrossRef]
- Kensche, A.; Basche, S.; Bowen, W.; Hannig, M.; Hannig, C. Fluorescence microscopic visualization of non cellular components during initial bioadhesion in situ. Arch. Oral Biol. 2013, 58, 1271–1281. [Google Scholar] [CrossRef]
- Attin, T.; Becker, K.; Hannig, C.; Buchalla, W.; Hilgers, R. Method to detect minimal amounts of calcium dissolved in acidic solutions. Caries Res. 2005, 39, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Becker, K.; Hannig, C.; Buchalla, W.; Wiegand, A. Suitability of a malachite green procedure to detect minimal amounts of phosphate dissolved in acidic solutions. Clin. Oral Investig. 2005, 9, 203–207. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flemming, J.; Meyer-Probst, C.T.; Hille, K.; Basche, S.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study. Molecules 2023, 28, 3803. https://doi.org/10.3390/molecules28093803
Flemming J, Meyer-Probst CT, Hille K, Basche S, Speer K, Kölling-Speer I, Hannig C, Hannig M. Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study. Molecules. 2023; 28(9):3803. https://doi.org/10.3390/molecules28093803
Chicago/Turabian StyleFlemming, Jasmin, Clara Theres Meyer-Probst, Kristin Hille, Sabine Basche, Karl Speer, Isabelle Kölling-Speer, Christian Hannig, and Matthias Hannig. 2023. "Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study" Molecules 28, no. 9: 3803. https://doi.org/10.3390/molecules28093803




