Effects of HAT-CN Layer Thickness on Molecular Orientation and Energy-Level Alignment with ZnPc
Abstract
1. Introduction
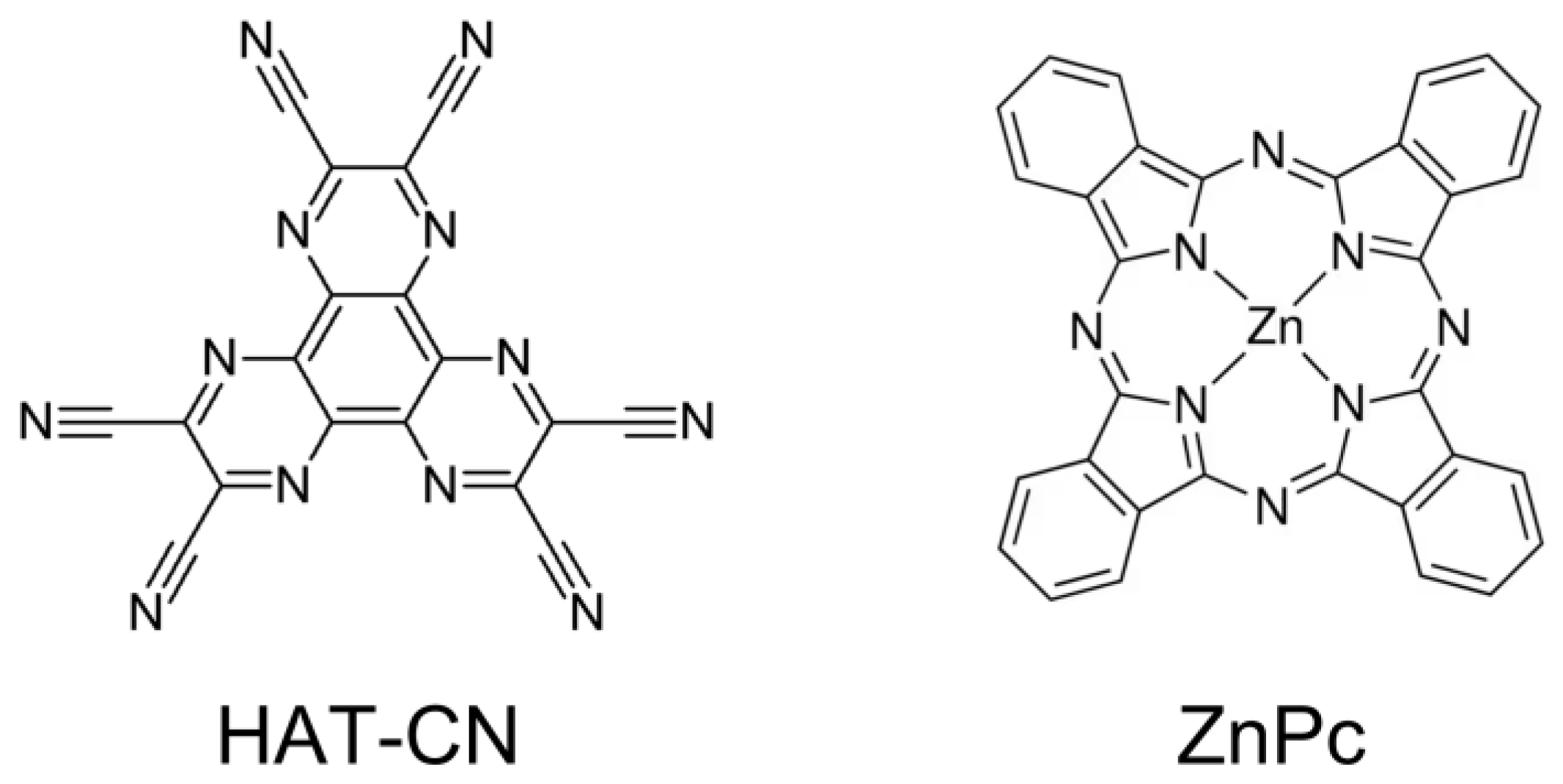
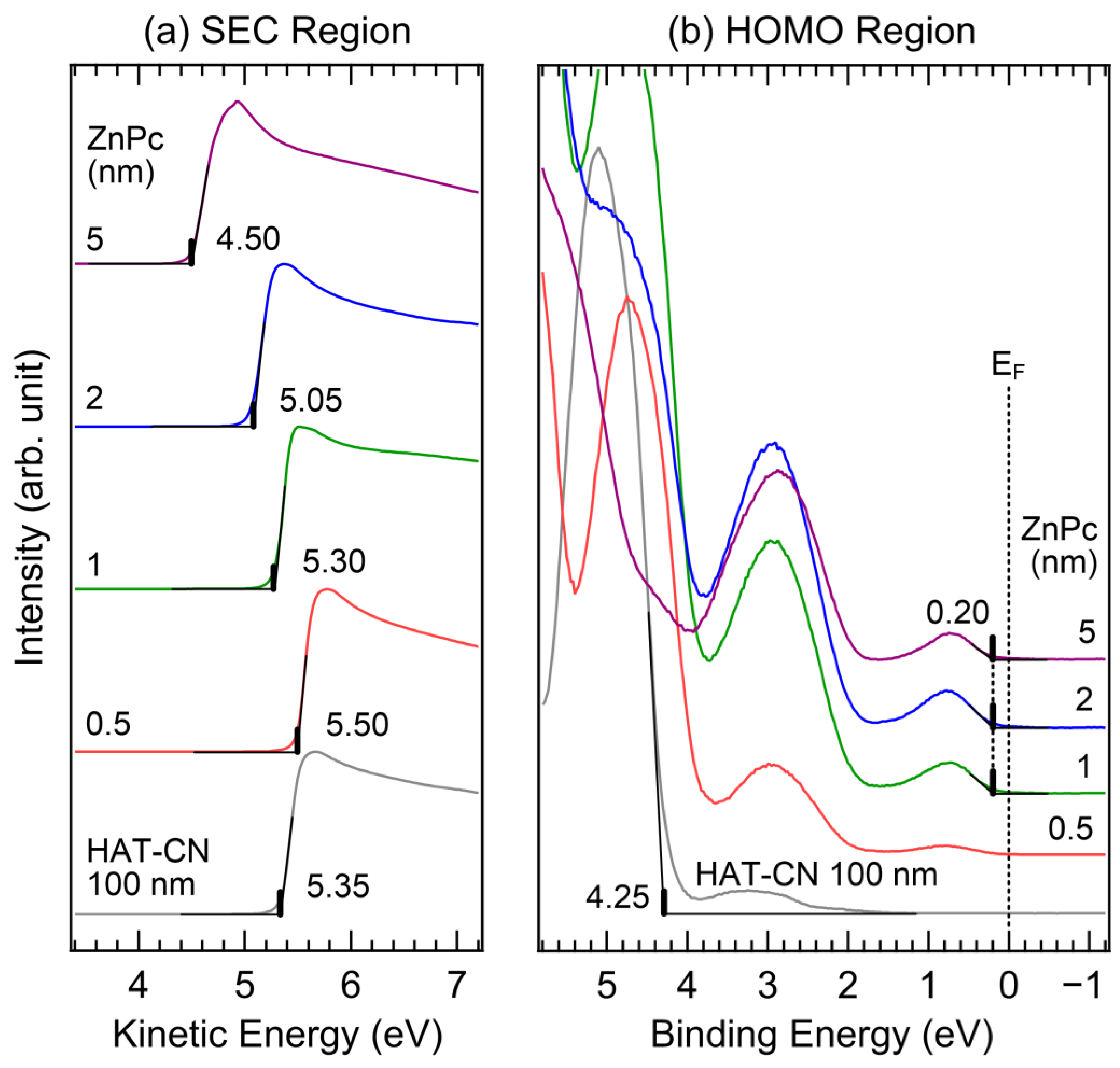
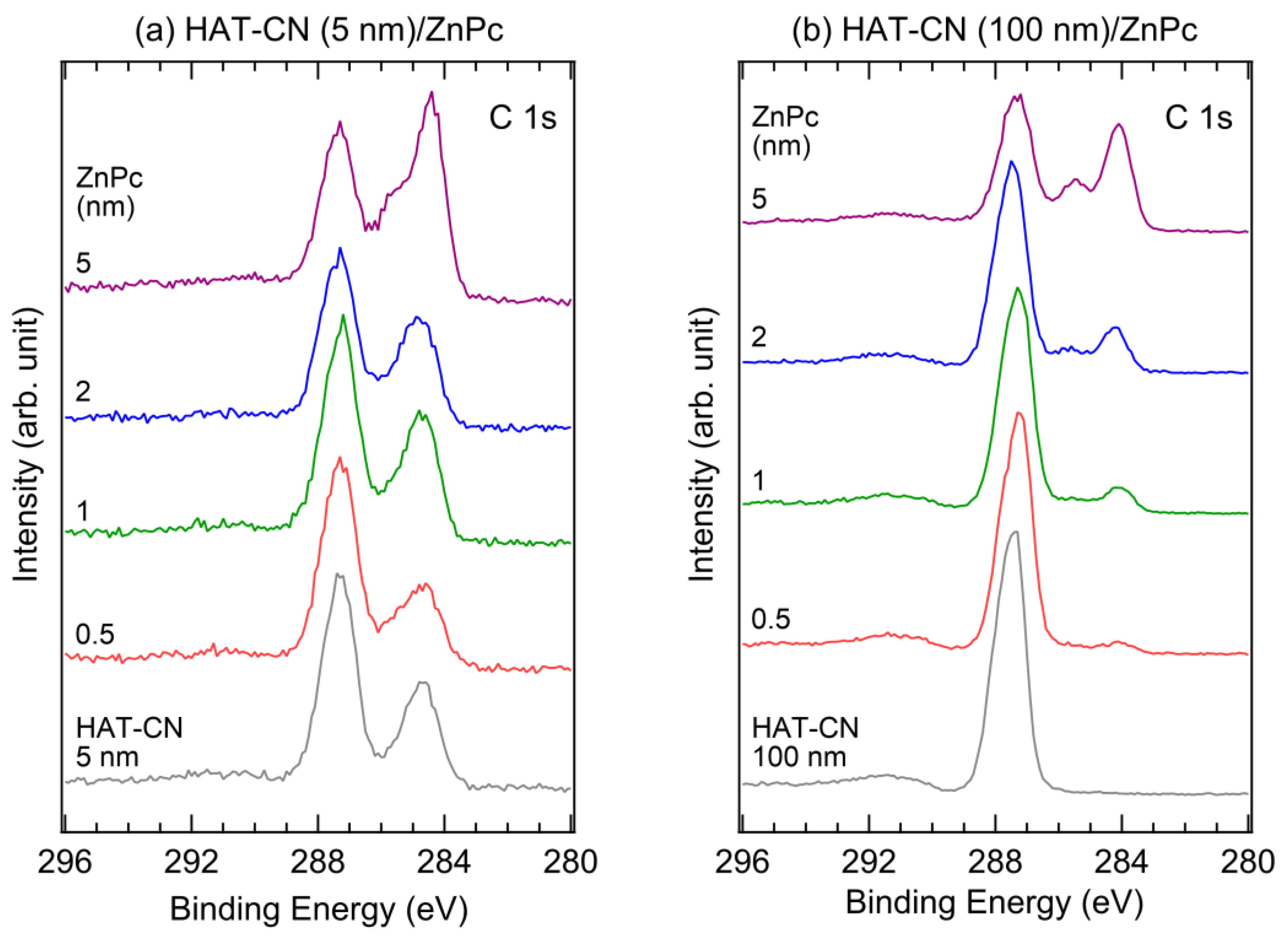
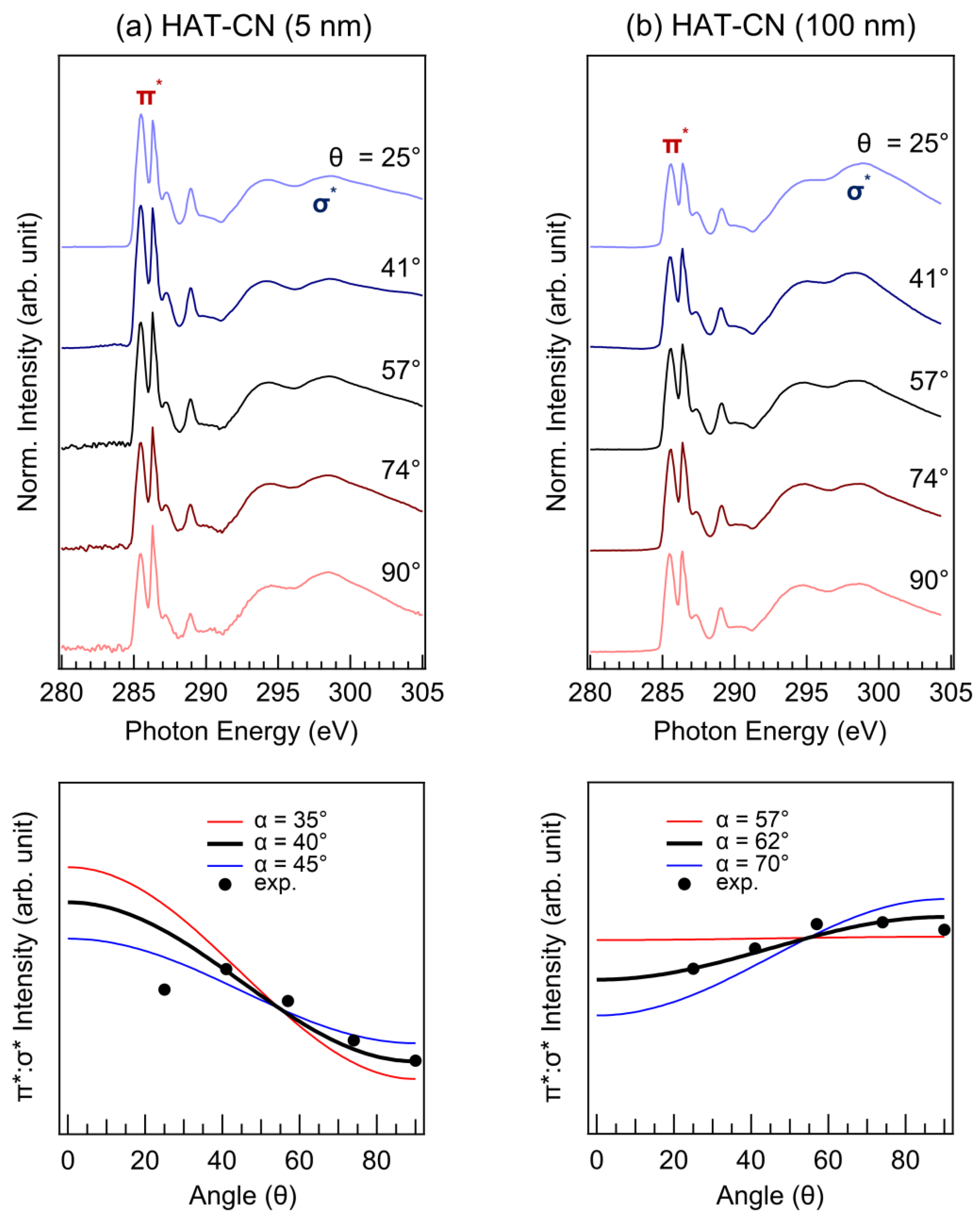
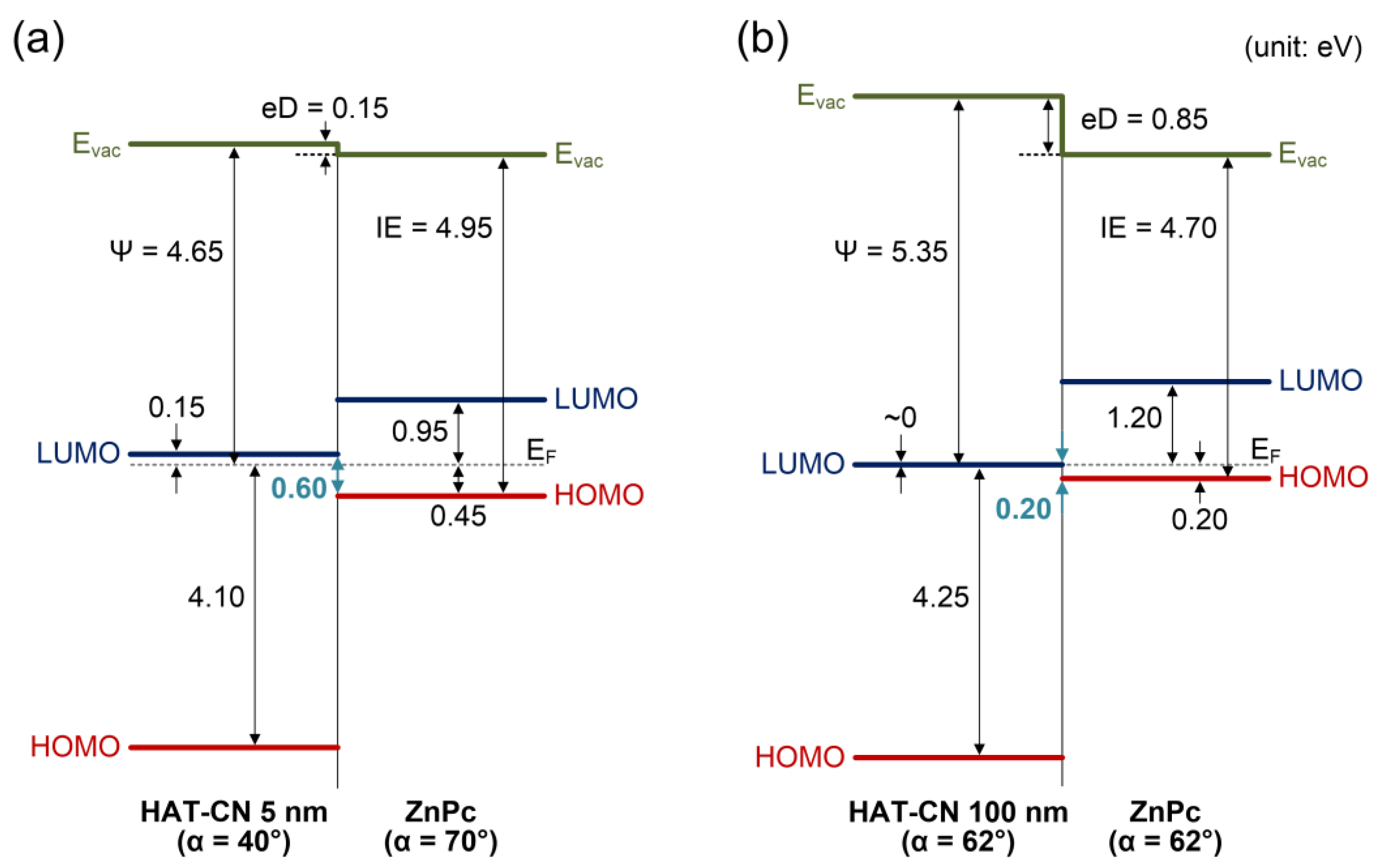
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Ling, H.; Liu, S.; Zheng, Z.; Yan, F. Organic flexible electronics. Small Methods 2018, 2, 1800070. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 2018, 8, 1701791. [Google Scholar] [CrossRef]
- Liu, K.; Ouyang, B.; Guo, X.; Guo, Y.; Liu, Y. Advances in flexible organic field-effect transistors and their applications for flexible electronics. NPJ Flex. Electron. 2022, 6, 1. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M. Conducting polymers for optoelectronic devices and organic solar cells: A review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Tavasli, A.; Gurunlu, B.; Gunturkun, D.; Isci, R.; Faraji, S. A review on solution-processed organic phototransistors and their recent developments. Electronics 2022, 11, 316. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy level alignment and interfacial electronic structures at organic/metal and organic/organic interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Kahn, A.; Koch, N.; Gao, W. Electronic structure and electrical properties of interfaces between metals and π-conjugated molecular films. J. Polym. Sci. B-Polym. Phys. 2003, 41, 2529–2548. [Google Scholar] [CrossRef]
- Koch, N. Organic electronic devices and their functional interfaces. ChemPhysChem 2007, 8, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-level alignment at organic/metal and organic/organic interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Lee, H.; Cho, S.W.; Yi, Y. Interfacial electronic structure for high performance organic devices. Curr. Appl. Phys. 2016, 16, 1533–1549. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, J.W.; Park, Y. Energy level alignment at a charge generation interface between 4,4′-bis(N-phenyl-1-naphthylamino)biphenyl and 1,4,5,8,9,11-hexaazatriphenylene-hexacarbonitrile. Appl. Phys. Lett. 2009, 94, 063305. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, Y.H.; Yi, Y.; Oh, H.-Y.; Kim, J.W. Insertion of an organic interlayer for hole current enhancement in inverted organic light emitting devices. Appl. Phys. Lett. 2010, 97, 063308. [Google Scholar] [CrossRef]
- Lee, H.; Cho, S.W.; Han, K.; Jeon, P.E.; Whang, C.-N.; Jeong, K.; Cho, K.; Yi, Y. The origin of the hole injection improvements at indium tin oxide/molybdenum trioxide/N,N′-bis(1-naphthyl)-N,N′-diphenyl-1,1′-biphenyl- 4,4′-diamine interfaces. Appl. Phys. Lett. 2008, 93, 043308. [Google Scholar] [CrossRef]
- Yi, Y.; Jeon, P.E.; Lee, H.; Han, K.; Kim, H.S.; Jeong, K.; Cho, S.W. The interface state assisted charge transport at the MoO3/metal interface. J. Chem. Phys. 2009, 130, 094704. [Google Scholar]
- Zhong, J.Q.; Mao, H.Y.; Wang, R.; Lin, J.D.; Zhao, Y.B.; Zhang, J.L.; Ma, D.G.; Chem, W. Ionization potential dependent air exposure effect on the MoO3/organic interface energy level alignment. Org. Electron. 2012, 13, 2793–2800. [Google Scholar] [CrossRef]
- Kröger, M.; Hamwi, S.; Meyer, J.; Riedl, T.; Kowalsky, W.; Kahn, A. Role of the deep-lying electronic states of MoO3 in the enhancement of of hole-injection in organic thin films. Appl. Phys. Lett. 2009, 95, 123301. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Park, S.; Yi, Y.; Cho, S.W.; Kim, J.W.; Kang, S.J. Hole injection enhancement of a single-walled carbon nanotube anode using an organic charge-generation layer. Carbon 2014, 71, 268–275. [Google Scholar] [CrossRef]
- Jeong, J.; Park, S.; Kang, S.J.; Lee, H.; Yi, Y. Impacts of molecular orientation on the hole injection barrier reduction: CuPc/HAT-CN/graphene. J. Phys. Chem. C 2016, 120, 2292–2298. [Google Scholar] [CrossRef]
- Yang, J.-P.; Bussolotti, F.; Li, Y.-Q.; Zeng, X.-H.; Kera, S.; Tang, J.-X.; Ueno, N. The role of gap states on energy level alignment at an α-NPD/HAT(CN)6 charge generation interface. Org. Electron. 2015, 24, 120–124. [Google Scholar] [CrossRef]
- Kim, J.-H.; Seo, J.; Kwon, D.-G.; Hong, J.-A.; Hwang, J.; Choi, H.K.; Moon, J.; Lee, J.-I.; Jung, D.Y.; Choi, S.-Y.; et al. Carrier injection efficiencies and energy level alignments of multilayer graphene anodes for organic light-emitting diodes with different hole injection layers. Carbon 2014, 79, 623–630. [Google Scholar] [CrossRef]
- Lin, H.-W.; Lin, W.-C.; Chang, J.-H.; Wu, C.-I. Solution-processed hexaazatriphenylene hexacarbonitrile as a universal hole-injection layer for organic light-emitting diodes. Org. Electron. 2013, 14, 1204–1210. [Google Scholar] [CrossRef]
- Duhm, S.; Heimel, G.; Salzmann, I.; Glowatzki, H.; Johnson, R.L.; Vollmer, A.; Rabe, P.J.; Koch, N. Orientation-dependent ionization energies and interface dipoles in ordered molecular assemblies. Nat. Mater. 2008, 7, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, H.; Chen, S.; Huang, Y.L.; Gao, X.Y.; Wee, A.T.S. Molecular orientation-dependent ionization potential of organic thin films. Chem. Mater. 2008, 20, 7017–7021. [Google Scholar] [CrossRef]
- Yamada, K.; Yanagisawa, S.; Koganezawa, T.; Mase, K.; Sato, N.; Yoshida, H. Impact of the molecular quadrupole moment on ionization energy and electron affinity of organic thin films: Experimental determination of electrostatic potential and electronic polarization energies. Phys. Rev. B 2018, 97, 245206. [Google Scholar] [CrossRef]
- Christodoulou, C.; Giannakopoulos, A.; Nardi, M.V.; Ligorio, G.; Oehzelt, M.; Chen, L.; Pasquali, L.; Timpel, M.; Giglia, A.; Nannarone, S.; et al. Tuning the work function of graphene-on-quartz with a high weight molecular acceptor. J. Phys. Chem. C 2014, 118, 4784–4790. [Google Scholar] [CrossRef]
- Cho, S.W.; Piper, L.F.J.; DeMasi, A.; Preston, A.R.H.; Smith, K.E.; Chauhan, K.V.; Sullivan, P.; Hatton, R.A.; Jones, T.S. Electronic structure of C60/phthalocyanine/ITO interfaces studied using soft X-ray spectroscopies. J. Phys. Chem. C 2010, 114, 1928–1933. [Google Scholar] [CrossRef]
- Kera, S.; Yamane, H.; Honda, H.; Fukagawa, H.; Okudaira, K.K.; Ueno, N. Photoelectron fine structures of uppermost valence band for well-characterized ClAl-phthalocyanine ultrathin film: UPS and MAES study. Surf. Sci. 2004, 566–568, 571–578. [Google Scholar] [CrossRef]
- Chen, W.; Qi, D.-C.; Huang, H.; Gao, X.; Wee, A.T.S. Organic–organic heterojunction interfaces: Effect of molecular orientation. Adv. Funct. Mater. 2011, 21, 410–424. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Dou, W.; Wang, P.; Ye, Q.; Yang, X.; Li, B.; Mao, H. Impact of graphene work function on the electronic structures at the interface between graphene and organic molecules. Nanomaterials 2019, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Maennig, B.; Pfeiffer, M.; Nollau, A.; Zhou, X.; Simon, P. Controlled p-type doping of polycrystalline and amorphous organic layers: Self-consistent description of conductivity and field-effect mobility by a microscopic percolation model. Phys. Rev. B 2001, 64, 195208. [Google Scholar] [CrossRef]
- Dey, A.; Singh, A.; Das, D.; Iyer, P.K. Photosensitive organic field effect transistors: The influence of ZnPc morphology and bilayer dielectrics for achieving a low operating voltage and low bias stress effect. Phys. Chem. Chem. Phys. 2016, 18, 32602–32609. [Google Scholar] [CrossRef] [PubMed]
- Warren, P.R.; Hardigree, J.F.M.; Lauritzen, A.E.; Nelson, J.; Riede, M. Tuning the ambipolar behaviour of organic field effect transistors via band engineering. AIP Adv. 2019, 9, 035202. [Google Scholar] [CrossRef]
- Kim, T.-M.; Kim, J.W.; Shim, H.-S.; Kim, J.-J. High efficiency and high photo-stability zinc-phthalocyanine based planar heterojunction solar cells with a double interfacial layer. Appl. Phys. Lett. 2012, 101, 113301. [Google Scholar] [CrossRef]
- Fleetham, T.; Choi, J.-Y.; Choi, H.W.; Alford, T.; Jeong, D.S.; Lee, T.S.; Lee, W.S.; Lee, K.-S.; Li, J.; Kim, I. Photocurrent enhancements of organic solar cells by altering dewetting of plasmonic Ag nanoparticles. Sci. Rep. 2015, 5, 14250. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.U.; Tahir, M.; Syed, W.A.; Aziz, F.; Wahab, F.; Said, S.M.; Sarker, M.R.; Ali, S.H.M.; Sabri, M.F.M. Fabrication and photovoltaic properties of organic solar cell based on zinc phthalocyanine. Energies 2020, 13, 962. [Google Scholar] [CrossRef]
- Braun, S.; De Jong, M.P.; Osikowicz, W.; Salaneck, W.R. Influence of the electrode work function on the energy level alignment at organic-organic interfaces. Appl. Phys. Lett. 2007, 91, 202108. [Google Scholar] [CrossRef]
- Oh, E.; Park, S.; Jeong, J.; Kang, S.J.; Lee, H.; Yi, Y. Energy level alignment at the interface of NPB/HAT-CN/graphene for flexible organic light-emitting diodes. Chem. Phys. Lett. 2017, 668, 64–68. [Google Scholar] [CrossRef]
- Kera, S.; Yabuuchi, Y.; Yamane, H.; Setoyama, H.; Okudaira, K.K.; Kahn, A.; Ueno, N. Impact of an interface dipole layer on molecular level alignment at an organic-conductor interface studied by ultraviolet photoemission spectroscopy. Phys. Rev. B 2004, 70, 085304. [Google Scholar] [CrossRef]
- Wang, Q.-K.; Wang, R.-B.; Shen, P.-F.; Li, C.; Li, Y.-Q.; Liu, L.-J.; Duhm, S.; Tang, J.-X. Energy level offsets at lead halide perovskite/organic hybrid interfaces and their impacts on charge separation. Adv. Mater. Interfaces 2015, 2, 1400528. [Google Scholar] [CrossRef]
- Jung, K.; Park, S.; Yoo, J.; Jung, N.E.; Moon, B.J.; Lee, S.H.; Yi, Y.; Lee, H. Elucidation of hole transport mechanism in efficient energy cascade organic photovoltaics using triple donor system. Appl. Surf. Sci. 2022, 576, 151747. [Google Scholar] [CrossRef]
- Grobosch, M.; Aristov, V.Y.; Molodtsova, O.V.; Schmidt, C.; Doyle, B.P.; Nannarone, S.; Knupfer, M. Engineering of the energy level alignment at organic semiconductor interfaces by intramolecular degrees of freedom: Transition metal phthalocyanines. J. Phys. Chem. C 2009, 113, 13219–13222. [Google Scholar] [CrossRef]
- Paoloni, D.; Di Filippo, G.; Cvetko, D.; Kladnik, G.; Morgante, A.; Ruocco, A. Strong chemical interaction and self-demetalation of zinc-phthalocyanine on Al(100). J. Phys. Chem. C 2020, 124, 22550–22558. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Jeong, K.; Yi, Y.; Lee, J.H.; Kim, J.W.; Cho, S.W. Hole injection enhancements of a CoPc and CoPc:NPB mixed layer in organic light-emitting devices. J. Phys. Chem. C 2012, 116, 13210–13216. [Google Scholar] [CrossRef]
- Wang, C.; Niu, D.; Xie, H.; Liu, B.; Wang, S.; Zhu, M.; Gao, Y. Electronic structures at the interface between CuPc and black phosphorus. J. Chem. Phys. 2017, 147, 064702. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, J. NEXAFS Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- McNeil, C.R.; Ade, H. Soft X-ray characterisation of organic semiconductor films. J. Mater. Chem. C 2013, 1, 187–201. [Google Scholar] [CrossRef]
- Cho, S.W.; DeMasi, A.; Preston, A.R.H.; Smith, K.E.; Piper, L.F.J.; Chauhan, K.V.; Jones, T.S. Probing the effect of relative molecular orientation on the photovoltaic device performance of an organic bilayer heterojunction using soft x-ray spectroscopies. Appl. Phys. Lett. 2012, 100, 263302. [Google Scholar] [CrossRef]
- Cho, S.W.; Newby, D.; DeMasi, A.; Smith, K.E.; Piper, L.F.J.; Jones, T.S. Determination of the individual atomic site contribution to the electronic structure of 3,4,9,10-perylene-tetracarboxylic-dianhydride (PTCDA). J. Chem. Phys. 2013, 139, 184711. [Google Scholar] [CrossRef]
- Chang, J.-F.; Shie, H.-S.; Yang, Y.-W.; Wang, C.-H. Study on correlation between structural and electronic properties of fluorinated oligothiophenes transistors by controlling film thickness. Crystals 2019, 9, 144. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, N.; Kim, J.; Im, Y.J.; Cho, S.W. Probing the molecular orientation of ZnPc on AZO using soft X-ray spectroscopies for organic photovoltaic applications. Appl. Sci. Converg. Technol. 2015, 24, 151–155. [Google Scholar] [CrossRef]
- Dimitrakopoulos, C.D.; Malenfant, P.R.L. Organic thin film transistors for large area electronics. Adv. Mater. 2002, 14, 99–117. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; Da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Charge transport in organic semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Thayer, G.E.; Sadowski, J.T.; Zu Heringdorf, F.M.; Sakurai, T.; Tromp, R.M. Role of surface electronic structure in thin film molecular ordering. Phys. Rev. Lett. 2005, 95, 256106. [Google Scholar] [CrossRef]
- Kowarik, S.; Gerlach, A.; Sellner, S.; Schreiber, F.; Cavalcanti, L.; Konovalov, O. Real-time observation of structural and orientational transitions during growth of organic thin films. Phys. Rev. Lett. 2006, 96, 125504. [Google Scholar] [CrossRef] [PubMed]
- Tietze, M.L.; Tress, W.; Pfützner, S.; Schünemann, C.; Burtone, L.; Riede, M.; Leo, K.; Vandewal, K.; Olthof, S.; Schulz, P.; et al. Correlation of open-circuit voltage and energy levels in zinc-phthalocyanine: C60 bulk heterojunction solar cells with varied mixing ratio. Phys. Rev. B 2013, 88, 085119. [Google Scholar] [CrossRef]
- Schlaf, R.; Parkinson, B.A.; Lee, P.A.; Nebesny, K.W.; Armstrong, N.R. HOMO/LUMO alignment at PTCDA/ZnPc and PTCDA/ClInPc heterointerfaces determined by combined UPS and XPS measurements. J. Phys. Chem. B 1999, 103, 2984–2992. [Google Scholar] [CrossRef]
- Deuermeier, J.; Fortunato, E.; Martins, R.; Klein, A. Energy band alignment at the nanoscale. Appl. Phys. Lett. 2017, 110, 051603. [Google Scholar] [CrossRef]
- Yoo, J.; Jung, K.; Jeong, J.; Hyun, G.; Lee, H.; Yi, Y. Energy level alignment at C60/DTDCTB/PEDOT:PSS interfaces in organic photovoltaics. Appl. Surf. Sci. 2017, 402, 41–46. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, E.; Hur, J.W.; Ko, J.Y.; Kim, T.G.; Hwang, J.Y.; Smith, K.E.; Lee, H.; Cho, S.W. Effects of HAT-CN Layer Thickness on Molecular Orientation and Energy-Level Alignment with ZnPc. Molecules 2023, 28, 3821. https://doi.org/10.3390/molecules28093821
Joo E, Hur JW, Ko JY, Kim TG, Hwang JY, Smith KE, Lee H, Cho SW. Effects of HAT-CN Layer Thickness on Molecular Orientation and Energy-Level Alignment with ZnPc. Molecules. 2023; 28(9):3821. https://doi.org/10.3390/molecules28093821
Chicago/Turabian StyleJoo, Eunah, Jin Woo Hur, Joon Young Ko, Tae Gyun Kim, Jung Yeon Hwang, Kevin E. Smith, Hyunbok Lee, and Sang Wan Cho. 2023. "Effects of HAT-CN Layer Thickness on Molecular Orientation and Energy-Level Alignment with ZnPc" Molecules 28, no. 9: 3821. https://doi.org/10.3390/molecules28093821
APA StyleJoo, E., Hur, J. W., Ko, J. Y., Kim, T. G., Hwang, J. Y., Smith, K. E., Lee, H., & Cho, S. W. (2023). Effects of HAT-CN Layer Thickness on Molecular Orientation and Energy-Level Alignment with ZnPc. Molecules, 28(9), 3821. https://doi.org/10.3390/molecules28093821







