Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications
Abstract
:1. Introduction
2. Preparation Methods of Cadmium Sulfide Nanoparticles
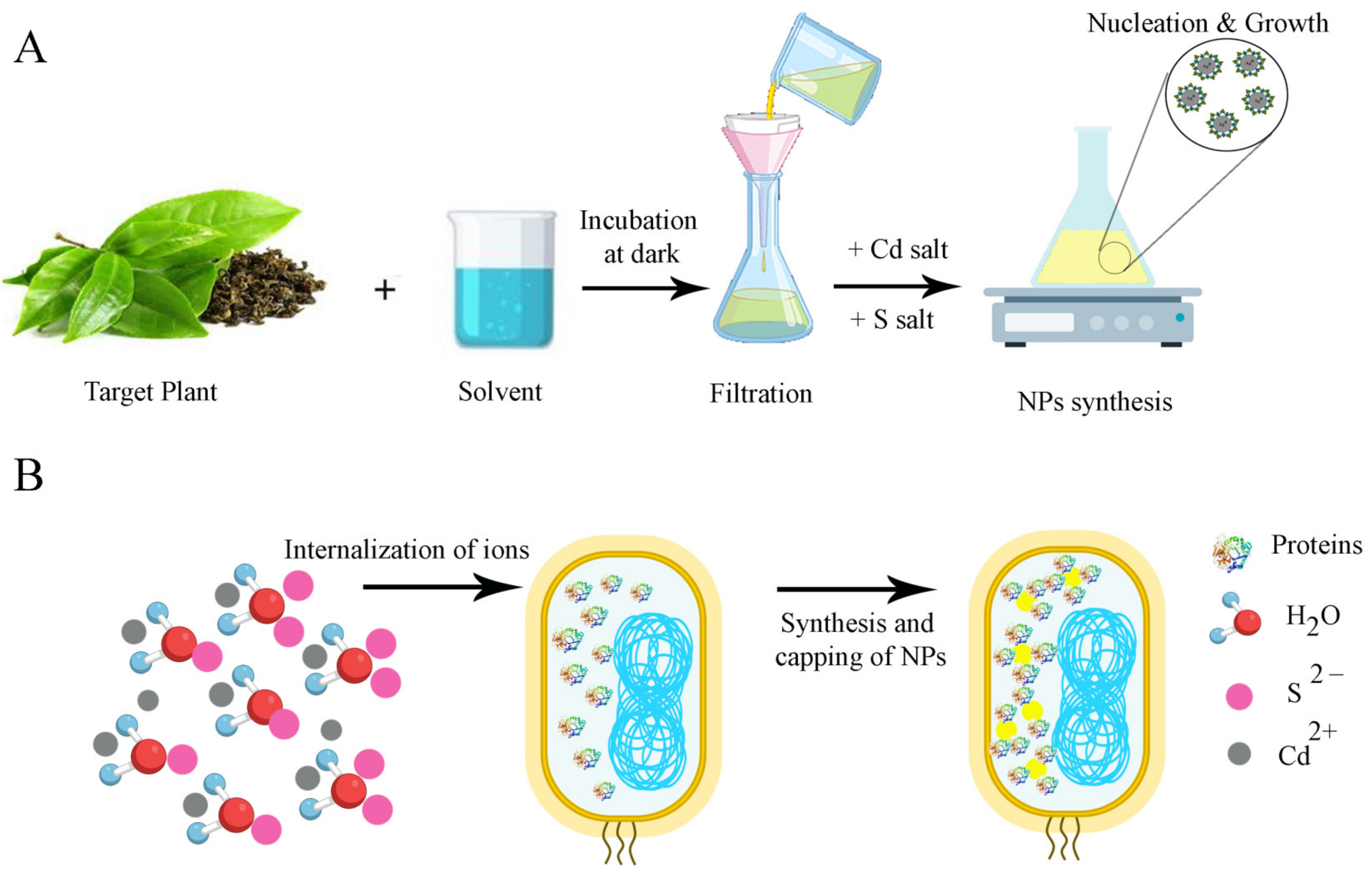
2.1. Biogenic Methods
2.2. Chemical Methods
2.2.1. Chemical Precipitation Method
2.2.2. Wet Chemical Synthesis
2.2.3. Solvothermal Synthesis
2.2.4. Chemical Reduction Method
2.2.5. Thermal Decomposition Technique
2.2.6. Sol–Gel Method
2.2.7. Sonochemical Method
2.2.8. Combustion
2.2.9. Micro-Emulsion Method
2.3. Physical Methods
2.3.1. Pulsed-Laser Ablation
2.3.2. Hydrothermal Method
2.3.3. Microwave-Assisted Method
2.3.4. Reflux Method
2.4. Physicochemical Method
Mechanochemical Processes
3. Characterization Methods of CdS NPs
4. Biomedical Applications
4.1. Anticancer Activity
4.2. Antimicrobial Activity
4.3. Bioimaging Application
4.4. Biosensor Application
5. Conclusions and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A Review on Biosynthesis of Metal Nanoparticles and Its Environmental Applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Saqib, S.; Faryad, S.; Afridi, M.I.; Arshad, B.; Younas, M.; Naeem, M.; Zaman, W.; Ullah, F.; Nisar, M.; Ali, S.; et al. Bimetallic Assembled Silver Nanoparticles Impregnated in Aspergillus Fumigatus Extract Damage the Bacterial Membrane Surface and Release Cellular Contents. Coatings 2022, 12, 1505. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Asghar, M.; Habib, S.; Zaman, W.; Hussain, S.; Ali, H.; Saqib, S. Synthesis and Characterization of Microbial Mediated Cadmium Oxide Nanoparticles. Microsc. Res. Tech. 2020, 83, 1574–1584. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Sedighi, M.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Kettiger, H.; Einfalt, T.; Huwyler, J.; Witzigmann, D. Controlled Tyrosine Kinase Inhibitor Delivery to Liver Cancer Cells by Gate-Capped Mesoporous Silica Nanoparticles. ACS Appl. Bio Mater. 2020, 3, 239–251. [Google Scholar] [CrossRef]
- Sedighi, M.; Sieber, S.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Huwyler, J.; Witzigmann, D. Rapid Optimization of Liposome Characteristics Using a Combined Microfluidics and Design-of-Experiment Approach. Drug. Deliv. Transl. Res. 2019, 9, 404–413. [Google Scholar] [CrossRef]
- Saqib, S.; Nazeer, A.; Ali, M.; Zaman, W.; Younas, M.; Shahzad, A.; Sunera; Nisar, M. Catalytic Potential of Endophytes Facilitates Synthesis of Biometallic Zinc Oxide Nanoparticles for Agricultural Application. Biometals 2022, 35, 967–985. [Google Scholar] [CrossRef]
- Titus, D.; James Jebaseelan Samuel, E.; Roopan, S.M. Chapter 12—Nanoparticle Characterization Techniques. In Green. Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–319. ISBN 978-0-08-102579-6. [Google Scholar]
- Dehghan, H.; Sedighi, M.; Jafari-Nozad, A.M.; Jafari, S.; Alemzadeh, E.; Farkhondeh, T.; Samarghandian, S. Gold Nanoparticles and Wound Healing in Rodents: A Systematic Study. Curr. Nanosci. 2023, 19, 1–10. [Google Scholar]
- Zheng, X.; Liu, Y.; Yang, Y.; Song, Y.; Deng, P.; Li, J.; Liu, W.; Shen, Y.; Tian, X. Recent Advances in Cadmium Sulfide-Based Photocatalysts for Photocatalytic Hydrogen Evolution. Renewables 2023, 1, 39–56. [Google Scholar] [CrossRef]
- Dabhane, H.; Ghotekar, S.; Tambade, P.; Pansambal, S.; Murthy, H.A.; Oza, R.; Medhane, V. A Review on Environmentally Benevolent Synthesis of CdS Nanoparticle and Their Applications. Environ. Chem. Ecotoxicol. 2021, 3, 209–219. [Google Scholar] [CrossRef]
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2023, 13, 36. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cadmium Pollution on Food Safety and Human Health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Huang, M.; Liu, C.; Cui, P.; Wu, T.; Feng, X.; Huang, H.; Zhou, J.; Wang, Y. Facet-Dependent Photoinduced Transformation of Cadmium Sulfide (CdS) Nanoparticles. Environ. Sci. Technol. 2021, 55, 13132–13141. [Google Scholar] [CrossRef]
- Qin, Z.; Yue, Q.; Liang, Y.; Zhang, J.; Zhou, L.; Hidalgo, O.B.; Liu, X. Extracellular Biosynthesis of Biocompatible Cadmium Sulfide Quantum Dots Using Trametes Versicolor. J. Biotechnol. 2018, 284, 52–56. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, L.; Li, Z.; An, W.; Liu, D.; Lin, J.; Tian, L.; Wang, X.; Liu, B.; Qi, W.; et al. The Potential Application of Raw Cadmium Sulfide Nanoparticles as CT Photographic Developer. Nanoscale Res. Lett. 2016, 11, 232. [Google Scholar] [CrossRef]
- Varmazyari, A.; Baris, O. Rapid Biosynthesis of Cadmium Sulfide (CdS) Nanoparticles Using Culture Supernatants of Viridibacillus Arenosi K64. BioNanoSci. 2022, 12, 191–202. [Google Scholar] [CrossRef]
- Shakibaie, M.; Riahi-Madvar, S.; Ameri, A.; Amiri-Moghadam, P.; Adeli-Sardou, M.; Forootanfar, H. Microwave Assisted Biosynthesis of Cadmium Nanoparticles: Characterization, Antioxidant and Cytotoxicity Studies. J. Clust. Sci. 2022, 33, 1877–1887. [Google Scholar] [CrossRef]
- Shivashankarappa, A.; Sanjay, K.R. Escherichia Coli-Based Synthesis of Cadmium Sulfide Nanoparticles, Characterization, Antimicrobial and Cytotoxicity Studies. Braz. J. Microbiol. 2020, 51, 939–948. [Google Scholar] [CrossRef]
- Jameel, M.; Rauf, M.A.; Khan, M.T.; Farooqi, M.K.; Alam, M.A.; Mashkoor, F.; Shoeb, M.; Jeong, C. Ingestion and Effects of Green Synthesized Cadmium Sulphide Nanoparticle on Spodoptera Litura as an Insecticidal and Their Antimicrobial and Anticancer Activities. Pestic. Biochem. Physiol. 2023, 190, 105332. [Google Scholar] [CrossRef]
- Harish, R.; Nisha, K.D.; Prabakaran, S.; Sridevi, B.; Harish, S.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y.; Vinniee, C.; Ganesh, M.R. Cytotoxicity Assessment of Chitosan Coated CdS Nanoparticles for Bio-Imaging Applications. Appl. Surf. Sci. 2020, 499, 143817. [Google Scholar] [CrossRef]
- Ramesh, S.; Narayanan, V. Wet Chemical Synthesis of Cadmium Sulphide Nanoparticles and Its Characterization. Chem. Sci. Trans. 2013, 2, S192–S194. [Google Scholar]
- Emami Moghaddam, S.A.; Ghadam, P.; Rahimzadeh, F. Biosynthesis of Cadmium Sulfide Nanoparticles Using Aqueous Extract of Lactobacillus Acidophilus along with Its Improvement by Response Surface Methodology. J. Clean. Prod. 2022, 356, 131848. [Google Scholar] [CrossRef]
- Sankhla, A.; Sharma, R.; Yadav, R.S.; Kashyap, D.; Kothari, S.L.; Kachhwaha, S. Biosynthesis and Characterization of Cadmium Sulfide Nanoparticles—An Emphasis of Zeta Potential Behavior Due to Capping. Mater. Chem. Phys. 2016, 170, 44–51. [Google Scholar] [CrossRef]
- El-Baz, A.F.; Sorour, N.M.; Shetaia, Y.M. Trichosporon Jirovecii–Mediated Synthesis of Cadmium Sulfide Nanoparticles. J. Basic. Microbiol. 2016, 56, 520–530. [Google Scholar] [CrossRef]
- Varmazyari, A.; Taghizadehghalehjoughi, A.; Baris, O.; Yilmaz, A.; Hacimuftuoglu, A. The Evaluation of the Cortex Neurons Viability in CdS Nanoparticles Induced Toxicity. Nanomed. J. 2021, 8, 211–219. [Google Scholar]
- Wang, B.; Zeng, C.; Chu, K.H.; Wu, D.; Yip, H.Y.; Ye, L.; Wong, P.K. Enhanced Biological Hydrogen Production from Escherichia Coli with Surface Precipitated Cadmium Sulfide Nanoparticles. Adv. Energy Mater. 2017, 7, 1700611. [Google Scholar] [CrossRef]
- Sandoval Cárdenas, D.I.; Gomez-Ramirez, M.; Rojas-Avelizapa, N.G.; Vidales-Hurtado, M.A. Synthesis of Cadmium Sulfide Nanoparticles by Biomass of Fusarium Oxysporum f. Sp. Lycopersici. J. Nano Res. 2017, 46, 179–191. [Google Scholar] [CrossRef]
- Sakpirom, J.; Kantachote, D.; Siripattanakul-Ratpukdi, S.; McEvoy, J.; Khan, E. Simultaneous Bioprecipitation of Cadmium to Cadmium Sulfide Nanoparticles and Nitrogen Fixation by Rhodopseudomonas Palustris TN110. Chemosphere 2019, 223, 455–464. [Google Scholar] [CrossRef]
- Shivashankarappa, A.; Sanjay, K.R. Study on Biological Synthesis of Cadmium Sulfide Nanoparticles by Bacillus Licheniformis and Its Antimicrobial Properties against Food Borne Pathogens. Nanosci. Nanotechnol. Res. 2015, 3, 6–15. [Google Scholar]
- Wang, L.; Chen, S.; Ding, Y.; Zhu, Q.; Zhang, N.; Yu, S. Biofabrication of Morphology Improved Cadmium Sulfide Nanoparticles Using Shewanella Oneidensis Bacterial Cells and Ionic Liquid: For Toxicity against Brain Cancer Cell Lines. J. Photochem. Photobiol. B Biol. 2018, 178, 424–427. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Elbaz, A.F.; El Badawy-, S.; Moussa, S.H. Anticancer and Antibacterial Activity of Cadmium Sulfide Nanoparticles by Aspergillus Niger. Adv. Polym. Technol. 2020, 2020, e4909054. [Google Scholar] [CrossRef]
- Ma, N.; Sun, C. Cadmium Sulfide Nanoparticle Biomineralization and Biofilm Formation Mediate Cadmium Resistance of the Deep-Sea Bacterium Pseudoalteromonas Sp. MT33b. Environ. Microbiol. Rep. 2021, 13, 325–336. [Google Scholar] [CrossRef]
- Abd Elsalam, S.S.; Taha, R.H.; Tawfeik, A.M.; El-Monem, A.; Mohamed, O.; Mahmoud, H.A. Antimicrobial Activity of Bio and Chemical Synthesized Cadmium Sulfide Nanoparticles. Egypt. J. Hosp. Med. 2018, 70, 1494–1507. [Google Scholar] [CrossRef]
- Tandon, S.; Vats, S. Microbial Biosynthesis of Cadmium Sulfide (Cds) Nanoparticles and Their Characterization. Eur. J. Pharm. Med. Res. 2016, 3, 545–550. [Google Scholar]
- Sekar, P.V.; Parvathi, V.D.; Sumitha, R. Green Nanotechnology in Cadmium Sulphide Nanoparticles and Understanding Its Toxicity and Antimicrobial Properties. Biomed. Res. 2019, 30, 805–809. [Google Scholar] [CrossRef]
- Ullah, A.; Rasheed, S.; Ali, I.; Ullah, N. Plant Mediated Synthesis of CdS Nanoparticles, Their Characterization and Application for Photocatalytic Degradation of Toxic Organic Dye. Chem. Rev. Lett. 2021, 4, 98–107. [Google Scholar] [CrossRef]
- Haq Bhat, I.U.; Yi, Y.S. Green Synthesis and Antibacterial Activity of Cadmium Sulfide Nanoparticles (CdSNPs) Using Panicum Sarmentosum. Asian J. Green Chem. 2019, 3, 455–469. [Google Scholar] [CrossRef]
- Shivaji, K.; Mani, S.; Ponmurugan, P.; Castro, C.S.D.; Davies, M.L.; Balasubramanian, M.G.; Pitchaimuthu, S. Green-Synthesis-Derived CdS Quantum Dots Using Tea Leaf Extract: Antimicrobial, Bioimaging, and Therapeutic Applications in Lung Cancer Cells. ACS Appl. Nano Mater. 2018, 1, 1683–1693. [Google Scholar] [CrossRef]
- Qutub, N.; Pirzada, B.M.; Umar, K.; Sabir, S. Synthesis of CdS Nanoparticles Using Different Sulfide Ion Precursors: Formation Mechanism and Photocatalytic Degradation of Acid Blue-29. J. Environ. Chem. Eng. 2016, 4, 808–817. [Google Scholar] [CrossRef]
- Mir, F.A.; Chattarjee, I.; Dar, A.A.; Asokan, K.; Bhat, G.M. Preparation and Characterizations of Cadmium Sulfide Nanoparticles. Optik 2015, 126, 1240–1244. [Google Scholar] [CrossRef]
- Devi, R.A.; Latha, M.; Velumani, S.; Oza, G.; Reyes-Figueroa, P.; Rohini, M.; Becerril-Juarez, I.G.; Lee, J.-H.; Yi, J. Synthesis and Characterization of Cadmium Sulfide Nanoparticles by Chemical Precipitation Method. J. Nanosci. Nanotechnol. 2015, 15, 8434–8439. [Google Scholar] [CrossRef]
- Senobari, S.; Nezamzadeh-Ejhieh, A. A Comprehensive Study on the Photocatalytic Activity of Coupled Copper Oxide-Cadmium Sulfide Nanoparticles. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 196, 334–343. [Google Scholar] [CrossRef]
- Bangi, U.K.M. Impact of Cadmium Salt Concentration on CdS Nanoparticles Synthesized by Chemical Precipitation Method. Chalcogenide Lett. 2020, 17, 537–547. [Google Scholar]
- Sheikh, H.N.; Khajuria, S.; Sanotra, S.; Khajuria, H.; Singh, A. Synthesis, Structural and Optical Characterization of Copper and Rare Earth Doped CdS Nanoparticles. Acta Chim. Slov. 2016, 63, 104–112. [Google Scholar] [CrossRef]
- Lacave, J.M.; Bilbao, E.; Gilliland, D.; Mura, F.; Dini, L.; Cajaraville, M.P.; Orbea, A. Bioaccumulation, Cellular and Molecular Effects in Adult Zebrafish after Exposure to Cadmium Sulphide Nanoparticles and to Ionic Cadmium. Chemosphere 2020, 238, 124588. [Google Scholar] [CrossRef]
- Dong, J.; Wu, J.; Jia, J.; Fan, L.; Lin, Y.; Lin, J.; Huang, M. Efficient Perovskite Solar Cells Employing a Simply-Processed CdS Electron Transport Layer. J. Mater. Chem. C 2017, 5, 10023–10028. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Saeed, S.R.; Abdullah, O.G. Synthesis and Characterization of CdS Nanoparticles Grown in a Polymer Solution Using In-Situ Chemical Reduction Technique. Int. J. Electrochem. Sci. 2017, 12, 3263–3274. [Google Scholar] [CrossRef]
- Gaur, R.; Jeevanandam, P. Effect of Anions on the Morphology of CdS Nanoparticles Prepared via Thermal Decomposition of Different Cadmium Thiourea Complexes in a Solvent and in the Solid State. N. J. Chem. 2015, 39, 9442–9453. [Google Scholar] [CrossRef]
- Gaur, R.; Jeevanandam, P. Evolution of Different Morphologies of CdS Nanoparticles by Thermal Decomposition of Bis(Thiourea)Cadmium Chloride in Various Solvents. J. Nanopart Res. 2015, 17, 156. [Google Scholar] [CrossRef]
- Seyghalkar, H.; Sabet, M.; Salavati-Niasari, M. Synthesis and Characterization of Cadmium Sulfide Nanoparticles via a Simple Thermal Decompose Method. High. Temp. Mater. Process. 2016, 35, 1013–1016. [Google Scholar] [CrossRef]
- Sankar, M.; Jothibas, M.; Muthuvel, A.; Rajeshwari, A.; Jeyakumar, S.J. Structural, Optical and Photocatalytic Degradation of Organic Dyes by Sol Gel Prepared Ni Doped CdS Nanoparticles. Surf. Interfaces 2020, 21, 100775. [Google Scholar] [CrossRef]
- Arya, S.; Sharma, A.; Singh, A.; Ahmed, A.; Mahajan, S. Preparation of CdS and CdS@Zn3(PO4)2 Nanocomposites by Sol-Gel Method: DFT Study and Effect of Temperature on Band Gap. Russ. J. Inorg. Chem. 2020, 65, 1424–1435. [Google Scholar] [CrossRef]
- Mahdi, H.S.; Parveen, A.; Azam, A. Microstructural and Optical Properties of Ni Doped CdS Nanoparticles Synthesized by Sol Gel Route. Mater. Today Proc. 2018, 5, 20636–20640. [Google Scholar] [CrossRef]
- Martínez-Alonso, C.; Cortina-Marrero, H.J.; Selene Coria-Monroy, C.; Arenas, M.C.; Nicho, M.E.; Hu, H. Solution Synthesized CdS Nanoparticles for Hybrid Solar Cell Applications. J. Mater. Sci Mater. Electron. 2015, 26, 5539–5545. [Google Scholar] [CrossRef]
- Bozkurt, P.A.; Derkuş, B. Synthesis and characterization of CdS nanorods by combined sonochemical-solvothermal method. Mater. Sci. -Pol. 2016, 34, 684–690. [Google Scholar] [CrossRef]
- Palanisamy, B.; Paul, B.; Chang, C. The Synthesis of Cadmium Sulfide Nanoplatelets Using a Novel Continuous Flow Sonochemical Reactor. Ultrason. Sonochem. 2015, 26, 452–460. [Google Scholar] [CrossRef]
- Ayodhya, D.; Venkatesham, M.; Kumari, A.S.; Bhagavanth Reddy, G.; Veerabhadram, G. One-Pot Sonochemical Synthesis of CdS Nanoparticles: Photocatalytic and Electrical Properties. Int. J. Ind. Chem. 2015, 6, 261–271. [Google Scholar] [CrossRef]
- Zulkafli, R.; Othman, N.K.; Hamid, M.A.A.; Jalar, A. Synthesis of CdS Nanoparticles via AOT-Water-n-Heptane Microemulsion Technique. Mater. Sci. Forum 2013, 756, 85–90. [Google Scholar] [CrossRef]
- García Guillén, G.; Zuñiga Ibarra, V.A.; Mendivil Palma, M.I.; Krishnan, B.; Avellaneda Avellaneda, D.; Shaji, S. Effects of Liquid Medium and Ablation Wavelength on the Properties of Cadmium Sulfide Nanoparticles Formed by Pulsed-Laser Ablation. ChemPhysChem 2017, 18, 1035–1046. [Google Scholar] [CrossRef]
- Abd, A.N.; Ismail, R.A.; Habubi, N.F. Characterization of CdS Nanoparticles Prepared by Laser Ablation in Methanol. J. Mater. Sci. Mater. Electron. 2015, 26, 9853–9858. [Google Scholar] [CrossRef]
- Goncharova, D.A.; Lapin, I.N.; Svetlichnyi, V.A. Synthesis of CdS Nanoparticles by Laser Ablation of Metallic Cadmium Target in Presence Different Precursors. Adv. Mater. Res. 2015, 1085, 182–186. [Google Scholar] [CrossRef]
- AL-Mamoori, M.H.K.; Mahdi, D.K.; Alshrefi, S.M. Synthesis and Spectroscopic Study of CdS Nanoparticles Using Hydrothermal Method. AIP Conf. Proc. 2018, 1968, 030011. [Google Scholar] [CrossRef]
- Cheng, G.; Ding, H.; Chen, G.; Shi, H.; Zhang, X.; Zhu, M.; Tan, W. Effects of Cadmium Sulfide Nanoparticles on Sulfate Bioreduction and Oxidative Stress in Desulfovibrio Desulfuricans. Bioresour. Bioprocess. 2022, 9, 35. [Google Scholar] [CrossRef]
- Zhong, W.; Tu, W.; Feng, S.; Xu, A. Photocatalytic H2 Evolution on CdS Nanoparticles by Loading FeSe Nanorods as Co-Catalyst under Visible Light Irradiation. J. Alloys Compd. 2019, 772, 669–674. [Google Scholar] [CrossRef]
- Choubey, S.K.; Tiwary, K.P. Structural, Morphological and Optical Investigation of CdS Nanoparticles Synthesized by Microwave Assisted Method. Dig. J. Nanomater. Biostruct. 2016, 11, 33–37. [Google Scholar]
- Darwish, M.; Mohammadi, A.; Assi, N. Microwave-Assisted Polyol Synthesis and Characterization of Pvp-Capped Cds Nanoparticles for the Photocatalytic Degradation of Tartrazine. Mater. Res. Bull. 2016, 74, 387–396. [Google Scholar] [CrossRef]
- Khan, A.; Shkir, M.; Manthrammel, M.A.; Ganesh, V.; Yahia, I.S.; Ahmed, M.; El-Toni, A.M.; Aldalbahi, A.; Ghaithan, H.; AlFaify, S. Effect of Gd Doping on Structural, Optical Properties, Photoluminescence and Electrical Characteristics of CdS Nanoparticles for Optoelectronics. Ceram. Int. 2019, 45, 10133–10141. [Google Scholar] [CrossRef]
- Bharti, D.B.; Bharati, A.V.; Wankhade, A.V. Synthesis, Characterization and Optical Property Investigation of CdS Nanoparticles. Luminescence 2018, 33, 1445–1449. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Yunus, R.B.; Navaseri, M. Characterization of CdS Nanoparticles Synthesized Using Microwave-Assisted Polyol Method. Adv. Mater. Res. 2013, 667, 122–127. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Chalapathi, U.; Kumar, M.; Subramanyam, K.; Vattikuti, S.V.P.; Pratap Reddy, M.S.; Park, S.-H. Enhanced Photocatalytic Activity and Hydrogen Evolution of CdS Nanoparticles through Er Doping. Ceram. Int. 2020, 46, 21728–21735. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Ai, Z.; Wu, L.; Zhang, Q. Mechanochemical Synthesis of CdS/MgAl LDH-Precursor as Improved Visible-Light Driven Photocatalyst for Organic Dye. Appl. Clay Sci. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Dutková, E.; Zorkovská, A.; Kováč, J.; Hronec, P.; Kováč, J.; Čaplovičová, M.; Mojžiš, J.; Mojžišová, G.; et al. CdS/ZnS Nanocomposites: From Mechanochemical Synthesis to Cytotoxicity Issues. Mater. Sci. Eng. C 2016, 58, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Shalabayev, Z.; Baláž, M.; Khan, N.; Nurlan, Y.; Augustyniak, A.; Daneu, N.; Tatykayev, B.; Dutková, E.; Burashev, G.; Casas-Luna, M.; et al. Sustainable Synthesis of Cadmium Sulfide, with Applicability in Photocatalysis, Hydrogen Production, and as an Antibacterial Agent, Using Two Mechanochemical Protocols. Nanomaterials 2022, 12, 1250. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Amin, T.; Katuva, S.; Kumari, M.; Selvaraj, K. Synthesis of Water Soluble CdS Nanoparticles and Study of Their DNA Damage Activity. Arab. J. Chem. 2017, 10, S3929–S3935. [Google Scholar] [CrossRef]
- MubarakAli, D.; Gopinath, V.; Rameshbabu, N.; Thajuddin, N. Synthesis and Characterization of CdS Nanoparticles Using C-Phycoerythrin from the Marine Cyanobacteria. Mater. Lett. 2012, 74, 8–11. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus Bisporus Mediated Biosynthesis of Copper Nanoparticles and Its Biological Effects: An in-Vitro Study. Colloid. Interface Sci. Commun. 2020, 35, 100254. [Google Scholar] [CrossRef]
- Rose, M.M.; Sheela Christy, R.; Asenath Benitta, T.; Thampi Thanka Kumaran, J. Phase Transitions in Cadmium Sulfide Nanoparticles. AIP Adv. 2021, 11, 085129. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, B.; Wu, Y.; Li, M.; Liu, X.; Ding, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. Facile High Throughput Wet-Chemical Synthesis Approach Using a Microfluidic-Based Composition and Temperature Controlling Platform. Front. Chem. 2020, 8, 579828. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Wu, X.-J.; Li, L.; Liu, J.; Zhang, H. Wet-Chemical Synthesis and Applications of Semiconductor Nanomaterial-Based Epitaxial Heterostructures. Nano-Micro Lett. 2019, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Dalvand, P.; Mohammadi, M.R. Controlling Morphology and Structure of Nanocrystalline Cadmium Sulfide (CdS) by Tailoring Solvothermal Processing Parameters. J. Nanopart Res. 2011, 13, 3011–3018. [Google Scholar] [CrossRef]
- Haider, M.S.; Badejo, A.C.; Shao, G.N.; Imran, S.M.; Abbas, N.; Chai, Y.G.; Hussain, M.; Kim, H.T. Sequential Repetitive Chemical Reduction Technique to Study Size-Property Relationships of Graphene Attached Ag Nanoparticle. Solid. State Sci. 2015, 44, 1–9. [Google Scholar] [CrossRef]
- Lopes, P.A.L.; Brandão Santos, M.; Mascarenhas, A.J.S.; Silva, L.A. Synthesis of CdS Nano-Spheres by a Simple and Fast Sonochemical Method at Room Temperature. Mater. Lett. 2014, 136, 111–113. [Google Scholar] [CrossRef]
- Liu, J.; Pu, X.; Zhang, D.; Seo, H.J.; Du, K.; Cai, P. Combustion Synthesis of CdS/Reduced Graphene Oxide Composites and Their Photocatalytic Properties. Mater. Res. Bull. 2014, 57, 29–34. [Google Scholar] [CrossRef]
- Manikandan, A.; Antony, S.A. A Novel Approach for the Synthesis and Characterization Studies of Mn 2+-Doped CdS Nanocrystals by a Facile Microwave-Assisted Combustion Method. J. Supercond. Nov. Magn. 2014, 27, 2725–2733. [Google Scholar] [CrossRef]
- Khan, J.; Ullah, H.; Sajjad, M.; Ali, A.; Thebo, K.H. Synthesis, Characterization and Electrochemical Performance of Cobalt Fluoride Nanoparticles by Reverse Micro-Emulsion Method. Inorg. Chem. Commun. 2018, 98, 132–140. [Google Scholar] [CrossRef]
- Wolf, S.; Feldmann, C. Microemulsions: Options To Expand the Synthesis of Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 15728–15752. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.H.; Ghows, N. Micro-Emulsion under Ultrasound Facilitates the Fast Synthesis of Quantum Dots of CdS at Low Temperature. Ultrason. Sonochem. 2011, 18, 127–134. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 295–328. ISBN 978-3-319-15338-4. [Google Scholar]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020, e8917013. [Google Scholar] [CrossRef]
- Byeon, J.H.; Kim, Y.-W. A Novel Polyol Method to Synthesize Colloidal Silver Nanoparticles by Ultrasonic Irradiation. Ultrason. Sonochem. 2012, 19, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, Y.-C.; Feldmann, C. Polyol Synthesis of Nanoparticles: Status and Options Regarding Metals, Oxides, Chalcogenides, and Non-Metal Elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- Aditha, S.K.; Kurdekar, A.D.; Chunduri, L.A.A.; Patnaik, S.; Kamisetti, V. Aqueous Based Reflux Method for Green Synthesis of Nanostructures: Application in CZTS Synthesis. MethodsX 2016, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. Rapid Mechanochemical Synthesis of Two New Cd(II)-Based Metal–Organic Frameworks with High Removal Efficiency of Congo Red. CrystEngComm 2014, 17, 686–692. [Google Scholar] [CrossRef]
- Gamboa, S.M.; Rojas, E.R.; Martínez, V.V.; Vega-Baudrit, J. Synthesis and Characterization of Silver Nanoparticles and Their Application as an Antibacterial Agent. Int. J. Biosen. Bioelectron. 2019, 5, 166–173. [Google Scholar]
- De La Cruz Terrazas, E.C.; Lazaro, R.A.; Gonzalez, M.M.; Luque, P.A.; Castillo, S.J.; Carrillo-Castillo, A. A simple method for the synthesis of cds nanoparticles using a novel surfactant. Chalcogenide Lett. 2015, 12, 147–153. [Google Scholar]
- Kouhbanani, M.A.J.; Beheshtkhoo, N.; Nasirmoghadas, P.; Yazdanpanah, S.; Zomorodianc, K.; Taghizadeh, S.; Amani, A.M. Green Synthesis of Spherical Silver Nanoparticles Using Ducrosia Anethifolia Aqueous Extract and Its Antibacterial Activity. J. Environ. Treat. Tech. 2019, 7, 461–466. [Google Scholar]
- Maver, U.; Velnar, T.; Gaberšček, M.; Planinšek, O.; Finšgar, M. Recent Progressive Use of Atomic Force Microscopy in Biomedical Applications. TrAC Trends Anal. Chem. 2016, 80, 96–111. [Google Scholar] [CrossRef]
- Cruz, G.G.D.L.; Rodriguez-Fragoso, P.; Mastache-Juarez, A.; Reyes-Esparza, J.; Lourdes, L.R.F. Doxorubicin-Bioconjugated Cadmium Sulfide Dextrin Quantum Dots for Imaging Cells. Indian. J. Pharm. Sci. 2020, 82, 230–241. [Google Scholar] [CrossRef]
- Hamid Rather, A.; Umair Wani, T.; Saleem Khan, R.; Abdal-hay, A.; Rather, S.; Macossay, J.; Sheikh, F.A. Recent Progress in the Green Fabrication of Cadmium Sulfide and Cadmium Oxide Nanoparticles: Synthesis, Antimicrobial and Cytotoxic Studies. Mater. Sci. Eng. B 2022, 286, 116022. [Google Scholar] [CrossRef]
- Varmazyari, A.; Taghizadehghalehjoughi, A.; Sevim, C.; Baris, O.; Eser, G.; Yildirim, S.; Hacimuftuoglu, A.; Buha, A.; Wallace, D.R.; Tsatsakis, A.; et al. Cadmium Sulfide-Induced Toxicity in the Cortex and Cerebellum: In Vitro and in Vivo Studies. Toxicol. Rep. 2020, 7, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Apykhtina, O.L.; Dybkova, S.M.; Sokurenko, L.M.; Chaikovsky, Y.B. Cytotoxic and Genotoxic Effects of Cadmium Sulfide Nanoparticles. Exp. Oncol. 2018, 40, 194–199. [Google Scholar] [CrossRef]
- Peng, W.; Luo, P.; Gui, D.; Jiang, W.; Wu, H.; Zhang, J. Enhanced Anticancer Effect of Fabricated Gallic Acid/CdS on the RGO Nanosheets on Human Glomerular Mesangial (IP15) and Epithelial Proximal (HK2) Kidney Cell Lines—Cytotoxicity Investigations. J. Photochem. Photobiol. B Biol. 2018, 178, 243–248. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, F.; Liu, L.; Wei, W.; Zhao, Z.; Sun, J. The Enhanced Photo-Thermal Therapy of Surface Improved Photoactive Cadmium Sulfide (CdS) Quantum Dots Entrenched Graphene Oxide Nanoflakes in Tumor Treatment. J. Photochem. Photobiol. B Biol. 2019, 192, 34–39. [Google Scholar] [CrossRef]
- MAKKAWI, A.J.J.; AYSA, N.H.; GASSIM, F.-A.G. Anticancer Activity of Zinc Oxide and Zinc Oxide/Cadmium Sulfide Nanocomposites. Asian J. Pharm. Clin. Res. 2019, 12, 535–539. [Google Scholar] [CrossRef]
- Akhtar, S.; Rehman, S.; Asiri, S.M.; Khan, F.A.; Baig, U.; Hakeem, A.S.; Gondal, M.A. Evaluation of Bioactivities of Zinc Oxide, Cadmium Sulfide and Cadmium Sulfide Loaded Zinc Oxide Nanostructured Materials Prepared by Nanosecond Pulsed Laser. Mater. Sci. Eng. C 2020, 116, 111156. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus Limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ashengroph, M.; Khaledi, A. Rapid Extracellular Synthesis of Cadmium Sulfide Nanoparticles by Pseudomonas Pseudoalcaligenes Cd11 and Study of Its Antibacterial Activity. Cell. Mol. Res. (Iran. J. Biol.) 2018, 31, 421–436. [Google Scholar]
- Tamire, A. The Synthesis of Cobalt Doped Cadmium Sulfide Nanoparticles by Solution Growth Method for Antimicrobial Activity. Ph.D. Thesis, Hawassa University, Awasa, Ethiopia, 2020. [Google Scholar]
- Calvo-Olvera, A.; De Donato-Capote, M.; Pool, H.; Rojas-Avelizapa, N.G. In Vitro Toxicity Assessment of Fungal-Synthesized Cadmium Sulfide Quantum Dots Using Bacteria and Seed Germination Models. J. Environ. Sci. Health Part A 2021, 56, 713–722. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.-S.G.; Calaminus, S.D.J.; Stasiuk, G.J. NIR-Quantum Dots in Biomedical Imaging and Their Future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef]
- Naranthatta, S.; Janardhanan, P.; Pilankatta, R.; Nair, S.S. Green Synthesis of Engineered CdS Nanoparticles with Reduced Cytotoxicity for Enhanced Bioimaging Application. ACS Omega 2021, 6, 8646–8655. [Google Scholar] [CrossRef]
- Stavitskaya, A.V.; Novikov, A.A.; Kotelev, M.S.; Kopitsyn, D.S.; Rozhina, E.V.; Ishmukhametov, I.R.; Fakhrullin, R.F.; Ivanov, E.V.; Lvov, Y.M.; Vinokurov, V.A. Fluorescence and Cytotoxicity of Cadmium Sulfide Quantum Dots Stabilized on Clay Nanotubes. Nanomaterials 2018, 8, 391. [Google Scholar] [CrossRef]
- Órdenes-Aenishanslins, N.; Anziani-Ostuni, G.; Monrás, J.P.; Tello, A.; Bravo, D.; Toro-Ascuy, D.; Soto-Rifo, R.; Prasad, P.N.; Pérez-Donoso, J.M. Bacterial Synthesis of Ternary CdSAg Quantum Dots through Cation Exchange: Tuning the Composition and Properties of Biological Nanoparticles for Bioimaging and Photovoltaic Applications. Microorganisms 2020, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Jiang, H.; Wang, X. Biosensors Based on Advanced Sulfur-Containing Nanomaterials. Sensors 2020, 20, 3488. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, N.; Rasheed, M.A.; Khan, M.; Maqbool, M.; Ahmad, M.; Karim, S.; Nisar, A.; Schmuki, P.; Cho, S.O.; Ali, G. Voltage-Switchable Biosensor with Gold Nanoparticles on TiO2 Nanotubes Decorated with CdS Quantum Dots for the Detection of Cholesterol and H2O2. ACS Appl. Mater. Interfaces 2021, 13, 3653–3668. [Google Scholar] [CrossRef] [PubMed]
- Ngamdee, K.; Ngeontae, W. Circular Dichroism Glucose Biosensor Based on Chiral Cadmium Sulfide Quantum Dots. Sens. Actuators B Chem. 2018, 274, 402–411. [Google Scholar] [CrossRef]
- Yuting, L.; Jing, Z.; Donghui, L. Preparation of Cadmium Sulfide Nanoparticles and Their Application for Improving the Properties of the Electrochemical Sensor for the Determination of Enrofloxacin in Real Samples. Chirality 2019, 31, 174–184. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Daniyal, W.M.E.M.M.; Mahdi, M.A. Sensitive Surface Plasmon Resonance Performance of Cadmium Sulfide Quantum Dots-Amine Functionalized Graphene Oxide Based Thin Film towards Dengue Virus E-Protein. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Ju, Y.; Hu, X.; Zang, Y.; Cao, R.; Xue, H. Amplified Photoelectrochemical DNA Biosensor Based on a CdS Quantum Dot/WS2 Nanosheet Heterojunction and Hybridization Chain Reaction-Mediated Enzymatic Hydrolysis. Anal. Methods 2019, 11, 2163–2169. [Google Scholar] [CrossRef]
- Dhyani, H.; Ali, M.A.; Pal, S.P.; Srivastava, S.; Solanki, P.R.; Malhotra, B.D.; Sen, P. Mediator-Free Biosensor Using Chitosan Capped CdS Quantum Dots for Detection of Total Cholesterol. RSC Adv. 2015, 5, 45928–45934. [Google Scholar] [CrossRef]
- Xia, L.; Xu, L.; Song, J.; Xu, R.; Liu, D.; Dong, B.; Song, H. CdS Quantum Dots Modified CuO Inverse Opal Electrodes for Ultrasensitive Electrochemical and Photoelectrochemical Biosensor. Sci. Rep. 2015, 5, 10838. [Google Scholar] [CrossRef]
- Kini, S.; Ganiga, V.; Kulkarni, S.D.; Chidangil, S.; George, S.D. Sensitive Detection of Mercury Using the Fluorescence Resonance Energy Transfer between CdTe/CdS Quantum Dots and Rhodamine 6G. J. Nanopart Res. 2018, 20, 232. [Google Scholar] [CrossRef]
- Isildak, I.; Navaeipour, F.; Afsharan, H.; Kanberoglu, G.S.; Agir, I.; Ozer, T.; Annabi, N.; Totu, E.E.; Khalilzadeh, B. Electrochemiluminescence Methods Using CdS Quantum Dots in Aptamer-Based Thrombin Biosensors: A Comparative Study. Microchim. Acta 2019, 187, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, J.; Zhao, P.; Guo, S.; Han, S. One-Step Synthesis of Water-Soluble CdS Quantum Dots for Silver-Ion Detection. ACS Omega 2021, 6, 7139–7146. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.; Zhou, H.; Xie, Z.; Zeng, X.; Liu, C. Cuprous Oxide Coated Silver/Graphitic Carbon Nitride/Cadmium Sulfide Nanocomposite Heterostructure: Specific Recognition of Carcinoembryonic Antigen through Sandwich-Type Mechanism. J. Colloid. Interface Sci. 2022, 616, 858–871. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxicity and Oxidative Stress Induced by Different Metallic Nanoparticles on Human Kidney Cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed]






| Methods | Importance of Method | Characterizations * | Morphology/Size (nm) | Microorganism or Plant | Year | Ref. | |
|---|---|---|---|---|---|---|---|
| Green | Green or biogenic synthesis | -Biocompatible; -Eco-friendly; -Quick process; -Economically affordable; -One-pot synthesis; Least hazardous; Does not require the use of stabilizing agents. | UV–visible spectra, SEM, EDX, FTIR, XRD | - | Escherichia coli | 2020 | [23] |
| UV–visible spectra, FESEM, XRD, FTIR | Spherical/~19.07±2.54 | Lactobacillus acidophilus | 2022 | [27] | |||
| UV–visible spectra, XRD, FTIR, TEM | non-spherical/5 ± 0.4 (Protein-capped NPs) and 11 ± 0.75 (Bare NPs) | Escherichia coli | 2015 | [28] | |||
| UV–visible spectra, SEM, EDX, TEM, XRD | Spherical/6–15 | Trichosporon jirovecii | 2015 | [29] | |||
| TEM, XRD, XPS | Spherical/4–12 | Viridi bacillus arenosi K64 | 2021 | [30] | |||
| UV–Vis spectra, TEM, EDX, SEM | -/15–20 | Escherichia coli | 2017 | [31] | |||
| UV-Vis and fluorescence spectra, TEM | Spherical/7–15 | Fusarium oxysporum f. sp. lycopersic | 2017 | [32] | |||
| UV-Vis spectra, HRTEM, EDS, FTIR | Spherical/3–6 | Rhodopseudomonas palustris TN110 | 2019 | [33] | |||
| UV–visible spectra, SEM, EDX, FTIR, XRD | - | Bacillus licheniformis | 2015 | [34] | |||
| XRD, FTIR, TEM, FESEM, EDX | Spherical/~15 | Shewanella oneidensis | 2017 | [35] | |||
| UV-Vis spectra, EDX, FTIR, XRD, DLS, TEM, Fluorescence spectrophotometer | Spherical/2–10 | Aspergillus niger | 2020 | [36] | |||
| TEM, SEM, EDX | -/- | Pseudoalteromonas sp. MT33b | 2021 | [37] | |||
| UV-Vis spectra, TEM, EDX, FTIR | Spherical/3.2–44.9 (E.coli) and 5.7–26.3 (K. pneumonia) | Escherichia coli E-30 and Klebsiella pneumoniae K-6 | 2018 | [38] | |||
| AFM, UV–visible spectra | -/The average size by P. aeruginos, B. lichenisformis, E. coli, F. oxysporum & A. terrus, was 17.86, 17.00, 17.86, 18.73 and 13.21 nm respectively | Pseudomonas aeruginosa, Bacillus Lichenisformis, Escherichia coli, Fusarium oxysporum and Aspergillus terrus | 2016 | [39] | |||
| SEM | -/15–20 | Bacillus licheniformis | 2019 | [40] | |||
| UV–visible spectra, FTIR, XRD, EDX, SEM, TEM | Spherical/2.5–8 | Dicliptera Roxburghiana plant | 2021 | [41] | |||
| UV–Vis spectra, FTIR, XRD, SEM, XRF, TGA | -/~4.6 estimated by XRRD pattern | Panicumsarmentosum | 2019 | [42] | |||
| SEM, EDX, HRTEM, FTIR, UV-Vis, and fluorescence emission spectra | Spherical/2–5 | Camellia sinensis | 2018 | [43] | |||
| Chemical | Chemical precipitation method | -Simple; -Short reaction time; -Requires a hazardous chemical agent; -Able to control the size and stability of NPs. | UV–visible spectra, XRD, FTIR, TEM, SEM, EDS, TGA, DTA, DTG | Spherical/<10 | - | 2015 | [44] |
| UV–visible spectra, XRD, FESEM, FTIR, PL, Raman measurement | Spherical/ few hundred to tens of nanometres | - | 2015 | [45] | |||
| XRD, FESEM, HRTEM, UV–visible spectra, Micro-Raman measurements, EDS | Spherical/15–85 | - | 2015 | [46] | |||
| UV–visible spectra, XRD, SEM, FTIR, EDX | Nano-rods/14.3–18.7 Spherical/6–8.8 | - | 2018 | [47] | |||
| UV-visible spectra, Raman Spectra, XRD, FESEM, EDX | Spherical/6.8–28 | - | 2020 | [48] | |||
| XRD, SEM, TEM, UV–visible spectra, PL, DLS | Spherical/15–42 | - | 2016 | [49] | |||
| Wet chemical synthesis | -Simple; One step; -Does not require a high temperature and potential chemical agent; -Quick process. | TEM, ICP-AES | -/5–10 | - | 2019 | [50] | |
| UV–visible spectra, XRD, FTIR, TEM, XPS, PL, DLS | Spherical/10-15 | - | 2019 | [25] | |||
| UV-Vis spectra, TEM, EDX, FTIR | -/8.77–16.50 | - | 2018 | [38] | |||
| Solvothermal synthesis | -Control of morphology, dimensions, and structure of nanomaterials; -Multiple steps; -Fabricates pure and clean nanoparticles with a high degree of crystallinity | XRD, XPS, FETEM, FESEM, UV/VIS/NIR spectra, PL | Irregular particle shape/Small particles less than 5 nm and large particles greater than 10 nm | - | 2017 | [51] | |
| Chemical reduction method | -Inexpensive; -Simple; | UV-Vis-NIR spectra, XRD, FTIR | -/- | - | 2017 | [52] | |
| -Formation of various morphologies; -Requires a high temperature; -Synthesis of the relatively stable particles that can be re-dispersed in nonpolar solvents easily. | -Formation of various morphologies; -Requires a high temperature; -Synthesis of the relatively stable particles that can be re-dispersed in nonpolar solvents easily. | XRD, FESEM, TEM, EDX, PL, CHNS elemental analyzer, TG/DTA, DRS | -Thermal decomposition of cadmium thiourea complexes in diphenyl ether: microspheres, pyramid-like and mixture of nanorods and NPs. -Solid state thermal decomposition of different cadmium thiourea complexes: microspheres, nanotube-like, flower-like and irregular shape | - | 2015 | [53] | |
| XRD, FTIR, TGA, EDX, FESEM, TEM, DRS, PL, UV–Vis–NIR spectra | -Pyramid-like morphology/ The height of the pyramid is about 400 nm, and the diameter of the base is about 300 nm -Sponge-like morphology/200–300 nm -Hexagonal disc-like particles/50–70 nm -Flower-like nanostructures/250–300 nm -Gypsum rose and rosette-like particles/300-400 nm | - | 2015 | [54] | |||
| XRD, SEM, TEM, FTIR, PL | -/30–40 | - | 2016 | [55] | |||
| Sol–gel method | -An appropriate method for the development of quality crystals with high surface area and different morphologies; -Fast and simple. | XRD, PL, SEM, HRTEM, FTIR | Spherical/24 | - | 2020 | [56] | |
| XRD, FESEM, TEM, EDS, FTIR, PL | Spherical/<10 | - | 2020 | [57] | |||
| XRD, TEM, UV–visible spectra | Spherical/9 (pure CdS) and 16 (Ni-doped CdS) | - | 2018 | [58] | |||
| Combustion | -Short-time reaction; -Does not require high temperature. | XRD, TEM, FTIR, PL, BET, DRS | -/6 nm for rinsed and 3 nm for washed samples | - | 2015 | [59] | |
| Sono-chemical Method | -Rapid reaction rates; -Controllable reaction condi-tions; -Able to form nanoparti-cles with high purity; -Quick in process. | XRD, TEM, EDX | Spherical/10 | - | 2016 | [60] | |
| XRD, TEM, EDX | Hexagonal platelets/19.3–22.9 | - | 2015 | [61] | |||
| UV–visible spectra, FTIR, XRD, TGA, DLS, SEM, TEM | -/6 | - | 2015 | [62] | |||
| Micro-emulsion method | -Multiple steps; -Does not require certain conditions. | TEM, UV-visible spectra | Spherical/49–89 | - | 2013 | [63] | |
| Physical | Pulsed laser ablation | -Facile; -Ecofriendly; -Able to control size and shape. | XRD, TEM, XPS, PL, UV–visible spectra | Spherical/21 ± 9.1 | - | 2016 | [64] |
| UV–visible spectra, XRD, SEM, AFM | Spherical, monopod, bipod, and tripod rods/40 nm for NPs synthesized with 1.76 J/cm2 (2.8–5.5) µm in length and (80–400) nm in diameter for NPS synthesized with 2.25 J/cm2 | - | 2015 | [65] | |||
| XRD, TEM | -/10–15 | - | 2015 | [66] | |||
| Hydrothermal method | -Highly pure, with controlled morphology of NPs; -Has a narrow size distribution and consists of single crystals; -Production of fine-grained powder; -High reaction rate of powders; -Good dispersion in liquid; -Almost pollution-free; -Does not require expensive and highly sophisticated equipment; | XRD, FESEM, EDS, FTIR, UV–Diffuse Reflectance spectroscopy, PL | Spherical/ 50.8 | - | 2018 | [67] | |
| UV–visible spectra, XRD, SEM | Spherical/50 & 150 | - | 2022 | [68] | |||
| XRD, SEM, XPS, UV–vis diffuse reflectance spectroscopy, PL, BET | -/~25 | - | 2019 | [69] | |||
| Microwave-assisted | -Short reaction time; -Gives a narrow particle size distribution of nanocrystals with a high purity; -Cheap; -Environmentally friendly. | XRD, UV–visible spectra, SEM, TEM | -/8–10 | - | 2016 | [70] | |
| SEM, XRD, FTIR | -/75–180 (uncapped CdS) 40–59 (PVP-capped CdS) | - | 2016 | [71] | |||
| XRD, FESEM, TEM, EDX, PL | Spherical/15–25 | - | 2019 | [72] | |||
| XRD, TEM, PL, UV–visible spectra | -/30–60 | - | 2018 | [73] | |||
| XRD, TEM, UV-Vis spectra | Spherical/8.9 nm at 10 min and 9.2 nm at 15 min irradiation time. | - | 2013 | [74] | |||
| Reflux method | -Simple; -Low cost; -Aqueous based. | XRD, TEM, FTIR, STEM, XPS, PL | -/5–8 | - | 2020 | [75] | |
| Physico-chemical | Mechanochemical processes | -The reaction occurs at low temperatures; -Particle size can be controlled by changing milling conditions and starting materials; -Reproducible; -Ensures a high yield -Simple and easy to operate. | XRD, SEM, UV/Vis/NIR Spectra, EDX, BET | -/- | - | 2018 | [76] |
| XRD, HRTEM | - / ~5 | - | 2016 | [77] | |||
| XRD, XPS, PL, UV–visible spectra, TEM, EDS, DLS, Raman spectroscopy | -/<10 | - | 2022 | [78] |
| Type of CdS NPs | Synthesis Method | Morphology and Size (nm) | Cancer (Cell Line) | Effects | Explanations | Year | Ref. |
|---|---|---|---|---|---|---|---|
| CdS QDs | Green synthesis (Camellia sinensis) | Spherical/2–5 | Human lung alveolar basal epithelial cell line (A549) | - With CdS QDs, the inhibition of A549 cells is gradually enhanced (A549 cell viability at 50 g/mL was 20%), and the effect is comparable to that of the medication cisplatin (A549 cell viability at 50 g/mL was 24%). | Owing to the high florescence emission and quantum confinement effect results, green-synthesized CdS QDs particles could interact with the phosphorous moieties in DNA, and then DNA replication is inactivated. | 2018 | [43] |
| CdS NPs | Green synthesis (Shewanellaoneidensis) | Spherical/15 | Rat glioma cell line (RG2) | - The cytotoxic effect of the biosynthesized CdS NPs in the presence of IL increased with increasing NP concentrations. (Cell viability of GR2 in 100 μM CdS NPs was 75% and for CdS/IL NPs was 65%). | The improved cellular uptake was due to the improved surface morphology and surface area of the NPs via the IL soft template action. | 2018 | [35] |
| Gallic acid/cadmium sulfide (GA/CdS) NPs fabricated on graphene oxide (GA/CdS-rGO) nanosheets | - | Spherical CdS NPs | Human glomerular mesangial cancer cells (IP15) | - In samples treated with GA/CdS-rGO, the number of viable cells was decreased, and 83.87% inhibition was seen. - The IC50 value for IP15 cells was 50 µg/mL, and 55.05% of inhibition was obtained in pure CdS nanoparticles on IP15 cells. | - Oxidative stress from ROS species, mitochondrial dysfunction, and an increase in intracellular Ca2+ levels are associated with the apoptosis of cancer cell types caused by CdS/GA. - The anticancer properties of GA/CdS nanocomposites are superior to those of unprocessed CdS NPs. | 2018 | [109] |
| CdS/rGO NPs (graphene oxide/CdS nanocomposite) | Solvothermal method | Spherical CdS NPs /~10 | Hela cells | - The IC50 value of the CdS NPs on the normal and cancer cells is about around 60 μg/mL. | - The characteristics of the nanocomposites are improved by adding CdS to the rGO matrix. - The created CdS/rGO nanocomposites were likewise very effective at killing tumor cells. | 2019 | [110] |
| ZnO-CdS NPs | Chemical synthesis | -/- | - Hepatocellular carcinoma (HepG2) - Mammary gland (MCF-7) - Epidermoid carcinoma (HEP2) - Colorectal carcinoma (HCT-116) - Rhabdomyosarcoma (RD) | - IC50 of ZnO-CdS NPs against human tumor cells HePG2, HCT-116, MCF-7, RD, and HeP2 was 9.26 µg, 5.64 µg, 7.90 µg, 9.51 µg, 10.17 µg, respectively. | - The anticancer results of ZnO-CdS NPs were comparable to the anticancer results of doxorubicin. - Probably the pathway of treatment with ZnO/CdS nanocomposites was based on ROS production. | 2019 | [111] |
| composite of Cd loaded on ZnO | Pulsed laser ablation in water media | Spherical/12 | Human colorectal carcinoma cells (HCT-116) | - The IC50 values for 10% CdS/ZnO, (0.10 µg/mL), 20% CdS/ZnO, (0.12 µg/mL), and CdS (O.13 µg/mL). | - CdS-loaded ZnO showed better anticancer activities than CdS. | 2020 | [112] |
| CdS NPs | Green synthesis (Aspergillusniger) | Spherical/2–10 | - Breast cancer (MCF7) - Lung cancer (A549) - Prostatic carcinoma (PC3) | - 50% inhibitory concentrations (IC50) CdS NPs against MCF7, PC3, and A549 cell lines of 190 g/mL, 246 g/mL, and 149 g/mL, respectively. | - Long-term exposure of CSNPs to an oxidizing environment can lead to CSNP decomposition and the release of Cd ions. | 2020 | [36] |
| CdS NPs | Green synthesis | -/- | Mus musculus skin melanoma (B16F10) and Human epidermoid carcinoma (A431) | - The cytotoxicity of CdS NPs on A431 cells was inhibited by 81.53% at 100 M, which was significantly more effective than 5-ALA, which inhibited A431 cells by 33.45% at 1 mM. | - CdS NPs have a less toxic effect on musculus skin melanoma (B16F10) than epidermoid carcinoma (A431) cell lines. - CdS NPs showed more cytotoxic effects on cancer cells compared with standard 5-aminolevulinic acid (5-ALA). | 2020 | [23] |
| Type of CdS NPs | Synthesis Method | Morphology/Size (nm) | Microorganism | Test Approach | Results | Year | Ref. |
|---|---|---|---|---|---|---|---|
| CdS NPs | Green synthesis (Pseudomonas pseudoalcaligenes strain Cd11) | Spherical/12–19 |
- Escherichia coli - Bacillus subtilis - Staphylococcus aureus - Pseudomonas aeruginosa - Lactobacillus plantarum - Pseudomonas fluorescens | Well-diffusion method | - The inhibitory effect of CdS NPs was observed. - The highest inhibitory effect on L. Plantarum was 2 mm inhibition The lowest inhibition was with inhibition and for P. Fluorescens. - The inhibitory effect on other bacteria studied was between 1 and 2 mm. | 2018 | [115] |
| CdS NPs | Green synthesis (Escherichia coli and Klebsiella pneumoniae) | Spherical/3.2–44.9 (E.coli) and 5.7–26.3 (K. pneumonia) |
- Aspergillus fumigatus - Aspergillus niger - Geotricum candidum - Candida albicans - Bacillus subtilis - Streptococcus pneumoniae -Staphylococcus aureus -Staphylococcus epidermidis - Pseudomonas aeruginosa - Escherichia coli - Proteus mirabilis - Klebsiella pneumoniae | Well-diffusion method | - CdS NPs synthesized had the maximum zone of inhibition against Bacillus subtilis (23.4 mm), Staphylococcus aureus (22.1 mm), Pseudomonas aeruginosa (21.4 mm), Escherichia coli (17.3 mm), Geotricum candidum (17.3 mm), Aspergillu sfumigatus (17.3 mm), and Aspergillus niger (14.7 mm). -In comparison to chemically produced CdS NPs, biogenic CdS NPs exhibited the highest levels of inhibition on the majority of strains. - Gram-positive bacteria displayed the strongest inhibition, followed by Gram-negative bacteria. | 2018 | [38] |
| CdS NPs | Green method (Panicum sarmentosum) | -/~4.6 estimated by XRD pattern |
-Staphylococcus aureus -Escherichia coli | Well-diffusion method | - CdS NPs showed antibacterial efficacy against Staphylococcus aureus and Escherichia coli. - The antibacterial property and the diameter of the bacterial inhibition zone increased with the increased dosage of the NPs. Gram-negative bacteria (Escherichia coli) were discovered to have higher CdS NPs resistance than Gram-positive bacteria (Staphylococcus aureus). | 2019 | [42] |
| Cobalt doped CdS NPs | Chemical method | Spherical/15–20 | -Escherichia coli - Staphylococcus aureus | Disk-diffusion method | - While Gram-positive bacteria can withstand the antibiotic effects of CdS nanoparticles, Gram-negative bacteria are killed by them. - The diameter of the zone for Gram-positive bacteria (30–40 mm). - The diameter of the zone for Gram-negative bacteria (6–41 mm). | 2020 | [116] |
| CdS NPs | Green synthesis (Aspergillus niger) | -/- | - Escherichia coli - Bacillus licheniformis - Pseudomonas aeruginosa - Bacillus cereus - Staphylococcus aureus - Fusarium oxysporum - Aspergillus flavus - Penicillium expansum | Well-diffusion methods | - Bacillus licheniformis, Escherichia coli, Bacillus cereus, and Pseudomonas aeruginosa have inhibition zones of 25.1 mm, 23.5 mm, 20.6 mm, and 13.6 mm, respectively. - CdS NPs outperformed common antibiotics ampicillin, trimethoprim, and cefotaxime in their ability to inhibit Pseudomonas aeruginosa, Bacillus cereus, and Escherichia coli. - Inhibition zone on Penicillium expansum 18.6 mm, Fusarium oxysporum 23.0 mm, and Aspergillus flavus 29.7 mm. - CdS NPs showed greater activity than fluconazole in Penicillium expansum. | 2020 | [23] |
| CdS NPs | Green synthesis (Aspergillus niger) | Spherical/2–10 | -
Escherichia coli - Pseudomonas vulgaris - Staphylococcus aureus - Bacillus subtilis - Candida albicans | Well-diffusion method | - Gram-positive bacteria were more affected by CdS NPs than Gram-negative ones, and CdS NPs had a substantial antibacterial effect on all of the bacterial pathogens that were studied. - Antimicrobial activity inhibition zone for Bacillus subtilis was 16 mm; for Staphylococcus aureus, it was 25 mm; for Escherichia coli, it was 14 mm; and for Proteus vulgaris, it was 16 mm. -No evidence of antimicrobial activity against Candida albicans was found. | 2020 | [36] |
| CdS QDs | Chemical and Green methods (synthesized by Fusarium oxysporum f. sp. lycopersici) | Spherical/4.08 ± 0.07 nm for biogenic NPs and 3.2±0.20 nm for chemical NPs | E. coli | Well-diffusion method | - In bacterial cells, biogenic CdS QDs had a less lethal effect than chemical CdS QDs. - As the NPs’ concentration increased, a decrease in cell viability was seen. - Compared to the control and the biological NPs, minimal cellular viability was obtained for the chemical nanoparticles in all treatments. | 2021 | [117] |
| Type of CdS NPs | The Synthesis Method of CdS NPs | Size (nm) | Type of Microscope/Cell type | Color | Results | Year | Ref. |
|---|---|---|---|---|---|---|---|
| - Halloysite nanotubes (HNTs): - HNTs-Azine-CdS - HNTs-NH2-CdS - HNTs-Azine-Cd0.7Zn0.3S | Cadmium sulfide and cadmium–zinc-sulfide QDs were stabilized on the halloysite. | 6–8 | Laser scanning microscopy/PC-3 cells | - Green (HNTs-Azine-CdS) - Yellow-red (HNTs-Azine-Cd0.7Zn0.3S) - Red (HNT-NH2-CdS) | - Bright and well-resolved fluorescence was observed in all cases. - Well distributed on the cells’ surfaces or inside them. | 2018 | [120] |
| Dark-field and epifluorescence microscopy/PC-3 cells | NT-NH2-CdS bright white spots, or yellow and red spots | - Appear as bright spots due to their good light-scattering properties. - The cell membrane and cytoplasm can also be seen. | |||||
| CdS QDs | Green synthesis using tea leaf extract (Camellia sinensis) | 2–5 | Fluorescence microscopy/A549 cancer cells | Yellow, red, and orange fluorescence correspond to early apoptotic cells, necrotic cells, and late apoptotic cells, respectively. | - CdS QDs produce effective intracellular fluorescence intensity. - The fluorescence emission is enhanced with CdS QDs concentration. This enhanced fluorescence is responsible for the high-contrast fluorescence bioimaging. | 2018 | [43] |
| CdS NPs and Chitosan-coated CdS NPs | Wet chemical method | 10–15 | Fluorescence microscopy/Jurkat cells | - | - Cell images are not clear enough; this might be due to the low incorporation of Chitosan-coated CdS NPs. | 2019 | [25] |
| CdS QDs capped with dextrin and bioconjugated with doxorubicin | Chemical synthesis | 5 | Confocal laser fluorescent microscope/HeLa cells | Green and red spectrum | - In cells treated with CdS-Dx/DOX QDs, the fluorescence was observed mainly in the cytoplasm and in lesser amounts in the nucleus. - The changes in the morphology of those cells treated with DOX alone and CdS-Dx/DOX QDs were clearly evident. - The high photostability of CdS-Dx/QDs, both alone and conjugated. | 2020 | [105] |
| CdS NPs | Green synthesis (Chromolaenaodorata, Plectranthusamboinicus, and Ocimumtenuiflorum) | - | Cell imaging with bright-field and fluorescence microscope/HeLa cells | Hela cells showed bright green fluorescence | - The use of cadmium sulfide nanoparticles synthesized by the green method is more suitable due to the lower toxicity of these compounds for cells. | 2021 | [119] |
| CdSAg NPs | Green synthesis (E. coli) | 5.49 nm for CdS NPs and 7.20 nm for CdSAg NPs | Confocal microscope/HeLa cells | Red fluorescent | - It is not associated with changes in the cell morphology. - The fluorescence is stable. | 2021 | [121] |
| Type of CdS NPs | Synthesis | Basis of the Test | Measured Substance | Detection Limit | Benefits | Year | Ref. |
|---|---|---|---|---|---|---|---|
| CdS QDs | CdS/WS2 nanosheets modified ITO electrode surface | Photoelectrochemical | DNA | 5 fM to 50 pM | This biosensor showed excellent analytical performances under optimized conditions, low detection limit, favorable selectivity, and satisfactory stability. | 2019 | [127] |
| CdS QDs | CdS QDs capped with Chitosan and Bioconjugated with enzyme | Electrochemical | Cholesterol | 0.64–12.9 mM | The synthesis of “enzyme-QDs-polymer” system is platform in bionanocomposite formation for electrochemical applications. | 2015 | [128] |
| CdS QDs | The CuO inverse opal photonic crystals were synthesized by the sol–gel method and modified with CdS QDs by successive ionic layer adsorption and reaction (SILAR). | Photoelectrochemical | Glucose | up to 4345μA mM−1 cm−2 | It showed strong stability, good reproducibility, excellent selectivity, and fast amperometric response. | 2015 | [129] |
| CdS QDs | The chiral CdS QDs (DPA/Cys-CdS QDs) were prepared by mixing cysteamine-capped CdS QDs (Cys-CdS QDs; achiral QDs) with D-penicillamine (DPA). | Circular dichroism spectroscopy (CD) | Glucose | 50–250 μM | - Detection of glucose by indirect measurement of the concentration of H2O2 generated by the enzymatic reaction of GOx and glucose. | 2018 | [124] |
| CdS QD | Core–shell CdTe/CdS QDs were synthesized by a simple one-pot chemical reduction method | Fluorescence resonance energy transfer (FRET) | Mercury | 0.1 nM to 2 μM | CdTe/CdS QDs exhibit fluorescence quenching as the mercury concentration increases, acting as an “OFF-sensor.” | 2018 | [130] |
| CdS nanocrystals | CdS-Au NPs were made by three different methods, namely the quenching method (QM), amplification method (AM), and ratiometric method (RM). | Electrochemiluminescence | Thrombin | QM:>92 pg.mL−1 | RM showed great selectivity and good authenticity in real samples. | 2019 | [131] |
| AM: >6.5 pg.mL−1 | |||||||
| RM: >500 fg.mL−1 | |||||||
| CdS QDs | Au/CdS-NH2GO/EDC-NHS/IgM thin film was prepared and deployed in an SPR-based optical sensor | Surface plasmon resonance (SPR) | Dengue virus E-protein | 0.001 nM/1 pM | - CdS-NH2GO thin films are beneficial to the improvement of the performance of SPR biosensor. | 2019 | [126] |
| CdS NPs | Modified electrode containing cadmium sulfide CdS NPs (CdS@enrofloxacin-tetraphenylboron) | Electrochemical | Enrofloxacin (ENR) | 10−2–10−7 mol·L−1 | - Good selectivity. - Reproducibility. - Response time (<40 s). - Lifetime (up to 12 weeks). - A pH range (3.3–7.2). - Can be a reference for ENR rapid and efficient determination. | 2019 | [125] |
| CdS QDs | A thin layer of Au NPs sputtered on CdS-QDs-decorated anodic titanium dioxide nanotubes (TNTs) was fabricated (Au/CdS QDs/TNTs) | Electrochemical | Cholesterol | 0.024−1.2 mM | - Good reproducibility. - Thermal stability. - Increased shelf life. | 2021 | [123] |
| H2O2 | 18.73−355.87 μM | ||||||
| CdS QDs | Potato extract was used as a stabilizer and modifier to synthesize CdS QDs (green synthesis) | Fluorescence resonance energy transfer (FRET) | Ag+ | 1–100 mg/L | For the prepared CdS QDs, a good fluorescence quenching effect was observed, indicating its potential application for the rapid detection of Ag+. | 2021 | [132] |
| CdS nanoarrays | Heterogeneous cuprous-oxide-coated silver (Ag@Cu2O) nanocomposites/graphitic carbon nitride (g-C3N4)/CdS nanoarrays structure was constructed | Photoelectrochemical (PEC) | Carcinoembryonic antigen (CEA) | 10−5–1 ng/mL | - High sensitivity. - Excellent anti-interference ability. - Favorable repeatability. - Good stability. | 2022 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.-A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules 2023, 28, 3857. https://doi.org/10.3390/molecules28093857
Ghasempour A, Dehghan H, Ataee M, Chen B, Zhao Z, Sedighi M, Guo X, Shahbazi M-A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules. 2023; 28(9):3857. https://doi.org/10.3390/molecules28093857
Chicago/Turabian StyleGhasempour, Alireza, Hamideh Dehghan, Mehrnaz Ataee, Bozhi Chen, Zeqiang Zhao, Mahsa Sedighi, Xindong Guo, and Mohammad-Ali Shahbazi. 2023. "Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications" Molecules 28, no. 9: 3857. https://doi.org/10.3390/molecules28093857
APA StyleGhasempour, A., Dehghan, H., Ataee, M., Chen, B., Zhao, Z., Sedighi, M., Guo, X., & Shahbazi, M.-A. (2023). Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules, 28(9), 3857. https://doi.org/10.3390/molecules28093857







