Selective Photooxidation of Valencene and Thymol with Nano-TiO2 and O2 as Oxidant
Abstract
:1. Introduction
2. Results
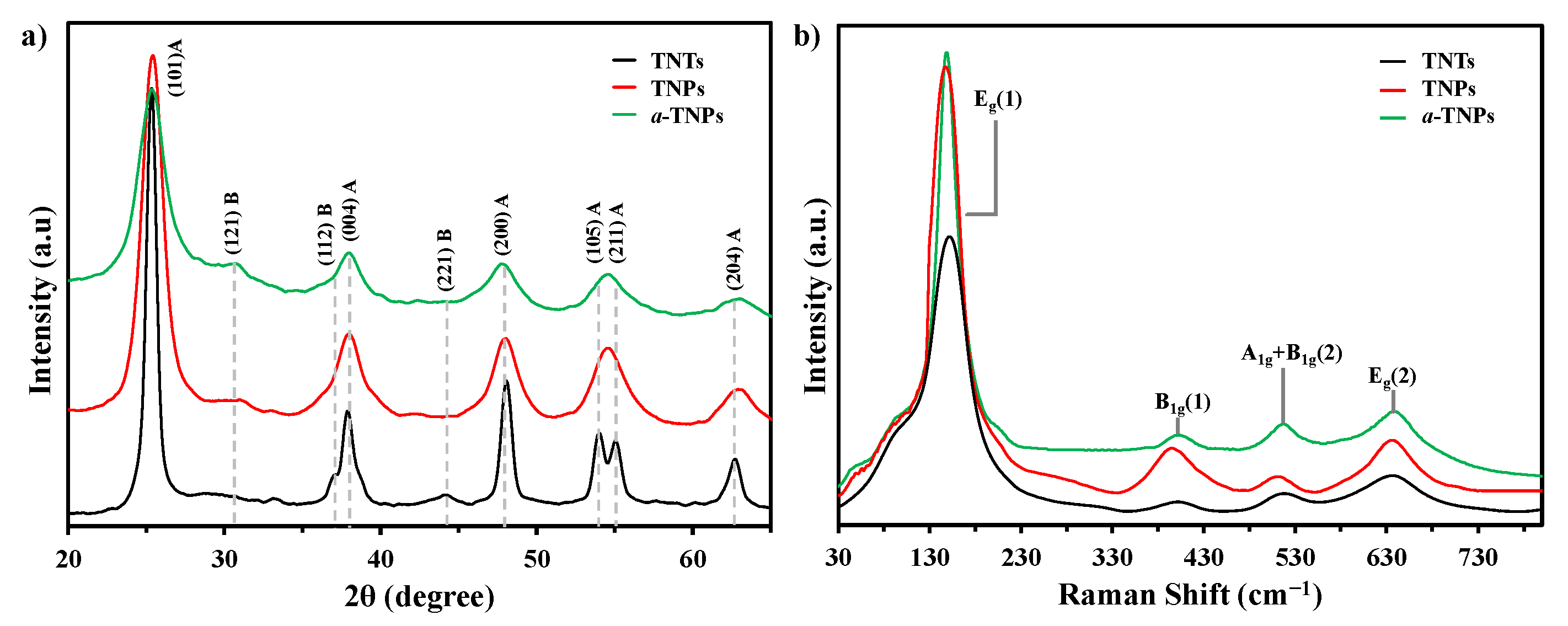
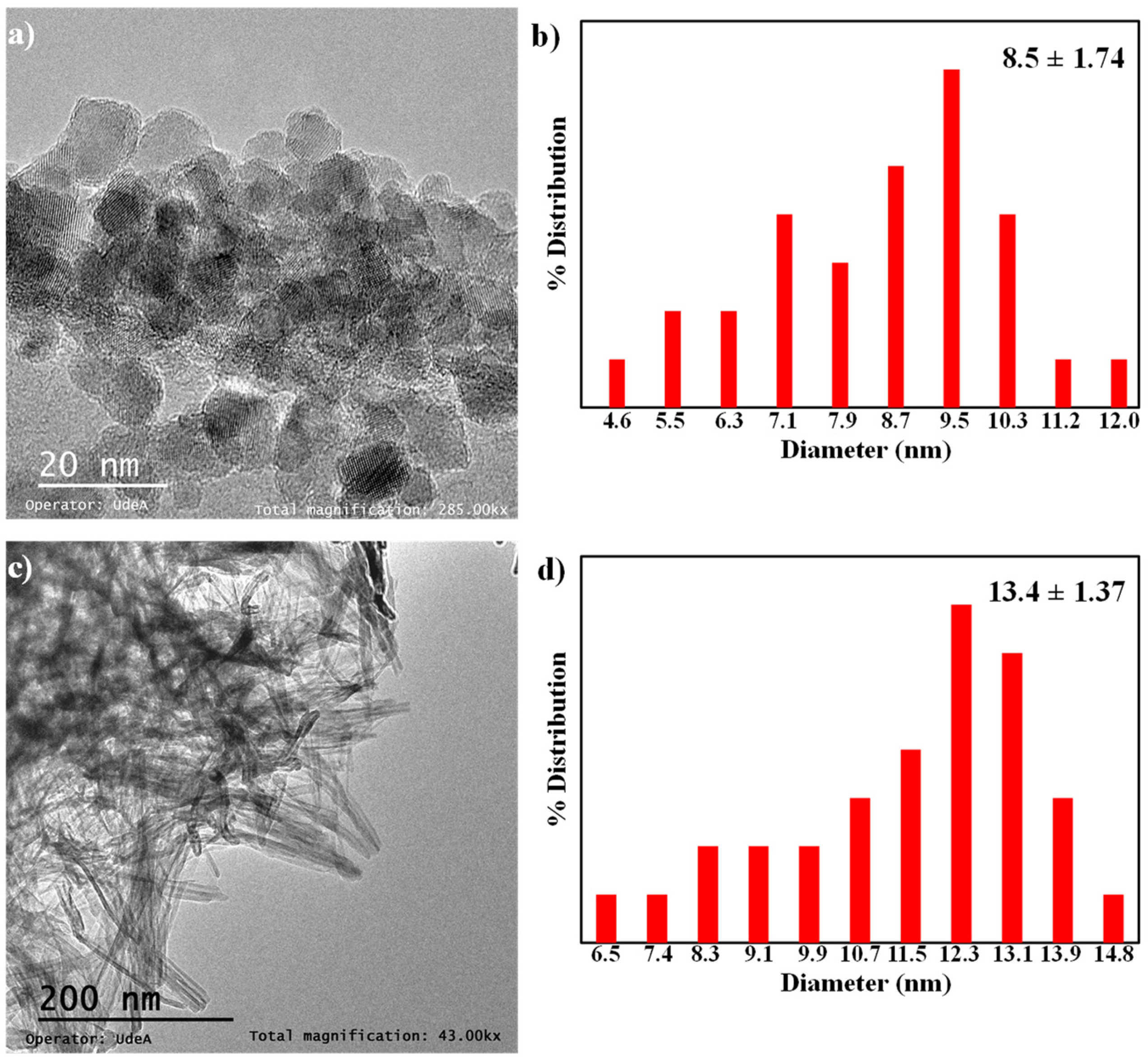
2.1. Characterization of the TiO2 Supports
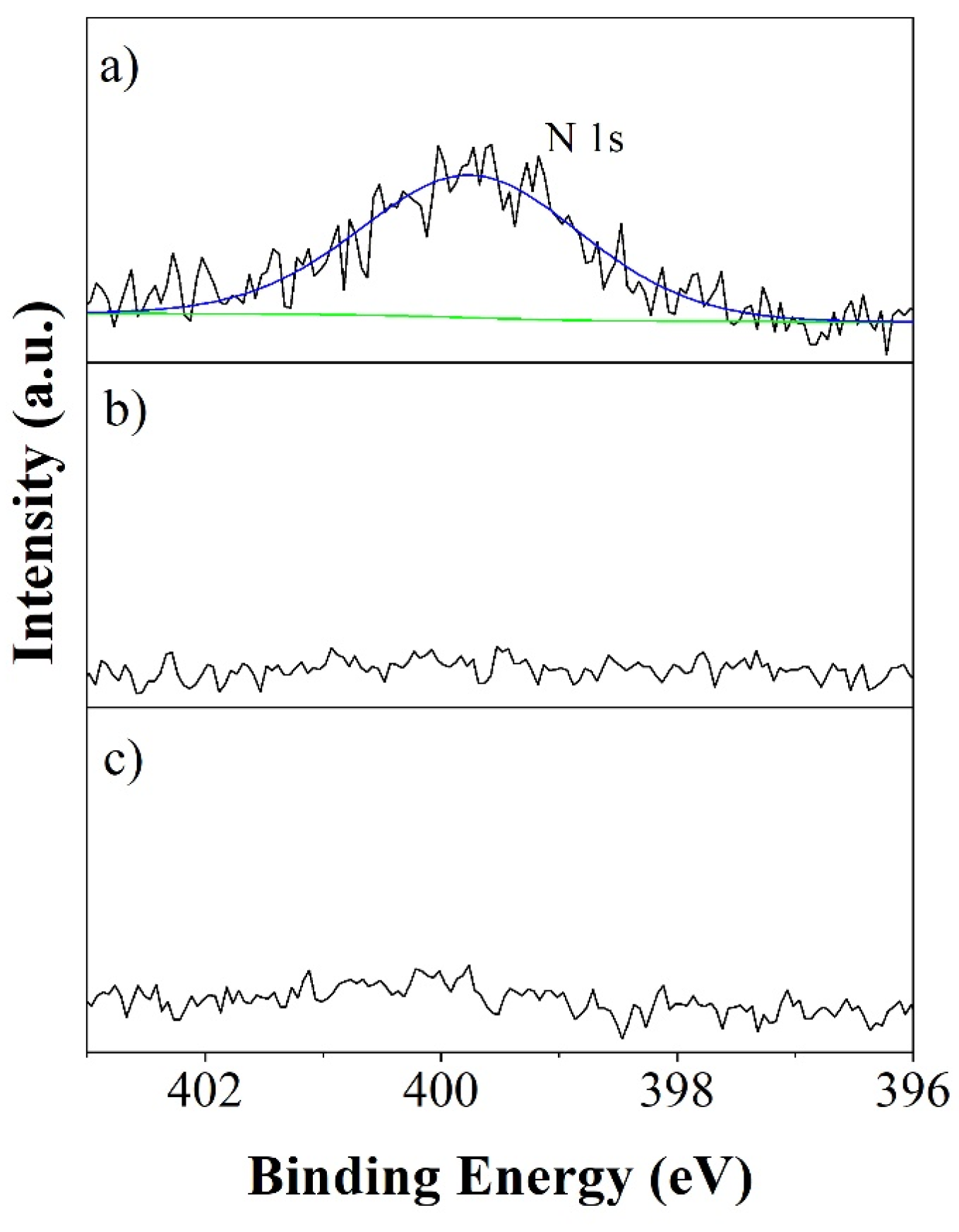
2.2. XPS Analysis
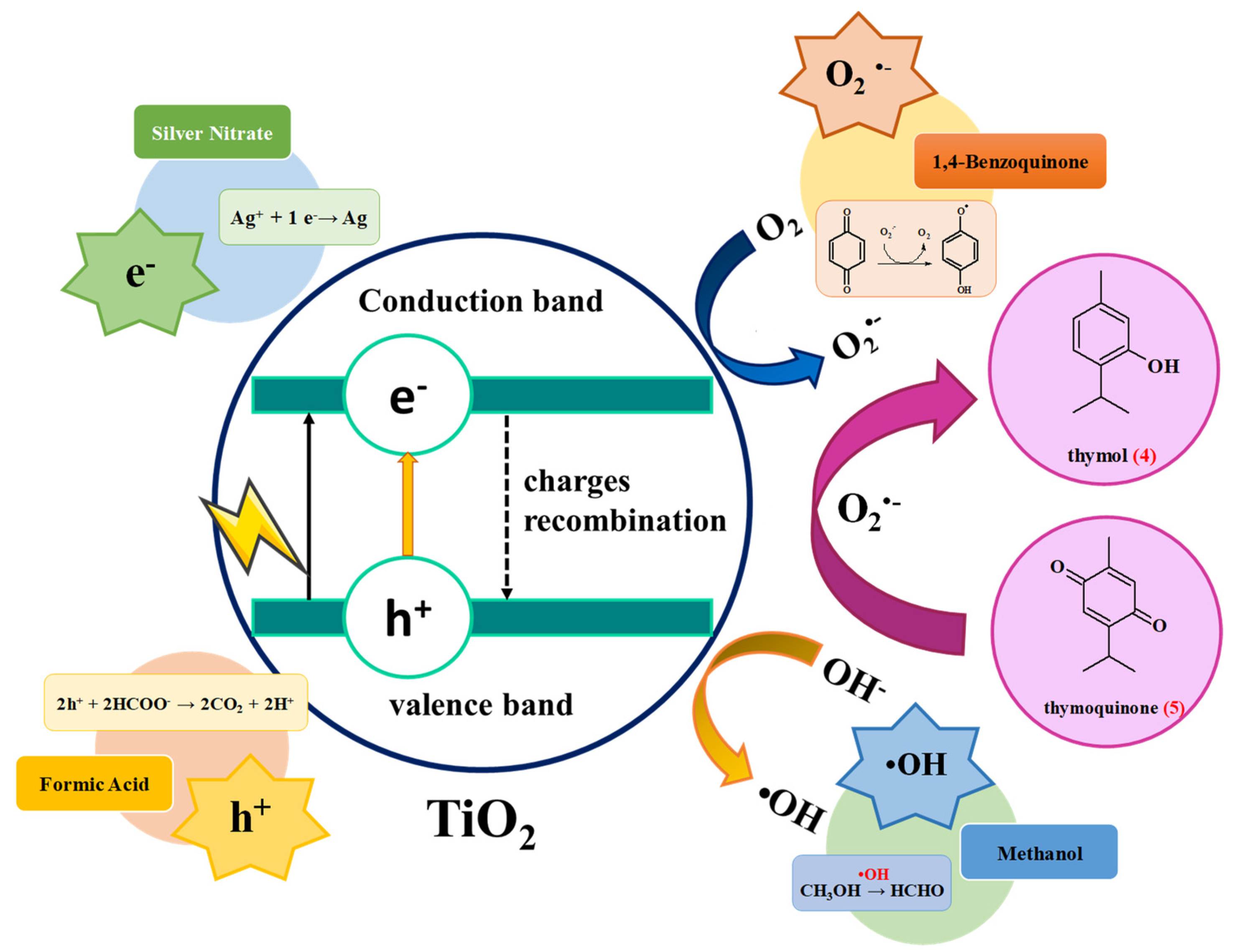
2.3. Valencene and Thymol Photooxidation
3. Discussion
4. Materials and Methods
4.1. TiO2 Nanotubes Preparation (TNTs)
4.2. Aminated TiO2 Nanoparticles (a-TNPs)
4.3. Catalyst Characterization
4.4. Catalytic Activity in the Photooxidation of Valencene and Thymol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shaffer, G.; Eschinasi, E.; Purzycki, K.; Doerr, A. Oxidations of valencene. J. Org. Chem. 1975, 40, 2181–2185. [Google Scholar] [CrossRef]
- Hong, B.; Lebeuf, R.; Delbaere, S.; Alsters, P.; Nardello-Rataj, V. One-Pot Synthesis of (+)-Nootkatone via Dark Singlet Oxygenation of Valencene: The Triple Role of the Amphiphilic Molybdate Catalyst. Catalysts 2016, 6, 184. [Google Scholar] [CrossRef]
- Asakawa, Y.; Hashimoto, T.; Noma, Y.; Furusawa, M. Modification of Valencene by Bio- and Chemical Transformation. Nat. Prod. Commun. 2013, 8, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Palmerín-Carreño, D.; Rutiaga-Quiñones, O.; Verde Calvo, J.; Prado-Barragán, A.; Huerta-Ochoa, S. Screening of Microorganisms for Bioconversion of (+)-Valencene to (+)-Nootkatone. LWT-Food Sci. Technol. 2015, 64, 788–793. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.D.; Guimaraes, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharm. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Goodner, K.L.; Mahattanatawee, K.; Plotto, A.; Sotomayor, J.A.; Jordan, M.J. Aromatic Profiles of Thymus Hyemalis and Spanish, T. Vulgaris Essential Oils by GC–MS/GC–O. Ind. Crops Prod. 2006, 24, 264–268. [Google Scholar] [CrossRef]
- Lee, S.-J.; Umano, K.; Shibamoto, T.; Kwang-Geun, L. Identification of Volatile Components in Basil (Ocimum basilicum L.) and Thyme Leaves (Thymus vulgaris L.) and Their Antioxidant Properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Fan, G.; Li, X. Production, Function, and Applications of the Sesquiterpenes Valencene and Nootkatone: A Comprehensive Review. J. Agric. Food Chem. 2023, 71, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Yanami, T.; Miyashita, M.; Yoshikoshi, A. Stereocontrolled Synthesis of (+)-Nootkatone from (−)-β-Pinene. J. Chem. Soc. Chem. Commun. 1979, 12, 525–527. [Google Scholar] [CrossRef]
- Yanami, T.; Miyashita, M.; Yoshikoshi, A. Synthetic Study of (+)-Nootkatone from (−)-β-Pinene. J. Org. Chem. 1980, 45, 607–612. [Google Scholar] [CrossRef]
- Sauer, A.M.; Crowe, W.E.; Henderson, G.; Laine, R.A. An Efficient and Economic Asymmetric Synthesis of (+)-Nootkatone, Tetrahydronootkatone, and Derivatives. Org. Lett. 2009, 11, 3530–3533. [Google Scholar] [CrossRef]
- Hunter, G.L.K.; Brogden, W.B., Jr. Conversion of Valencene to Nootkatone. J. Food Sci. 1965, 30, 876–878. [Google Scholar] [CrossRef]
- Wilson, C.W.; Shaw, P.E. Synthesis of Nootkatone from Valencene. J. Agric. Food Chem. 1978, 26, 1430–1432. [Google Scholar] [CrossRef]
- Salvador, J.; Clark, J. The Allylic Oxidation of Unsaturated Steroids by Tert-Butyl Hydroperoxide Using Surface Functionalised Silica Supported Metal Catalysts. Green Chem. 2002, 4, 352–356. [Google Scholar] [CrossRef]
- Salvador, J.; Clark, J. The Allylic Oxidation of Unsaturated Steroids by Tert-Butyl Hydroperoxide Using Homogeneous and Heterogeneous Cobalt Acetate. Chem. Commun. 2001, 4, 33–34. [Google Scholar] [CrossRef]
- Garcia-Cabeza, A.L.; Marin-Barrios, R.; Moreno-Dorado, F.J.; Ortega, M.J.; Massanet, G.M.; Guerra, F.M. Allylic Oxidation of Alkenes Catalyzed by a Copper-Aluminum Mixed Oxide. Org. Lett. 2014, 16, 1598–1601. [Google Scholar] [CrossRef]
- De Melo, C.; Moreira, A.; Da Silva, V.; Robles-Azocar, P.; DeFreitas-Silva, G. Manganese Complex Catalyst for Valencene Oxidation: The First use of Metalloporphyrins for the Selective Production of Nootkatone. Inorg. Chim. Acta 2020, 515, 120031. [Google Scholar] [CrossRef]
- Girhard, M.; Machida, K.; Itoh, M.; Schmid, R.D.; Arisawa, A.; Urlacher, V.B. Regioselective Biooxidation of (+)-Valencene by Recombinant E. coli Expressing CYP109B1 from Bacillus Subtilis in a Two-Liquid-Phase System. Microb. Cell Fact. 2009, 8, 1–12. [Google Scholar] [CrossRef]
- Sakamaki, H.; Itoh, K.-I.; Taniai, T.; Kitanaka, S.; Takagi, Y.; Chai, W.; Horiuchi, C.A. Biotransformation of Valencene by cultured Cells of Gynostemma pentaphyllum. J. Mol. Catal. B Enzym. 2005, 32, 103–106. [Google Scholar] [CrossRef]
- Furusawa, M.; Hashimoto, T.; Noma, Y.; Asakawa, Y. Highly Efficient Production of Nootkatone, the Grapefruit aroma from Valencene, by Biotransformation. Chem. Pharm. Bull. 2005, 53, 1513–1514. [Google Scholar] [CrossRef]
- Krügener, S.; Krings, U.; Zorn, H.; Berger, R.G. A Dioxygenase of Pleurotus Sapidus Transforms (+)-Valencene Regio-Specifically to (+)-Nootkatone via a Stereo-Specific Allylic Hydroperoxidation. Bioresour. Technol. 2010, 101, 457–462. [Google Scholar] [CrossRef]
- Rickert, A.; Krombach, V.; Hamers, O.; Zorn, H.; Maison, W. Enzymatic Allylic Oxidations with a Lyophilizate of the Edible Fungus Pleurotus Sapidus. Green Chem. 2012, 14, 639–644. [Google Scholar] [CrossRef]
- Kaspera, R.; Krings, U.; Nanzad, T.; Berger, R.G. Bioconversion of (+)-Valencene in Submerged Cultures of the Ascomycete Chaetomium Globosum. Appl. Microbiol. Biotechnol. 2005, 67, 477–483. [Google Scholar] [CrossRef]
- Tuğçe, G.; Yasemin, Ç.R.; Bengü, K.; Hayrettin, T. Oxidation of Thymol and Carvacrol to Thymoquinone with KHSO5 Catalyzed by Iron Phthalocyanine Tetrasulfonate in a Methanol–Water Mixture. Catal. Lett. 2016, 146, 2306–2312. [Google Scholar] [CrossRef]
- Kani, I. Oxidation of thymol catalysed by a water-soluble Cu(II)-Adipate-Diphenylamine Complex in a Biphasic Medium. Polyhedron 2023, 230, 116237. [Google Scholar] [CrossRef]
- Dockal, E.R.; Cass, Q.B.; Brocksom, T.J.; Brocksom, U.; Corrêa, A.G. A Simple and Efficient Synthesis of Thymoquinone and Methyl P-Benzoquinone. Synth. Commun. 1985, 15, 1033. [Google Scholar] [CrossRef]
- Martins, R.R.L.; Neves, M.G.P.M.S.; Silvestre, A.J.D.; Silva, A.M.S. Oxidation of Aromatic Monoterpenes with Hydrogen Peroxide Catalysed by Mn(III) Porphyrin Complexes. J. Mol. Catal. A Chem. 1999, 137, 41–47. [Google Scholar] [CrossRef]
- Skrobot, F.C.; Valente, A.A.; Neves, G.; Rosa, I.; Rocha, J.; Cavaleiro, J.A.S. Monoterpenes Oxidation in the Presence of Y Zeolite-Entrapped Manganese(III) Tetra(4-N-benzylpyridyl)porphyrin. J. Mol. Catal. A Chem. 2003, 201, 211–222. [Google Scholar] [CrossRef]
- Neves, C.M.B.; Tomé, J.P.C.; Hou, Z.; Dehaen, W.; Hoogenboom, R.; Neves, M.G.P.M.S.; Simoes, M.M.Q. Oxidation of Monoterpenes Catalysed by a Water-Soluble MnIII PEG-Porphyrin in a Biphasic Medium. Chem. Cat. Chem. 2018, 10, 2804. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Linares, N.; Serrano, E.; García-Martínez, J. Visible-Light-Activated Black Organotitanias: How Synthetic Conditions Influence Their Structure and Photocatalytic Activity. Chem. Plus Chem. 2018, 83, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Linares, N.; Garcia-Martínez, J.; Berenguer, J. Sol-Gel Coordination Chemistry: Building Catalysts from the Bottom-Up. Chem. Cat. Chem. 2013, 5, 844–860. [Google Scholar] [CrossRef]
- Rico, M.; Sepúlveda, Á.; Serrano, E.; Lalinde, E.; Berenguer, J.; García, J. Organotitanias: A Versatile Approach for Band Gap Reduction in Titania Based Materials. J. Mater. Chem. 2014, 2, 9497–9504. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies 2017, 10, 1624. [Google Scholar] [CrossRef]
- Rabbani, M.; Bathaee, H.; Rahimi, R.; Maleki, A. Photocatalytic Degradation of p-Nitrophenol and Methylene blue Using Zn-TCPP/Ag Doped Mesoporous TiO2 under UV and Visible Light Irradiation. Desalination Water Treat. 2016, 57, 25848–25856. [Google Scholar] [CrossRef]
- Sugiawati, V.A.; Vacandio, F.; Galeyeva, A.; Kurbatov, A.P.; Djenizian, T. Enhanced Electrochemical Performance of Electropolymerized Self-Organized TiO2 Nanotubes Fabricated by Anodization of Ti Grid. Front. Phys. 2019, 7, 179. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Samriti; Cho, J.; Prakash, J. Hydrothermal Synthesis of TiO2 Nanorods: Formation Chemistry, Growth Mechanism, and Tailoring of Surface Properties for Photocatalytic Activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Ouidri, S.; Guillard, C.; Caps, V.; Khalaf, H. Epoxidation of Olefins on Photoirradiated TiO2-Pillared Clays. Appl. Clay Sci. 2010, 48, 431–437. [Google Scholar] [CrossRef]
- Kitano, S.; Tanaka, A.; Hashimoto, K.; Kominami, H. Selective Oxidation of Alcohols in Aqueous Suspensions of Rhodium Ion-Modified TiO2 Photocatalysts under Irradiation of Visible Light. Phys. Chem. Phys. Chem. 2014, 16, 12554–12559. [Google Scholar] [CrossRef]
- Molina-Reyes, J.; Romero-Moran, A.; Uribe-Vargas, H.; Lopez-Ruiz, B.; Sanchez-Salas, J.; Ortega, E. Study on the Photocatalytic Activity of Titanium Dioxide Nanostructures: Nanoparticles, Nanotubes and Ultra-Thin Films. Catal. Today 2020, 341, 2–12. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nano-tubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Zhou, J.; Sheng, W.; Lang, X. Extending Aromatic Acids on TiO2 for Cooperative Photocatalysis with Triethylamine: Violet Light-Induced Selective Aerobic Oxidation of Sulfides. Chin. Chem. Lett. 2022, 33, 3733–3738. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A Review on Non Metal Ion Doped Titania for the Photocatalytic Degradation of Organic Pollutants under UV/Solar Light: Role of Photogenerated Charge Carrier Dynamics in Enhancing the Activity. Appl. Catal. B Environ. 2013, 140-141, 559–587. [Google Scholar] [CrossRef]
- Lin, H.; Rumaiz, A.K.; Schulz, M.; Huang, C.P.; Shah, S.I. Hydrogen Generation Under Visible Light Using Nitrogen Doped Titania Anodes. J. Appl. Phys. 2010, 107, 124305. [Google Scholar] [CrossRef]
- Piumetti, M.; Freyria, F.S.; Armandi, M.; Geobaldo, F.; Garrone, E.B.; Bonelli, B. Fe- and V-doped Mesoporous Titania Prepared by Direct Synthesis: Characterization and Role in the Oxidation of AO7 by H2O2 in the Dark. Catal. Today 2014, 227, 71–79. [Google Scholar] [CrossRef]
- Benito, H.; Sánchez, T.; Alamilla, R.; Hernández, J.; Robles, G.; Paraguay, F. Synthesis and Physicochemical Characterization of Titanium Oxide and Sulfated Titanium Oxide Obtained by Thermal Hydrolysis of Titanium Tetrachloride. Braz. J. Chem. Eng. 2014, 31, 737–745. [Google Scholar] [CrossRef]
- Leong, K.; Monash, P.; Ibrahim, S.; Saravanan, P. Solar Photocatalytic Activity of Anatase TiO2 Nanocrystals Synthesized by Non-Hydrolitic Sol–Gel Method. Sol. Energy 2014, 101, 321–332. [Google Scholar] [CrossRef]
- Burtrand, I.L.; Wang, X.; Bhave, R.; Hu, M. Synthesis of Brookite TiO2 Nanoparticles by Ambient Condition Sol Process. Mater. Lett. 2006, 60, 1179–1183. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Toxqui-Teran, A.; Vega-Becerra, O.; Miki-Yoshida, M.; Rojas-Villalobos, M.; García-Guaderrama, M.; Aguilar-Martínez, J.A. Low-Temperature Synthesis and Characterization of Anatase TiO2 Nanoparticles by an Acid Assisted Sol-Gel Method. J. Alloy. Compd. 2015, 647, 627–636. [Google Scholar] [CrossRef]
- García-Contreras, L.A.; Flores-Flores, J.O.; Arenas-Alatorre, J.A.; Chávez-Carvayar, J.A. Synthesis, Characterization and Study of the Structural Change of Nanobelts of TiO2 (H2Ti3O7) to Nanobelts with Anatase, Brookite and Rutile Phases. J. Alloy. Compd. 2022, 923, 166236. [Google Scholar] [CrossRef]
- Nachit, W.; Ahsaine, H.A.; Ramzi, Z.; Touhtouh, S.; Goncharova, I.; Benkhouja, K. Photocatalytic Activity of Anatase-Brookite TiO2 Nanoparticles Synthesized by Sol Gel Method at Low Temperature. Opt. Mater. 2022, 129, 112256. [Google Scholar] [CrossRef]
- Martínez, H.; Cáceres, M.; Martínez, F.; Páez-Mozo, E.; Valange, S.; Castellanos, N. Photo-Epoxidation of Cyclohexene, Cyclooctene and 1-Octene with Molecular Oxygen Catalyzed by Dichloro Dioxo-(4,4′-dicarboxylato-2,2′-bipyridine) Molybdenum(VI) Grafted on Mesoporous TiO2. J. Mol. Catal. 2016, 423, 248–255. [Google Scholar] [CrossRef]
- Martínez, H.; Amaya, Á.; Páez-Mozo, E.; Martínez, F. Highly Efficient Epoxidation of α-Pinene with O2 Photocatalyzed by Dioxomo(VI) Complex Anchored on TiO2 Nanotubes. Microp. Mesopor. Mat. 2018, 265, 202–210. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of Gases, with Special Reference to the Evaluation of Surface area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Abdel-Monem, Y.K. Efficient Nanophotocatalyt of Hydrothermally Synthesized Anatase TiO2 Nanoparticles from Its Analogue Metal Coordinated Precursor. J. Mater. Sci. Mater. Electron. 2016, 27, 5723–5728. [Google Scholar] [CrossRef]
- Sarvari, N.; Mohammadi, M.R. Influence of Photoanode Architecture on Light Scattering Mechanism and Device Performance of Dye-Sensitized Solar Cells Using TiO2 Hollow Cubes and Nanoparticles. J. Taiwan Inst. Chem. Eng. 2018, 86, 81–91. [Google Scholar] [CrossRef]
- Hejazi, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Aminated TiO2 Nanotubes as a Photoelectrochemical Water Splitting Photoanode. Catal. Today 2017, 281, 189–197. [Google Scholar] [CrossRef]
- Murphy, A. Band-Gap Determination from Diffuse Reflectance Measurements of Semiconductor Films, and Application to Photoelectrochemical Water-Splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Yao, M.; Yao, X. Band Structure Design of Semiconductors for Enhanced Photocatalytic Activity: The Case of TiO2. Prog. Nat. Sci. Mater. Int. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C. Neodymium Doped TiO2 Nanoparticles by Sol-Gel Method for Antibacterial and Photocatalytic Activity. Mater. Sci. Semicond. Process. 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Komaraiah, D.; Madhukar, P.; Vijayakumar, Y.; Ramana Reddy, M.; Sayanna, R. Photocatalytic Degradation Study of Methylene Blue by Brookite TiO2 Thin Film under Visible Light Irradiation. Mater. Today Proc. 2016, 3, 3770–3778. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.; Barrio, L.; Campos-Martin, J.; Fierro, J. Silylation and Surface Properties of Chemically Grafted Hydrophobic Silica. J. Colloid Interface Sci. 2004, 277, 146–153. [Google Scholar] [CrossRef]
- Sarigul, G.; Gómez-Palos, I.; Linares, N.; García-Martínez, J.; Costa, R.D.; Serrano, E. The Use of N^N Ligands as an Alternative Strategy for the Sol–Gel Synthesis of Visible-Light Activated Titanias. J. Mater. Chem. C 2020, 8, 12495–12508. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-Free Rutile and Anatase TiO2 Nanocrystals as Electron Extraction layers for High Performance Inverted Polymer Solar Cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Aita, Y.; Komatsu, M.; Yin, S.; Sato, T. Phase-Compositional Control and Visible Light Photocatalytic Activity of Nitrogen Doped Titania via Solvothermal Process. J. Solid State Chem. 2004, 177, 3235–3238. [Google Scholar] [CrossRef]
- Retamoso, C.; Escalona, N.; González, M.; Barrientos, L.; Allende-González, P.; Stancovich, S.; Serpell, R.; Fierro, J.L.G.; Lopez, M. Effect of Particle Size on the Photocatalytic Activity of Modified Rutile Sand (TiO2) for the Discoloration of Methylene Blue in Water. J. Photochem. Photobiol. A 2019, 378, 136–141. [Google Scholar] [CrossRef]
- Jackman, M.J.; Thomas, A.G.; Muryn, C. Photoelectron Spectroscopy Study of Stoichiometric and Reduced Anatase TiO2 (101) Surfaces: The Effect of Subsurface Defects on Water Adsorption at Near-Ambient Pressures. J. Phys. Chem. C 2015, 119, 13682–13690. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al Nashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Dionysiou, D.D. Use of Selected Scavengers for the Determination of NF-TiO2 Reactive Oxygen Species During the Degradation of Microcystin-LR Under Visible Light Irradiation. J. Mol. Catal. A 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B.; Xiao, X.; Gu, F.; Zhang, R. Roles of the Active Species Involved in the Photocatalytic Oxidation of Benzyl Alcohol into Benzaldehyde on TiO2 Under UV Light: Experimental and DFT Studies. J. Mol. Catal. A 2016, 420, 82–87. [Google Scholar] [CrossRef]
- Goto, H.; Hanada, Y.; Ohno, T.; Matsumura, M. Quantitative Analysis of Superoxide Ion and Hydrogen Peroxide Produced from Molecular Oxygen on Photoirradiated TiO2 Particles. J. Catal. 2004, 225, 223–229. [Google Scholar] [CrossRef]
- Denicourt-Nowicki, A.; Rauchdi, M.; Ait Ali, M.; Roucoux, A. Catalytic Oxidation Processes for the Upgrading of Terpenes: State-of-the-Art and Future Trends. Catalysts 2019, 9, 893. [Google Scholar] [CrossRef]
- Martinez, H.; Amaya, A.A.; Paez-Mozo, E.A.; Martinez-Ortega, F. Aminothiazole Ligand-Type Dioxo-Mo(VI) Complex Anchored on TiO2 Nanotubes for Selective Oxidation of Monoterpenes with Light and O2. Top. Catal. 2022, 65, 1088–1101. [Google Scholar] [CrossRef]
- Martinez, Q.H.; Amaya, Á.; Paez-Mozo, E.; Martinez, O.F.; Valange, S. Photo-Assisted O-Atom Transfer to Monoterpenes with Molecular Oxygen and a dioxoMo(VI) Complex Immobilized on TiO2 Nanotubes. Catal. Today 2020, 375, 441–457. [Google Scholar] [CrossRef]
- Martínez, H.; Paez-Mozo, E.A.; Martínez-Ortega, F. Selective Photo-Epoxidation of (R)-(+)- and (S)-(−)-Limonene by Chiral and Non-Chiral Dioxo-Mo(VI) Complexes Anchored on TiO2-Nanotubes. Top. Catal. 2021, 64, 36–50. [Google Scholar] [CrossRef]
- Munir, S.; Dionysiou, D.D.; Khan, S.B.; Shah, S.M.; Adhikari, B.; Shah, A. Development of Photocatalysts for Selective and Efficient Organic Transformations. J. Photochem. Photobiol. B Biol. 2015, 148, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Bayraktar, O.; Yılmaz, S. Liquid-Phase Oxidation of Carvacrol Using Zeolite-Encapsulated Metal Complexes. Ind. Eng. Chem. Res. 2006, 45, 54–61. [Google Scholar] [CrossRef]
- Palmisano, G.; Augugliaro, V.; Pagliaro, M.; Palmisano, L. Photocatalysis: A Promising Route for 21st Century Organic Chemistry. Chem. Commun. 2007, 33, 3425–3437. [Google Scholar] [CrossRef]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous Photocatalytic Organic Synthesis: State-of-the-Art and Future Perspectives. Green Chem. 2016, 18, 5391. [Google Scholar] [CrossRef]









| Sample | SBET (m2·g−1) | Vp (cm3·g−1) | Dp (nm) |
|---|---|---|---|
| TNTs | 289.81 | 0.60 | 13.1 |
| TNPs | 220.43 | 0.51 | 11.8 |
| a-TNPs | 188.73 | 0.62 | 13.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, H.; Neira, J.; Amaya, Á.A.; Páez-Mozo, E.A.; Martínez Ortega, F. Selective Photooxidation of Valencene and Thymol with Nano-TiO2 and O2 as Oxidant. Molecules 2023, 28, 3868. https://doi.org/10.3390/molecules28093868
Martínez H, Neira J, Amaya ÁA, Páez-Mozo EA, Martínez Ortega F. Selective Photooxidation of Valencene and Thymol with Nano-TiO2 and O2 as Oxidant. Molecules. 2023; 28(9):3868. https://doi.org/10.3390/molecules28093868
Chicago/Turabian StyleMartínez, Henry, Jane Neira, Álvaro A. Amaya, Edgar A. Páez-Mozo, and Fernando Martínez Ortega. 2023. "Selective Photooxidation of Valencene and Thymol with Nano-TiO2 and O2 as Oxidant" Molecules 28, no. 9: 3868. https://doi.org/10.3390/molecules28093868





