Three Component One-Pot Synthesis and Antiproliferative Activity of New [1,2,4]Triazolo[4,3-a]pyrimidines
Abstract
:1. Introduction
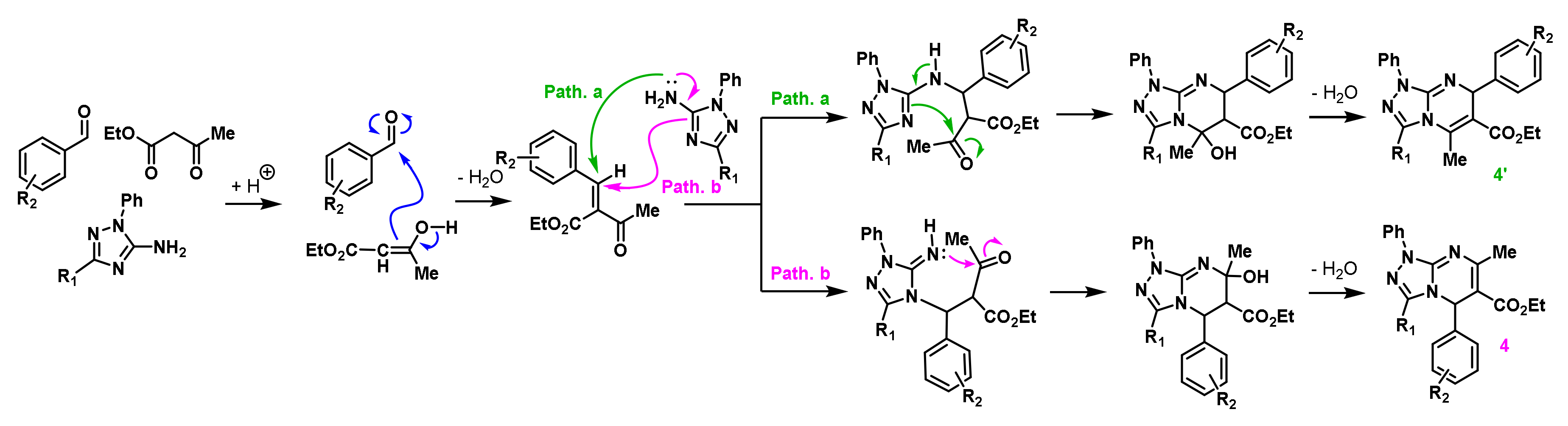
2. Results and Discussion
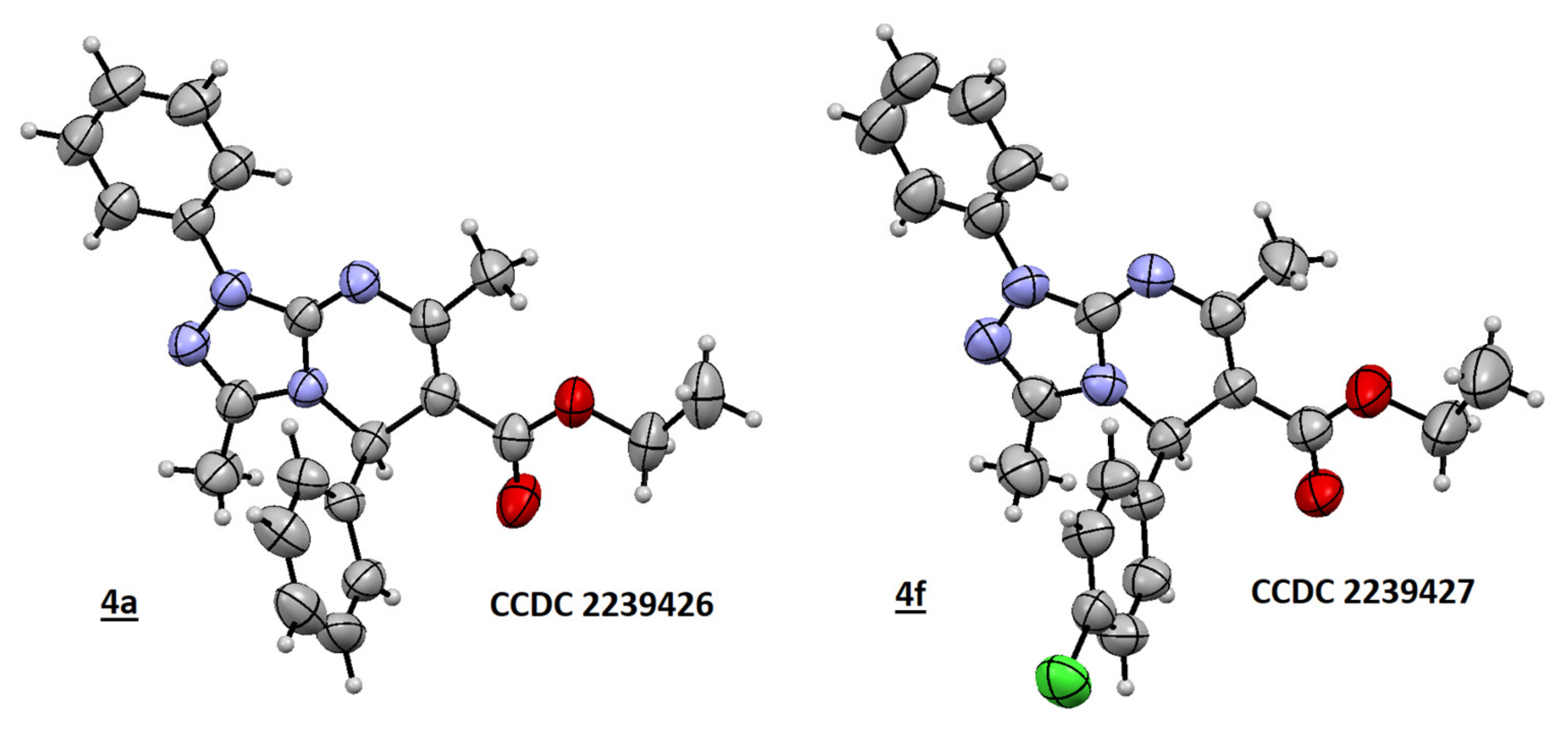
2.1. Chemistry
2.2. Biological Activity: Anticancer Activity In Vitro
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Synthesis of [1,2,4]triazolo[4,3-a]pyrimidines 4a–n
3.2. Pharmacology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jha, M.; Guy, S.; Chou, T.-Y. Microwave assisted synthesis of indole-annulated dihydropyrano [3,4-c]chromene derivatives via hetero-Diels–Alder reaction. Tetrahedron. Lett. 2011, 52, 4337–4341. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pinheiro, E.M.C.; Muri, E.M.F.; Pessôa, J.C.; Cadorini, M.A.; Greco, S.J. Biological activities of [1,2,4]Triazolo[1,5-a]pyrimidines and analogs. Med. Chem. Res. 2020, 29, 1751–1776. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.-H.; Wang, S.-Q.; Zhao, W.; Chen, X.-B.; Yu, B. FDA-approved pyrimidine-fused bicyclic heterocycles for cancer therapy: Synthesis and clinical application. Eur. J. Med. Chem. 2021, 214, 113218. [Google Scholar] [CrossRef] [PubMed]
- Rai, U.S.; Isloor, A.M.; Shetty, P.; Vijesh, A.M.; Prabhu, N.; Isloor, S.; Thiageeswaran, M.; Hoong-Kun, F. Novel chromeno [2,3-b]pyrimidine derivatives as potential anti-microbial agents. Eur. J. Med. Chem. 2010, 45, 2695–2699. [Google Scholar]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A review: Pharmacological aspects of metal based 1,2,4-triazole derived Schiff bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef]
- Salgado, A.; Varela, C.; Collazo, A.M.G.; Pevarello, P. Differentiation between [1,2,4]Triazolo[1,5-a]pyrimidine and [1,2,4]Triazolo[4,3-a]pyrimidine regioisomers by 1H-15N HMBC experiments. Magn. Reson. Chem. 2010, 48, 614–622. [Google Scholar] [CrossRef]
- Fischer, G. Recent progress in 1,2,4-Triazolo[1,5-a]pyrimidine chemistry. Adv. Heterocycl. Chem. 2008, 95, 143–219. [Google Scholar]
- Shaaban, M.R.; Saleh, T.S.; Farag, A.M. Synthesis and antimicrobial evaluation of novel pyrazolo[1,5-a]pyrimidine, Triazolo[1,5-a]pyrimidine and pyrimido[1,2-a]benzimidazole derivatives. Heterocycles 2007, 71, 1765–1777. [Google Scholar] [CrossRef]
- Boutaleb-Charki, S.; Marín, C.; Maldonado, C.R.; Rosales, M.J.; Urbano, J.; Guitierrez-Sánchez, R.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. Copper (II) complexes of [1,2,4]Triazolo[1,5-a]pyrimidine derivatives as potential anti-parasitic agents. Drug. Metab. Lett. 2009, 3, 35–44. [Google Scholar] [CrossRef]
- Ruisi, G.; Canfora, L.; Bruno, G.; Rotondo, A.; Mastropietro, T.F.; Debbia, E.A.; Girasolo, M.A.; Megna, B. Triorganotin(IV) derivatives of 7-amino-2-(methylthio)-[1,2,4]Triazolo[1,5-a]pyrimidine-6-carboxylic acid. Synthesis, spectroscopic characterization, in vitro antimicrobial activity and X-ray crystallography. J. Organomet. Chem. 2010, 695, 546–551. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; Shaaban, M.; Shaaban, K.A.; El-Bondkly, A.M.; Laatsch, H. Essramycin: A first triazolopyrimidine antibiotic isolated from nature. J. Antibiot. 2008, 61, 149–157. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Khalil, A.K.; Abbass, E.M.; El-Naggar, A.M. Design, synthesis of new pyrimidine derivatives as anticancer and antimicrobial agents. Synth. Commun. 2017, 47, 1441–1457. [Google Scholar] [CrossRef]
- El-Zahar, M.I.; Abd El-Karim, S.S.; Haiba, M.E.; Khedr, M.A. Synthesis, antitumor activity and molecular docking study of novel benzofuran-2-yl pyrazole pyridine derivatives. Acta Pol. Pharm. Drug Res. 2011, 68, 357–373. [Google Scholar]
- Prakash, O.; Bhardwaj, V.; Kumar, R.; Tyagi, P.; Aneja, K.R. Organoiodine (III) mediated synthesis of 3-aryl/hetryl-5,7-dimethyl-1,2,4-Triazolo[4,3-a]pyrimidines as antibacterial agents. Eur. J. Med. Chem. 2004, 39, 1073–1077. [Google Scholar] [CrossRef]
- Khera, M.K.; Cliffe, I.A.; Mathur, T.; Prakash, O. Synthesis and in vitro activity of novel 1,2,4-Triazolo[4,3-a]pyrimidine oxazolidinone antibacterial agents. Bioorg. Med. Chem. Lett. 2011, 21, 2887–2889. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Khare, R.; Gupta, G.K.; Beniwal, V. Synthesis, docking study, and DNA photocleavage activity of some pyrimidinyl hydrazones and 3-(quinolin-3-yl)-5,7-dimethyl-1,2,4- Triazolo[4,3-a]pyrimidine derivatives. Med. Chem. Res. 2015, 24, 1830–1841. [Google Scholar] [CrossRef]
- Kamal, R.; Kumar, V.; Kumar, R.; Bhardwaj, J.K.; Saraf, P.; Kumari, P.; Bhardwaj, V. Design, Synthesis, and Screening of Triazolopyrimidine–Pyrazole Hybrids as Potent Apoptotic Inducers. Arch. Pharm. Chem. 2017, 350, e1700137. [Google Scholar] [CrossRef]
- Oukoloff, K.; Lucero, B.; Francisco, K.R.; Brunden, K.R.; Ballatore, C. 1,2,4-Triazolo[1,5-a]pyrimidines in drug design. Eur. J. Med. Chem. 2019, 165, 332–346. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Qu, R.-Y.; Chen, Q.; Yang, J.-F.; Cong-Wei, N.; Zhen, X.; Yang, G.-F. Triazolopyrimidines as a new herbicidal lead for combating weed resistance associated with acetohydroxyacid synthase mutation. J. Agric. Food Chem. 2016, 64, 4845–4857. [Google Scholar] [CrossRef]
- Ochoa, C.; Goya, P. Six-membered ring systems: Triazines, tetrazines and fused ring polyaza systems. Prog. Heterocycl. Chem. 2000, 12, 294–316. [Google Scholar]
- Hajos, G.; Riedl, Z. Bicyclic 5–6 systems with one bridgehead (ring junction) nitrogen atom: Three extra heteroatoms 2:1. Compr. Heterocycl. Chem. III 2008, 11, 671–763. [Google Scholar]
- Yet, L. Five-membered ring sysyems: With more than one N atom. Prog. Heterocycl. Chem. 2009, 21, 224–260. [Google Scholar]
- Fizer, M.; Slivka, M. Synthesis of [1,2,4-Triazolo[1,5-a]pyrimidine (microreview). Chem. Heterocycl. Compd. 2016, 52, 155–157. [Google Scholar] [CrossRef]
- Fischer, G. Recent advances in 1,2,4-Triazolo[1,5-a]pyrimidine chemistry. Adv. Heterocycl. Chem. 2019, 128, 1–101. [Google Scholar]
- El Ashry, S.H.; Awad, L.F.; Badawy, M.E.I.; Rabea, E.I.; Ibrahim, N.A.; Abd Al Moaty, M.N. Synthesis, antibacterial, antioxidant, and molecular docking studies of 6-methylpyrimidin-4(3H)-one and oxo-1,2,4-Triazolo[4,3-a]pyrimidine derivative. J. Mol. Struct. 2022, 1249, 131551. [Google Scholar] [CrossRef]
- El Ashry, S.H.; Awad, L.F.; Teleb, M.; Ibrahim, N.A.; Abu-Serie, M.M.; Abd Al Moaty, M.N. Structure-based design and optimization of pyrimidine- and 1,2,4-Triazolo[4,3-a]pyrimidine-based matrix metalloproteinase-10/13 inhibitors via Dimroth rearrangement towards targeted polypharmacology. Bioorg. Chem. 2020, 96, 103616. [Google Scholar] [CrossRef]
- Bowack, D.A.; Lapworth, A. CLXXIX.-Hydrizino-halides derived from oxalic acid. J. Chem. Soc., Trans. 1905, 87, 1854–1869. [Google Scholar] [CrossRef]
- Silpa, L.; Niepceron, N.; Laurent, F.; Brossier, F.; Penichon, M.; Enguehard-Gueiffier, C.; Abarbri, M.; Silvestre, A.; Petrignet, J. Synthesis and evaluation of the anticoccidial activity of trifluoropyrido[1,2-a]pyrimidin-2-one derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 114–120. [Google Scholar] [CrossRef]
- Jismy, B.; Allouchi, H.; Guillaumet, G.; Akssira, M.; Abarbri, M. Concise and efficient access to 5,7- Disubstituted pyrazolo[1,5-a]pyrimidines by Pd-Catalyzed sequential arylation, alkynylation and SNAr reaction. Eur. J. Org. Chem. 2017, 2017, 6168–6178. [Google Scholar] [CrossRef]
- Jismy, B.; Guillaumet, G.; Akssira, M.; Knez, D.; Abarbri, M. A simple and a practical strategy to synthesize new 5-trifluoromethyl-7-substituted Imidazo[1,2-a]pyrimidines and benzimidazo[1,2-a]pyrimidines, and their biological evaluation test. New. J. Chem. 2019, 43, 9961–9968. [Google Scholar] [CrossRef]
- Jismy, B.; El Qami, A.; Pislar, A.; Frlan, R.; Kos, J.; Gobec, S.; Knez, D.; Abarbri, M. Pyrimido[1,2-b]indazole derivatives: Selective inhibitors of human monoamine oxidase B with neuroprotective activity. Eur. J. Med. Chem. 2021, 209, 112911. [Google Scholar] [CrossRef]
- Deshmukh, M.B.; Salunkhe, S.M.; Patil, D.R.; Anbhule, P.V. A novel and efficient one step synthesis of 2-amino- 5-cyano-6-hydroxy-4-aryl pyrimidines and their anti-bacterial activity. Eur. J. Med. Chem. 2009, 44, 2651–2654. [Google Scholar] [CrossRef]
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Weber, L.; Illgen, K.; Almstetter, M. Discovery of new multi component reactions with combinatorial methods. Synlett 1999, 3, 366–374. [Google Scholar] [CrossRef]
- Dandia, A.; Singh, R.; Singh, D.; Arya, K. Facile regioselective green synthesis of Triazolo[4,3-a]pyrimidines in aqueous medium. Lett. Org. Chem. 2009, 6, 100–105. [Google Scholar] [CrossRef]
- Ablajan, K.; Kamil, W.; Tuoheti, A.; Sun Wan-Fu, S. An Efficient Three Component One-Pot Synthesis of 5-Amino-7-aryl-7,8-dihydro-[1,2,4]Triazolo[4,3-a]-pyrimidine-6-carbonitriles. Molecules 2012, 17, 1860–1869. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Zhidovinova, M.S.; Rusinov, G.L.; Ovchinnikova, I.G. Aminoazoles in the three-component synthesis of 7-substituted 6-ethoxycarbonyl-5-methyl-4,7-dihydroazolo[1,5-a]pyrimidines. Russ. Chem. Bull. 2003, 52, 1768–1769. [Google Scholar] [CrossRef]
- El Rady, E.A. Three-component uncatalyzed eco-friendly reactions for one-pot Synthesis of 4,7-dihydro[1,2,4]Triazolo[1,5-a]pyrimidine derivatives. J. Hetrocycl. Chem. 2014, 51, 869–875. [Google Scholar] [CrossRef]
- Deposit Numbers CCDC 2239427(for 4a) and CCDC 2239426 (for 4f) Contain Additional Crystallographic Data for This Article. These Data are Provided Free of Charge by the Joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures. Available online: www.ccdc.cam.ac.uk/structures (accessed on 15 March 2023).
- Hodeify, R.; Siddiqui, S.S.; Matar, R.; Vazhappilly, C.G.; Merheb, M.; Al Zouabi, H.; Marton, J. Modulation of calcium-binding proteins expression and cisplatin chemosensitivity by calcium chelation in human breast cancer MCF-7 cells. Heliyon 2021, 7, e06041. [Google Scholar] [CrossRef]
- Aouali, M.; Mhalla, D.; Allouche, F.; El Kaim, L.; Tounsi, S.; Trigui, M.; Chabchoub, F. Synthesis, antimicrobial and antioxidant activities of imidazotriazoles and new multicomponent reaction toward 5-amino-1-phenyl[1,2,4]triazole derivatives. Med. Chem. Res. 2015, 24, 2732–2741. [Google Scholar] [CrossRef]
- Msalbi, D.; Elloumi-Mseddi, J.; Hakim, B.; Sahli, E.; Aifa, S. Chemical alternative for cell identification and cross-contamination detection. 3 Biotech. 2022, 12, 78. [Google Scholar] [CrossRef] [PubMed]




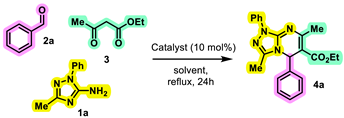 | |||
|---|---|---|---|
| Entry | Solvent | Catalyst | Yield (%) a |
| 1 | H2O | APTS | Trace |
| 2 | Ethanol | APTS | 75 |
| 3 | Ethanol | HCl | 45 |
| 4 | Ethanol | Acetic acid | 10 |
| 5 | Ethanol | Piperidine | Trace |
| 6 | CH3CN | APTS | 50 |
| 7 | Fusion state | - | 10 |
| Compound | IC50 (μM) | Compound | IC50 (μM) | ||
|---|---|---|---|---|---|
| MDA-MB-231 | MCF-7 | MDA-MB-231 | MCF-7 | ||
| 4a | 41.30 ± 1.8 | 43.86 ± 3.1 | 4h | >100 | >100 |
| 4b | 38.31 ± 2 | 36.28 ± 3.3 | 4i | 43.88 ± 1 | >100 |
| 4c | 17.83 ± 2 | 20.33 ± 1.7 | 4j | 23.97 ± 2.7 | 19.73 ± 1.5 |
| 4d | 44.63 ± 0.3 | 34.20 ± 1 | 4k | 53.45 ± 4.9 | 48.45 ± 1.1 |
| 4e | 62.26 ± 1.7 | >100 | 4l | >100 | >100 |
| 4f | 43.79 ± 1.8 | 32.09 ± 2.8 | 4m | 84.43 ± 3.8 | >100 |
| 4g | 39.57 ± 0.3 | 51.26 ± 0.7 | 4n | 58.34 ± 3.4 | 46.34 ± 2 |
| Cisplatin | 38.6 ± 0.8 | 83.1 ± 1.3 | Cisplatin | 38.6 ± 0.8 | 83.1 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Hassen, M.; Msalbi, D.; Jismy, B.; Elghali, F.; Aifa, S.; Allouchi, H.; Abarbri, M.; Chabchoub, F. Three Component One-Pot Synthesis and Antiproliferative Activity of New [1,2,4]Triazolo[4,3-a]pyrimidines. Molecules 2023, 28, 3917. https://doi.org/10.3390/molecules28093917
Ben Hassen M, Msalbi D, Jismy B, Elghali F, Aifa S, Allouchi H, Abarbri M, Chabchoub F. Three Component One-Pot Synthesis and Antiproliferative Activity of New [1,2,4]Triazolo[4,3-a]pyrimidines. Molecules. 2023; 28(9):3917. https://doi.org/10.3390/molecules28093917
Chicago/Turabian StyleBen Hassen, Manel, Dhouha Msalbi, Badr Jismy, Fares Elghali, Sami Aifa, Hassan Allouchi, Mohamed Abarbri, and Fakher Chabchoub. 2023. "Three Component One-Pot Synthesis and Antiproliferative Activity of New [1,2,4]Triazolo[4,3-a]pyrimidines" Molecules 28, no. 9: 3917. https://doi.org/10.3390/molecules28093917
APA StyleBen Hassen, M., Msalbi, D., Jismy, B., Elghali, F., Aifa, S., Allouchi, H., Abarbri, M., & Chabchoub, F. (2023). Three Component One-Pot Synthesis and Antiproliferative Activity of New [1,2,4]Triazolo[4,3-a]pyrimidines. Molecules, 28(9), 3917. https://doi.org/10.3390/molecules28093917








