Cold-Sintered ZnO Ceramic Composites Co-Doped with Polytetrafluoroethylene and Oxides
Abstract
:1. Introduction
2. Results and Discussion
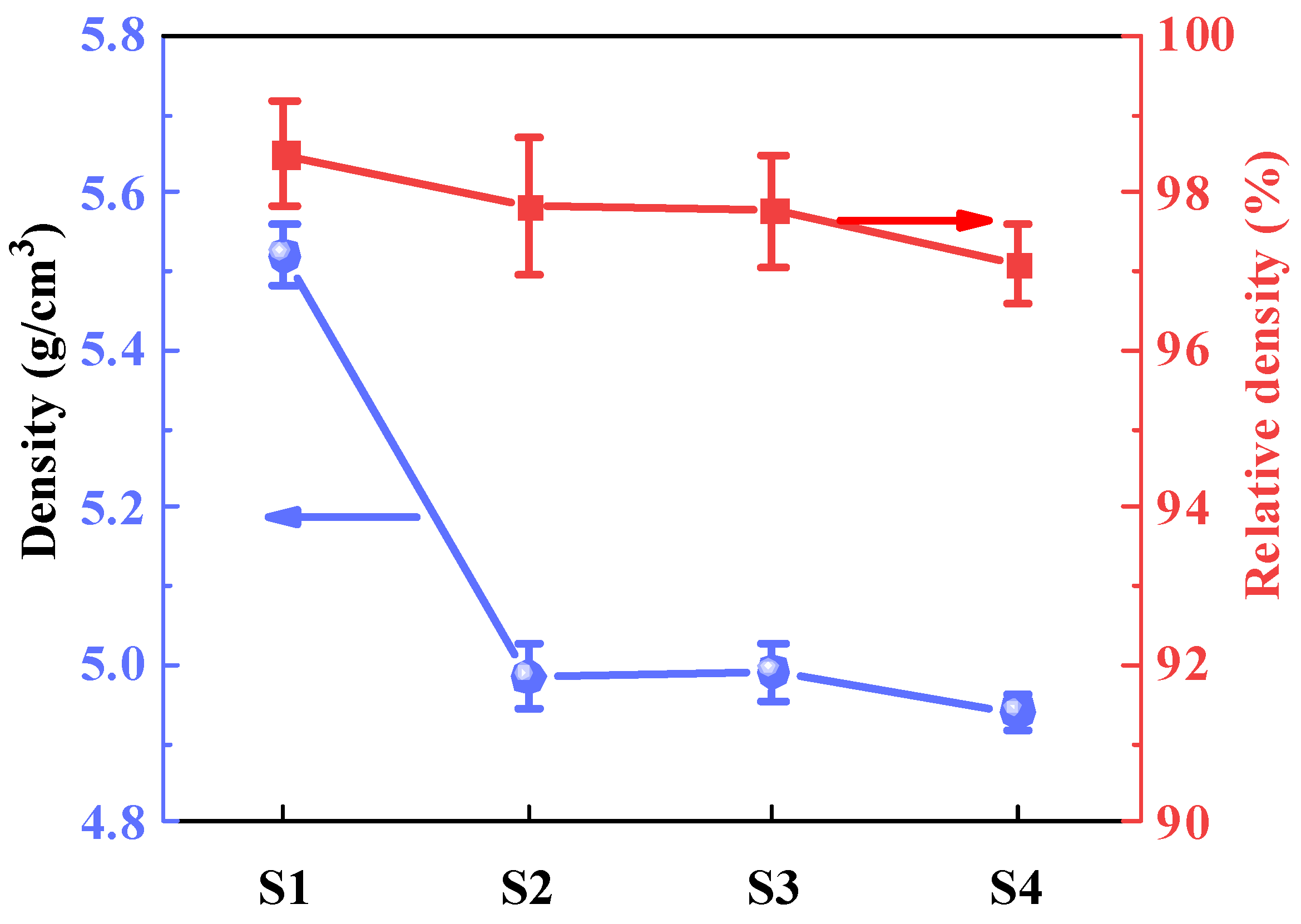
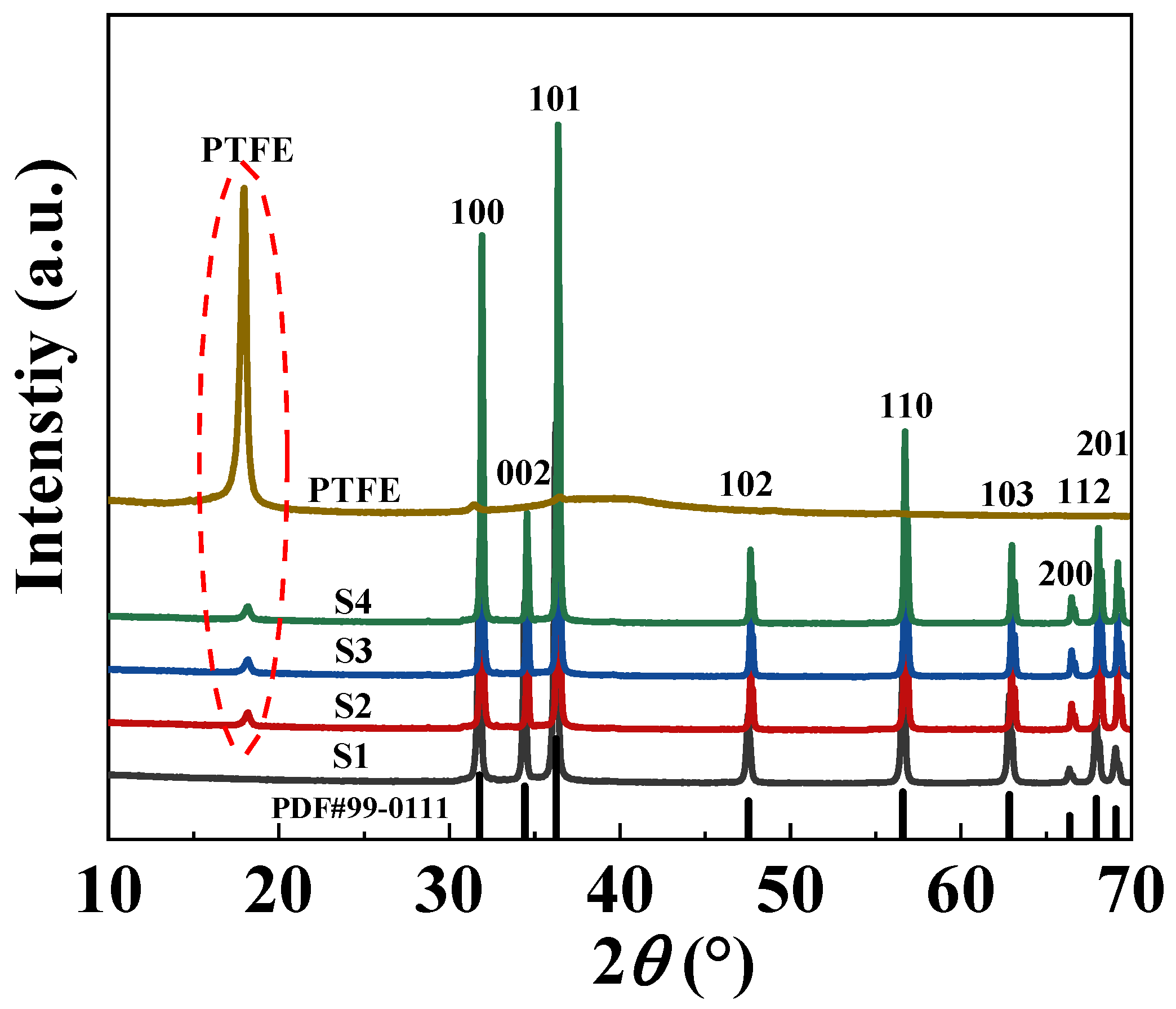
2.1. Density and Crystal Structure
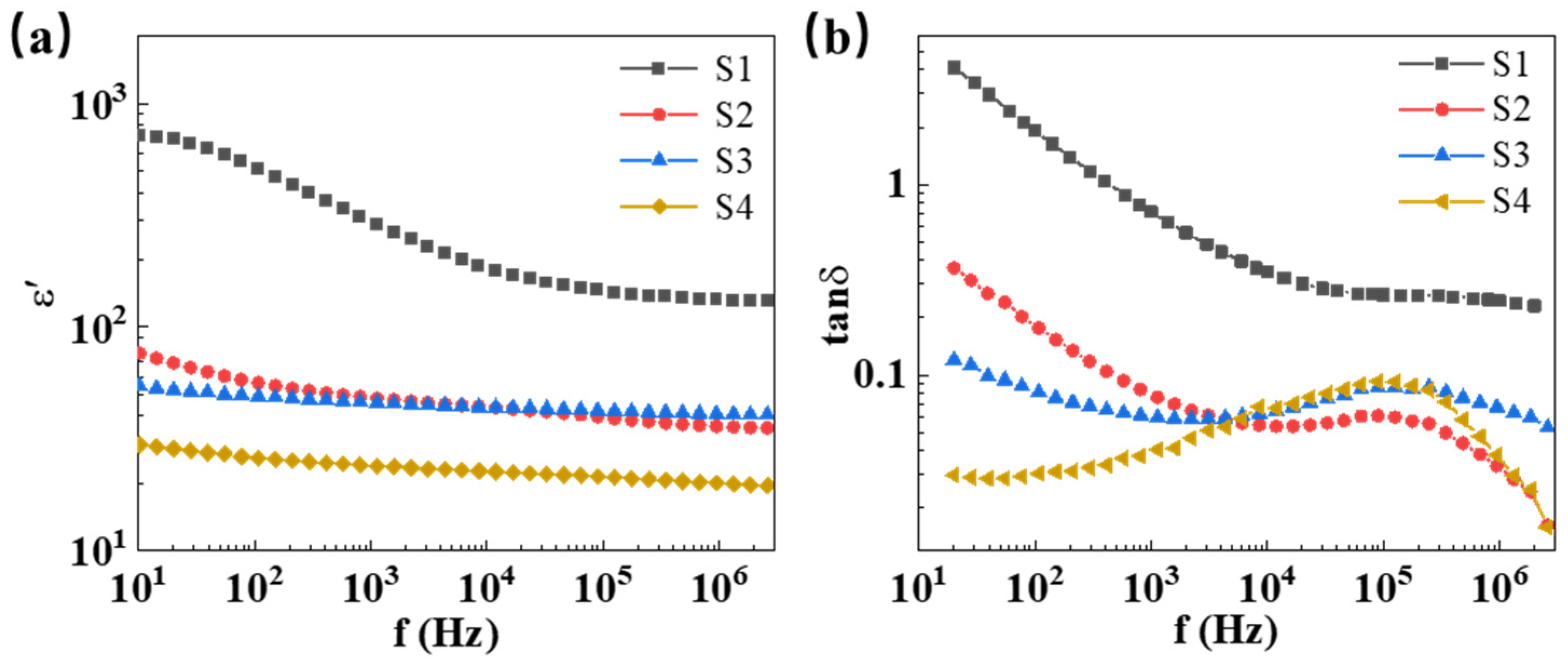
2.2. Microstructure and Dielectric Properties
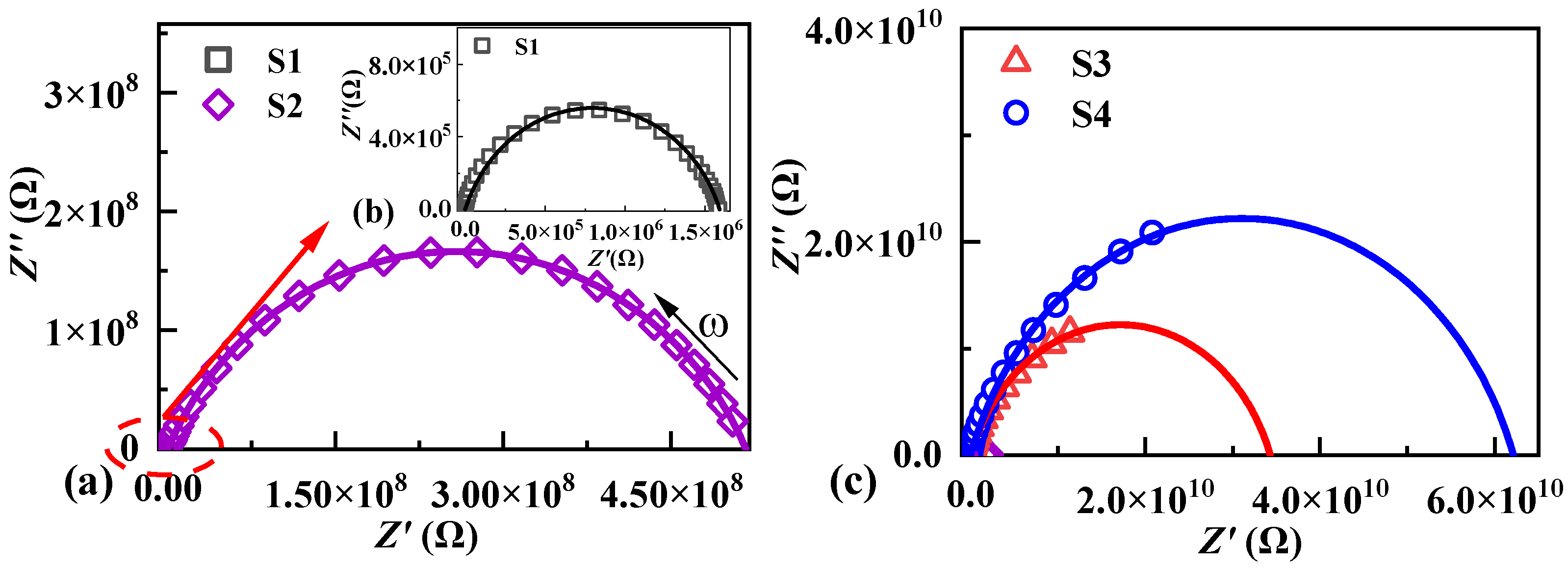
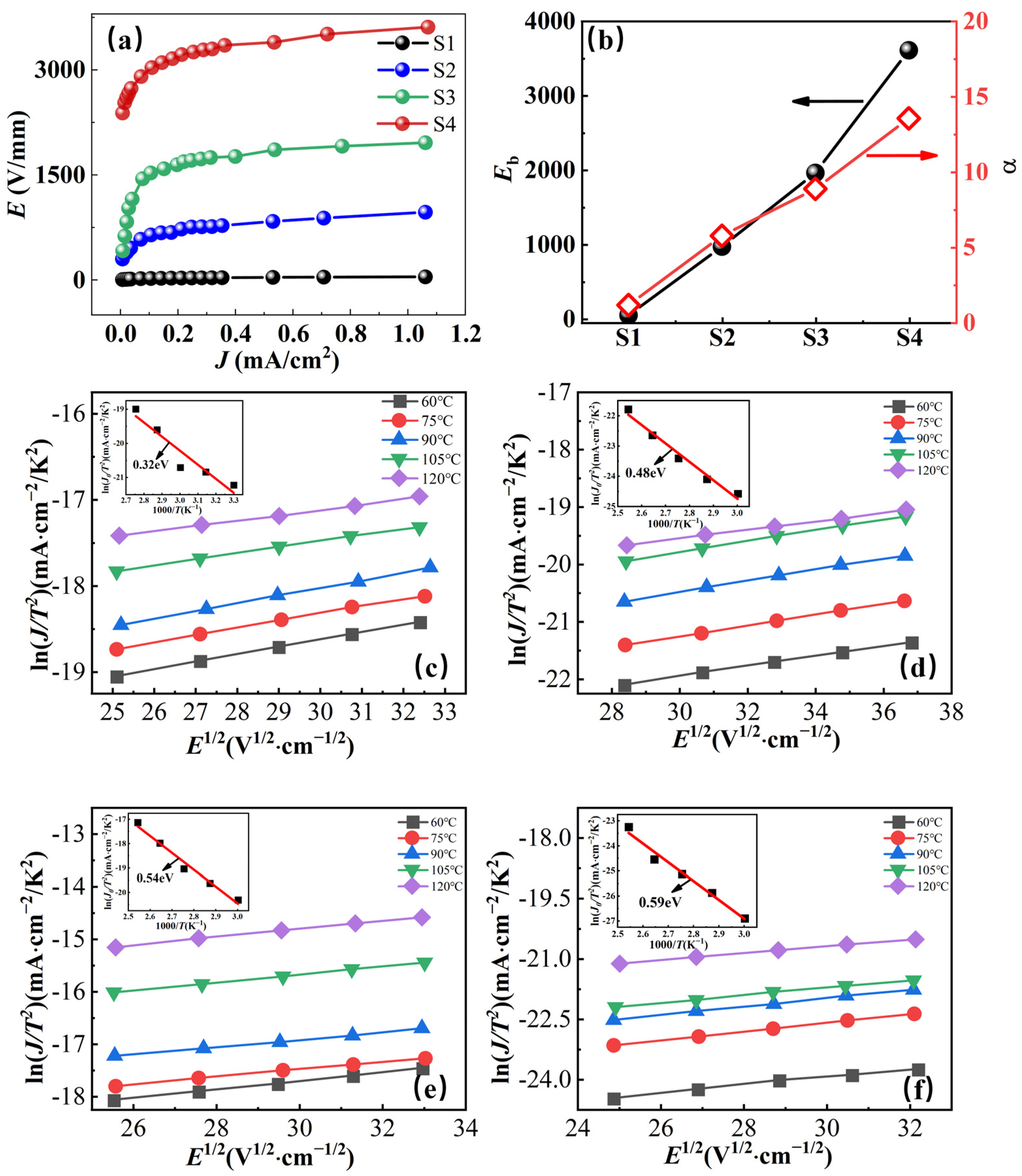
2.3. Nonlinear Ohmic Characteristics and Schottky Barriers
3. Experimental Procedure
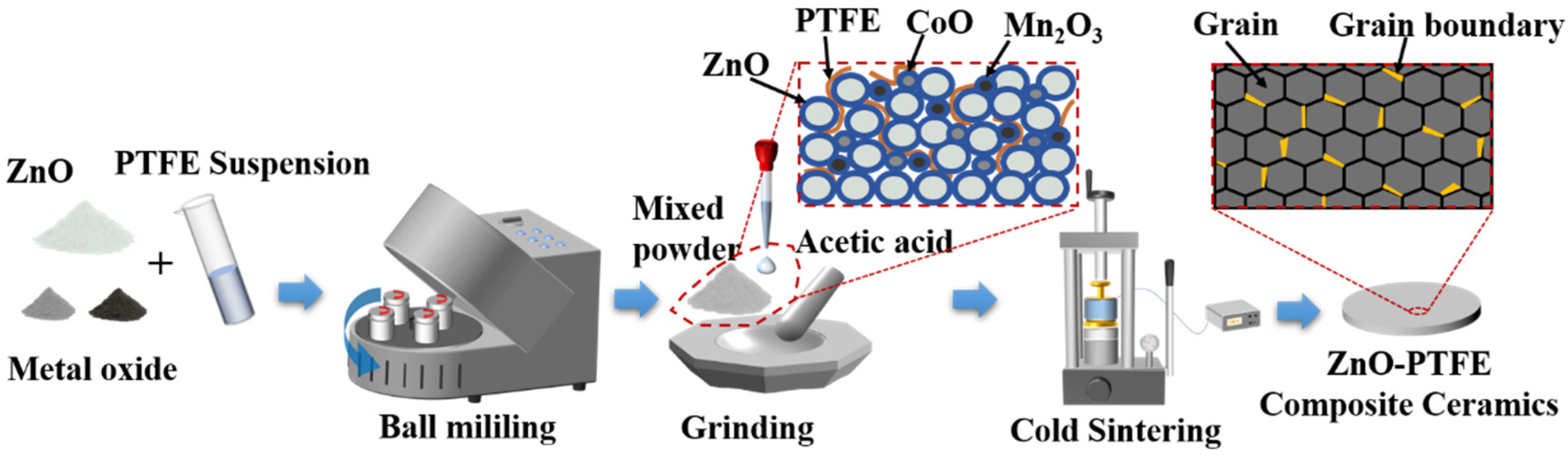
3.1. Sample Preparation
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Żydek, A.; Wermiński, M.; Trybula, M.E. Description of Grain Boundary Structure and Topology in Nanocrystalline Aluminum Using Voronoi Analysis and Order Parameter. Comput. Mater. Sci. 2021, 197, 110660. [Google Scholar] [CrossRef]
- Chen, I.W.; Wang, X.H. Sintering Dense Nanocrystalline Ceramics without Final-Stage Grain Growth. Nature 2000, 404, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Van Swygenhoven, H.; Weertman, J.R. Deformation in Nanocrystalline Metals. Mater. Today 2006, 9, 24–31. [Google Scholar] [CrossRef]
- Vladimirov, I.; Kühn, M.; Geßner, T.; May, F.; Weitz, R.T. Energy Barriers at Grain Boundaries Dominate Charge Carrier Transport in an Electron-Conductive Organic Semiconductor. Sci. Rep. 2018, 8, 14868. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Guo, J.; Hao, J.; Zhao, X.; Randall, C.A.; Wang, H. Cold Sintered Composites Consisting of PEEK and Metal Oxides with Improved Electrical Properties via the Hybrid Interfaces. Compos. Part B Eng. 2021, 226, 109349. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.G. Materials and Methods for Interface Engineering toward Stable and Efficient Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, T.; Rufner, J.F.; LaGrange, T.B.; Campbell, G.H.; Lavernia, E.J.; Schoenung, J.M.; van Benthem, K. Metal/Ceramic Interface Structures and Segregation Behavior in Aluminum-Based Composites. Acta Mater. 2015, 95, 254–263. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of Zinc Oxide Varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Greuter, F.; Blatter, G. Electrical Properties of Grain Boundaries in Polycrystalline Compound Semiconductors. Semicond. Sci. Technol. 1990, 5, 111–137. [Google Scholar] [CrossRef]
- Recnik, A.; Bernik, S.; Daneu, N. Microstructural Engineering of ZnO-Based Varistor Ceramics. J. Mater. Sci. 2012, 47, 1655–1668. [Google Scholar] [CrossRef]
- Masteghin, M.G.; Varela, J.A.; Orlandi, M.O. Controlling the Breakdown Electric Field in SnO2 Based Varistors by the Insertion of SnO2 Nanobelts. J. Eur. Ceram. Soc. 2017, 37, 1535–1540. [Google Scholar] [CrossRef]
- Staykov, A.; Tellez, H.; Druce, J.; Wu, J.; Ishihara, T.; Kilner, J. Electronic Properties and Surface Reactivity of SrO-Terminated SrTiO3 and SrO-Terminated Iron-Doped SrTiO3. Sci. Technol. Adv. Mater. 2018, 19, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, X.; Sun, J.; Ren, L.; Liao, R.; Yang, L.; Li, W. Enhanced Electrical Properties of ZnO Varistor Ceramics by Spark Plasma Sintering: Role of Annealing. Ceram. Int. 2020, 46, 15076–15083. [Google Scholar] [CrossRef]
- Yan, M.F.; Rhodes, W.W. Preparation and Properties of TiO2 Varistors. Appl. Phys. Lett. 1982, 40, 536–537. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, K.; Li, J.; Huang, S. Optimized Dual-Function Varistor-Capacitor Ceramics of Core-Shell Structured xBi2/3Cu3Ti4O12/(1-x)CaCu3Ti4O12 Composites. J. Eur. Ceram. Soc. 2020, 40, 3437–3444. [Google Scholar] [CrossRef]
- Tian, T.; Zheng, L.; Podlogar, M.; Zeng, H.; Bernik, S.; Xu, K.; Ruan, X.; Shi, X.; Li, G. Novel Ultrahigh-Performance ZnO-Based Varistor Ceramics. ACS Appl. Mater. Interfaces 2021, 13, 35924–35929. [Google Scholar] [CrossRef] [PubMed]
- Dillip, G.R.; Banerjee, A.N.; Joo, S.W. Template-Based Synthesis of Hollow Nanotubular ZnO Structures and Nonlinear Electrical Properties under Field-Induced Trap-Assisted Tunneling. J. Phys. Chem. C 2020, 124, 28371–28386. [Google Scholar] [CrossRef]
- Triboulet, R.; Perrière, J. Epitaxial Growth of ZnO Films. Prog. Cryst. Growth Charact. Mater. 2003, 47, 65–138. [Google Scholar] [CrossRef]
- Jood, P.; Mehta, R.J.; Zhang, Y.; Peleckis, G.; Wang, X.; Siegel, R.W.; Borca-Tasciuc, T.; Dou, S.X.; Ramanath, G. Al-Doped Zinc Oxide Nanocomposites with Enhanced Thermoelectric Properties. Nano Lett. 2011, 11, 4337–4342. [Google Scholar] [CrossRef]
- Cai, K.F.; Müller, E.; Drašar, C.; Mrotzek, A. Preparation and Thermoelectric Properties of Al-Doped ZnO Ceramics. Mater. Sci. Eng. B 2003, 104, 45–48. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener Model Description of Ferromagnetism in Zinc-Blende Magnetic Semiconductors. Science 2000, 287, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, H.; Lu, L.; Jiang, F.; Yang, C. Preparation and Properties of AZO Thin Films on Different Substrates. Prog. Nat. Sci. Mater. Int. 2010, 20, 44–48. [Google Scholar] [CrossRef]
- Clarke, D.R. Varistor ceramics. J. Am. Ceram. Soc. 1999, 82, 485–502. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, T.; Ikuhara, Y. Atomic Structures and Electrical Properties of ZnO Grain Boundaries. J. Am. Ceram. Soc. 2007, 90, 337–357. [Google Scholar] [CrossRef]
- Matsuoka, M. Nonohmic Properties of Zinc Oxide Ceramics. Jpn. J. Appl. Phys. 1971, 10, 736. [Google Scholar] [CrossRef]
- Hembram, K.; Rao, T.N.; Srinivasa, R.S.; Kulkarni, A.R. High Performance Varistors Prepared from Doped ZnO Nanopowders Made by Pilot-Scale Flame Spray Pyrolyzer: Sintering, Microstructure and Properties. J. Eur. Ceram. Soc. 2015, 35, 3535–3544. [Google Scholar] [CrossRef]
- Daneu, N.; Rečnik, A.; Bernik, S. Grain-Growth Phenomena in ZnO Ceramics in the Presence of Inversion Boundaries. J. Am. Ceram. Soc. 2011, 94, 1619–1626. [Google Scholar] [CrossRef]
- He, J. Metal Oxide Varistors: From Microstructure to Macro-Characteristics; Tsinghua University Press: Beijing, China, 2019; ISBN 978-3-527-33382-0. [Google Scholar]
- Bernik, S. Low-temperature sintering of ZnO–Bi2O3-based varistor ceramics for enhanced microstructure development and current-voltage characteristics. Ceram.–Silik. 2018, 62, 8–14. [Google Scholar] [CrossRef]
- Subasri, R.; Asha, M.; Hembram, K.; Rao, G.V.N.; Rao, T.N. Microwave Sintering of Doped Nanocrystalline ZnO and Characterization for Varistor Applications. Mater. Chem. Phys. 2009, 115, 677–684. [Google Scholar] [CrossRef]
- Macary, L.S.; Kahn, M.L.; Estournès, C.; Fau, P.; Trémouilles, D.; Bafleur, M.; Renaud, P.; Chaudret, B. Size Effect on Properties of Varistors Made from Zinc Oxide Nanoparticles through Low Temperature Spark Plasma Sintering. Adv. Funct. Mater. 2009, 19, 1775–1783. [Google Scholar] [CrossRef]
- Arbatti, M.; Shan, X.; Cheng, Z.-Y. Ceramic–Polymer Composites with High Dielectric Constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric Composites for Powder-Based Additive Manufacturing: Materials and Applications. Prog. Polym. Sci. 2019, 91, 141–168. [Google Scholar] [CrossRef]
- Funahashi, S.; Guo, J.; Guo, H.; Wang, K.; Baker, A.L.; Shiratsuyu, K.; Randall, C.A. Demonstration of the Cold Sintering Process Study for the Densification and Grain Growth of ZnO Ceramics. J. Am. Ceram. Soc. 2017, 100, 546–553. [Google Scholar] [CrossRef]
- Guo, J.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Kupp, E.R.; Messing, G.L.; Randall, C.A. Cold Sintering: A Paradigm Shift for Processing and Integration of Ceramics. Angew. Chem. 2016, 128, 11629–11633. [Google Scholar] [CrossRef]
- Guo, J.; Floyd, R.; Lowum, S.; Maria, J.-P.; Herisson de Beauvoir, T.; Seo, J.-H.; Randall, C.A. Cold Sintering: Progress, Challenges, and Future Opportunities. Annu. Rev. Mater. Res. 2019, 49, 275–295. [Google Scholar] [CrossRef]
- Guo, J.; Berbano, S.S.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Randall, C.A. Cold Sintering Process of Composites: Bridging the Processing Temperature Gap of Ceramic and Polymer Materials. Adv. Funct. Mater. 2016, 26, 7115–7121. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, X.; Herisson De Beauvoir, T.; Seo, J.-H.; Berbano, S.S.; Baker, A.L.; Azina, C.; Randall, C.A. Recent Progress in Applications of the Cold Sintering Process for Ceramic–Polymer Composites. Adv. Funct. Mater. 2018, 28, 1801724. [Google Scholar] [CrossRef]
- Sohrabi Baba Heidary, D.; Lanagan, M.; Randall, C.A. Contrasting Energy Efficiency in Various Ceramic Sintering Processes. J. Eur. Ceram. Soc. 2018, 38, 1018–1029. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, J.; Sun, J.; Guo, J.; Dursun, S.; Wang, K.; Randall, C.A. Cold Sintering ZnO Based Varistor Ceramics with Controlled Grain Growth to Realize Superior Breakdown Electric Field. J. Eur. Ceram. Soc. 2021, 41, 430–435. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, J.; Wang, K.; Herisson De Beauvoir, T.; Li, B.; Randall, C.A. Introducing a ZnO–PTFE (Polymer) Nanocomposite Varistor via the Cold Sintering Process. Adv. Eng. Mater. 2018, 20, 1700902. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Tsuji, K.; Wang, K.; Bang, S.H.; Randall, C.A. Sintering Mechanisms and Dielectric Properties of Cold Sintered (1-x) SiO2—x PTFE Composites. J. Eur. Ceram. Soc. 2019, 39, 4743–4751. [Google Scholar] [CrossRef]
- Sada, T.; Tsuji, K.; Ndayishimiye, A.; Fan, Z.; Fujioka, Y.; Randall, C.A. High Permittivity BaTiO3 and BaTiO3-Polymer Nanocomposites Enabled by Cold Sintering with a New Transient Chemistry: Ba(OH)2∙8H2O. J. Eur. Ceram. Soc. 2021, 41, 409–417. [Google Scholar] [CrossRef]
- Wurst, J.C.; Nelson, J.A. Lineal Intercept Technique for Measuring Grain Size in Two-Phase Polycrystalline Ceramics. J. Am. Ceram. Soc. 1972, 55, 109. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, Y.; Kang, S.; Li, Y.; Cheng, L.; Ren, C.; Guo, J.; Wang, X.; Yang, L.; Liao, R. Comparative Study on Energy Efficiency and Densification of ZnO Ceramics Using Various Sintering Processes. J. Mater. Sci. Mater. Electron. 2023, 34, 1798. [Google Scholar] [CrossRef]
- Sinclair, D.C.; West, A.R. Impedance and Modulus Spectroscopy of Semiconducting BaTiO3 Showing Positive Temperature Coefficient of Resistance. J. Appl. Phys. 1989, 66, 3850–3856. [Google Scholar] [CrossRef]
- Xiang, F.; Wang, H.; Yao, X. Preparation and Dielectric Properties of Bismuth-Based Dielectric/PTFE Microwave Composites. J. Eur. Ceram. Soc. 2006, 26, 1999–2002. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Li, H.; Li, S. Intrinsic and Extrinsic Defect Relaxation Behavior of ZnO Ceramics. J. Appl. Phys. 2012, 111, 124106. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, R.; Liang, N.; Yang, L.; Li, J.; Li, J. Role of Defects in Determining the Electrical Properties of ZnO Ceramics. J. Appl. Phys. 2014, 116, 014103. [Google Scholar] [CrossRef]

| Composite Powder | ZnO | PTFE | CoO | Mn2O3 |
|---|---|---|---|---|
| P1 | 100 | 0 | 0 | 0 |
| P2 | 85 | 15 | 0 | 0 |
| P3 | 84 | 15 | 1 | 0 |
| P4 | 83.5 | 15 | 1 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Yang, Y.; Kang, S.; Li, Y.; Hou, X.; Ren, C.; Wang, X.; Zhao, X. Cold-Sintered ZnO Ceramic Composites Co-Doped with Polytetrafluoroethylene and Oxides. Molecules 2024, 29, 129. https://doi.org/10.3390/molecules29010129
Xiao Y, Yang Y, Kang S, Li Y, Hou X, Ren C, Wang X, Zhao X. Cold-Sintered ZnO Ceramic Composites Co-Doped with Polytetrafluoroethylene and Oxides. Molecules. 2024; 29(1):129. https://doi.org/10.3390/molecules29010129
Chicago/Turabian StyleXiao, Yongjian, Yang Yang, Shenglin Kang, Yuchen Li, Xinyuan Hou, Chengjun Ren, Xilin Wang, and Xuetong Zhao. 2024. "Cold-Sintered ZnO Ceramic Composites Co-Doped with Polytetrafluoroethylene and Oxides" Molecules 29, no. 1: 129. https://doi.org/10.3390/molecules29010129
APA StyleXiao, Y., Yang, Y., Kang, S., Li, Y., Hou, X., Ren, C., Wang, X., & Zhao, X. (2024). Cold-Sintered ZnO Ceramic Composites Co-Doped with Polytetrafluoroethylene and Oxides. Molecules, 29(1), 129. https://doi.org/10.3390/molecules29010129






