Metabolomics Analysis of Sodium Salicylate Improving the Preservation Quality of Ram Sperm
Abstract
:1. Introduction
2. Results
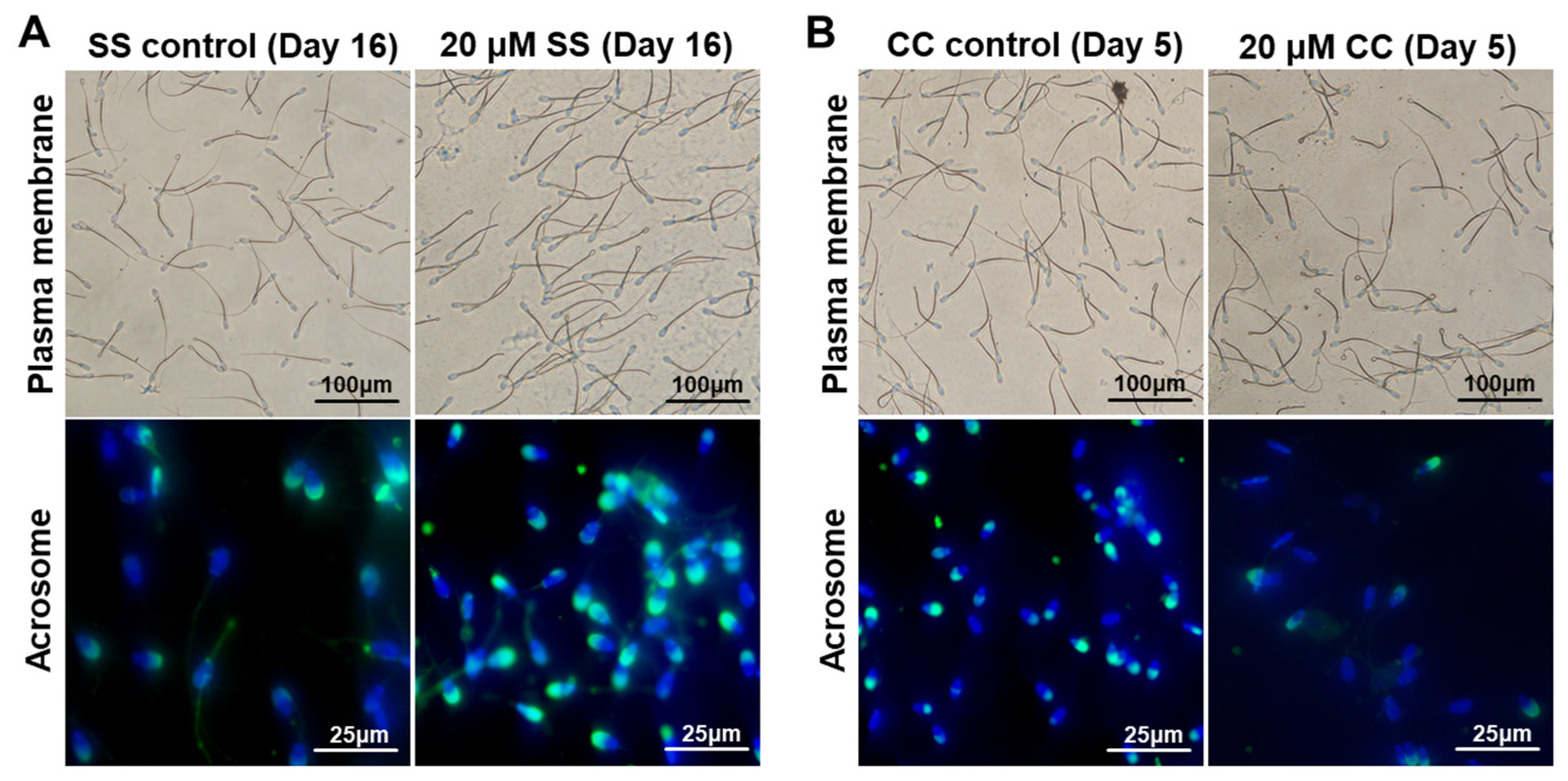
2.1. Effects of SS on Sperm Quality
2.2. Effects of CC on Sperm Quality
2.3. Effects of SS and CC on the Antioxidant Ability
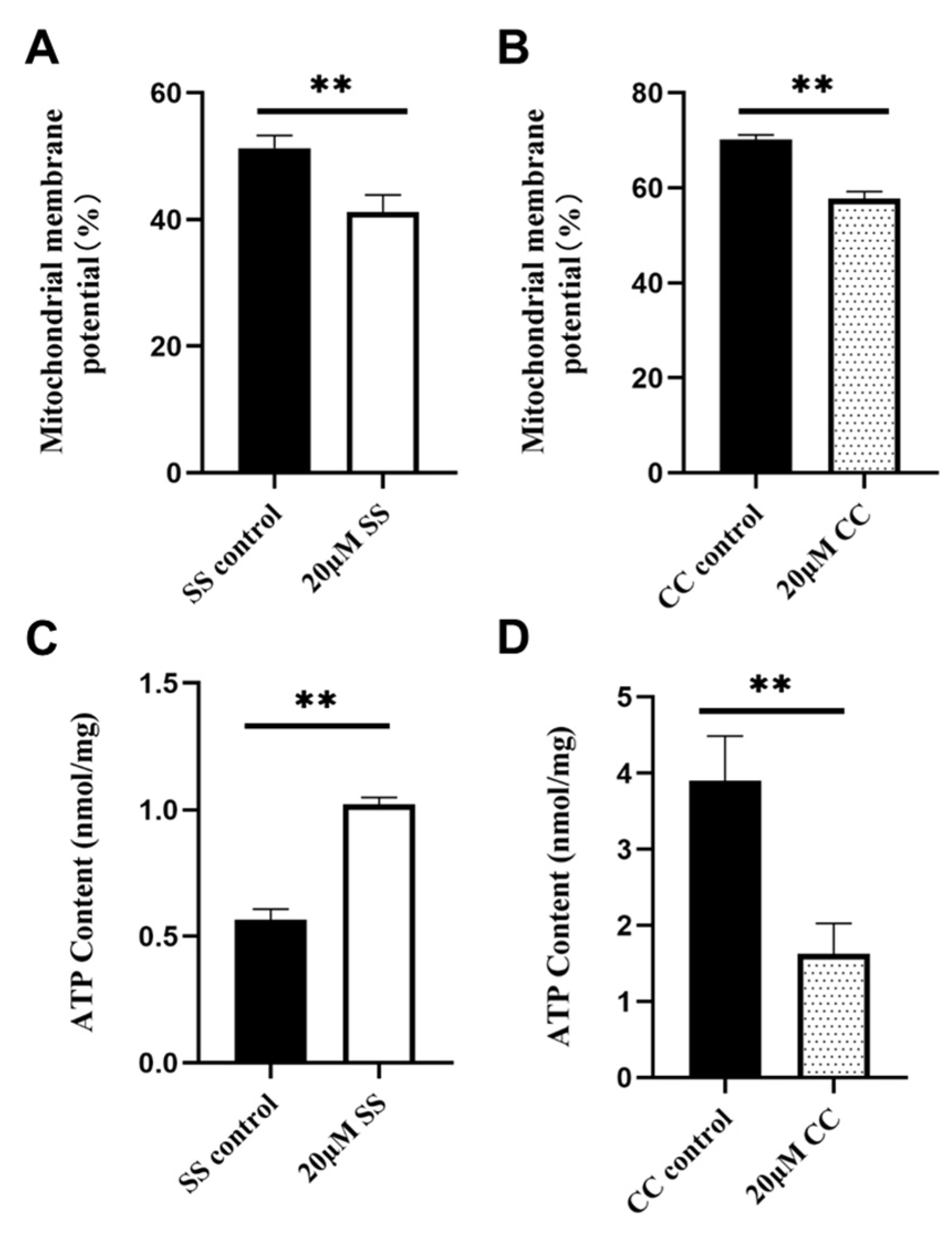
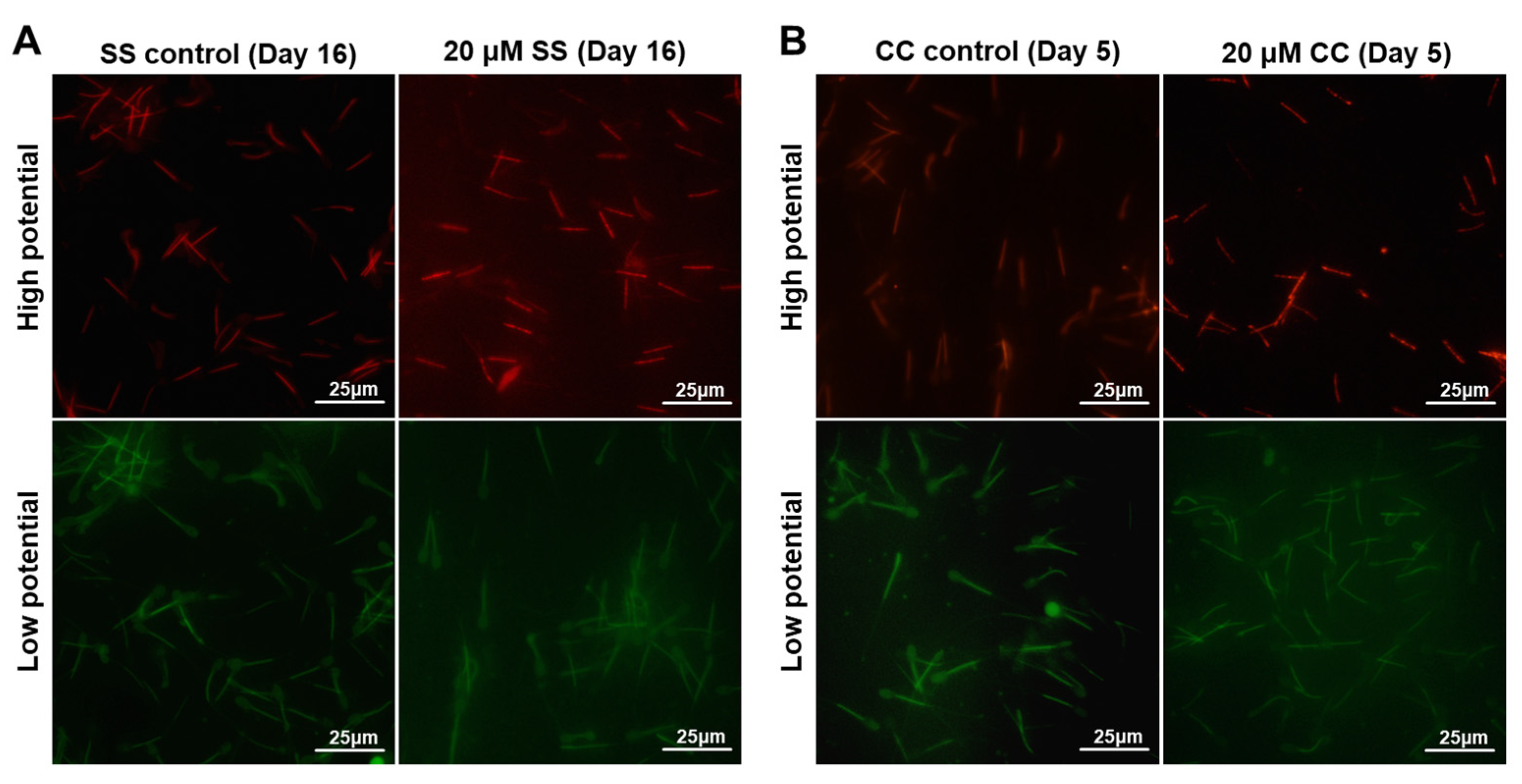
2.4. Effects of SS and CC on Mitochondrial Energy Metabolism
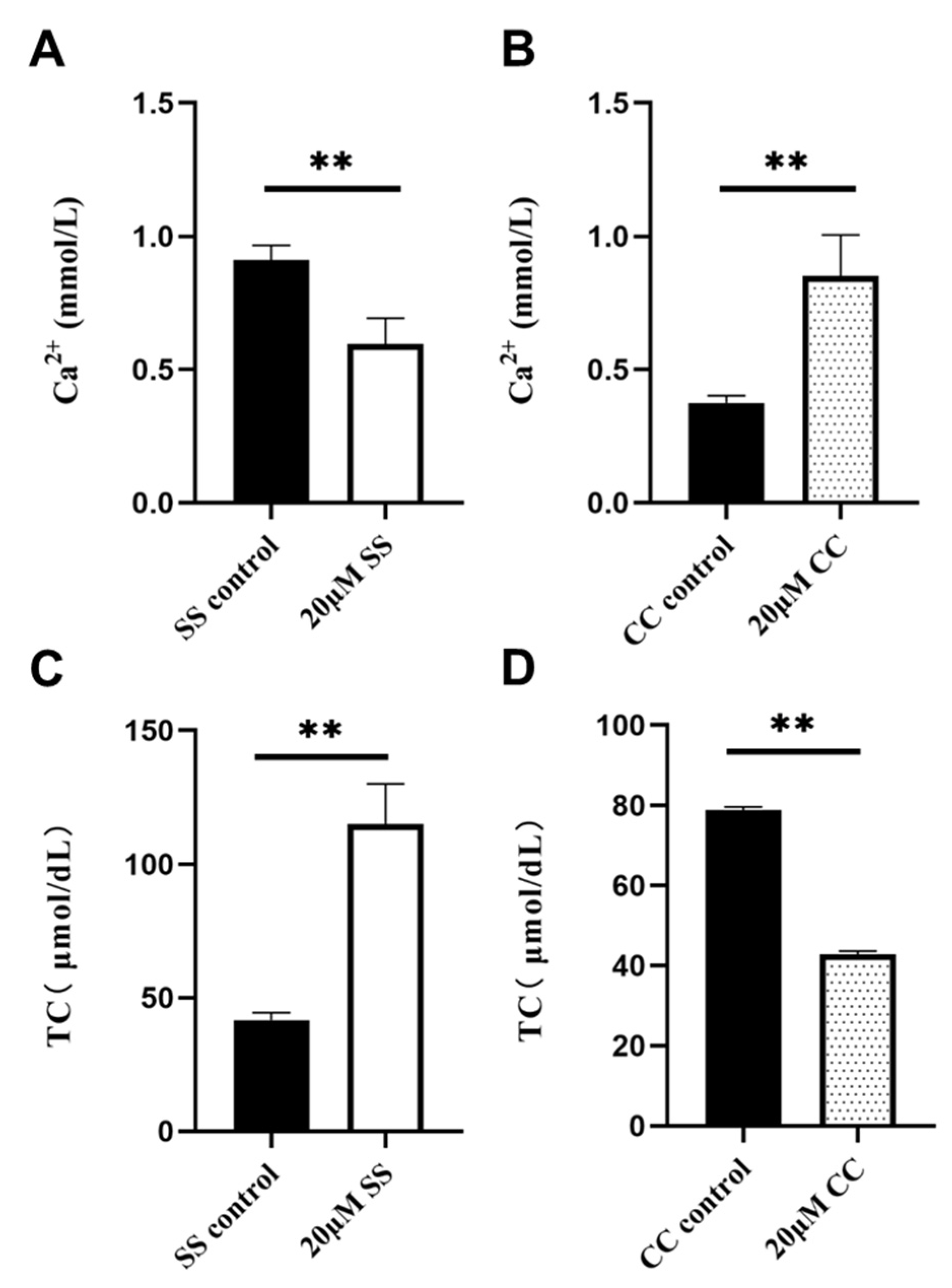
2.5. Ca2+ and TC Content Detection
2.6. Ultrastructural Analysis
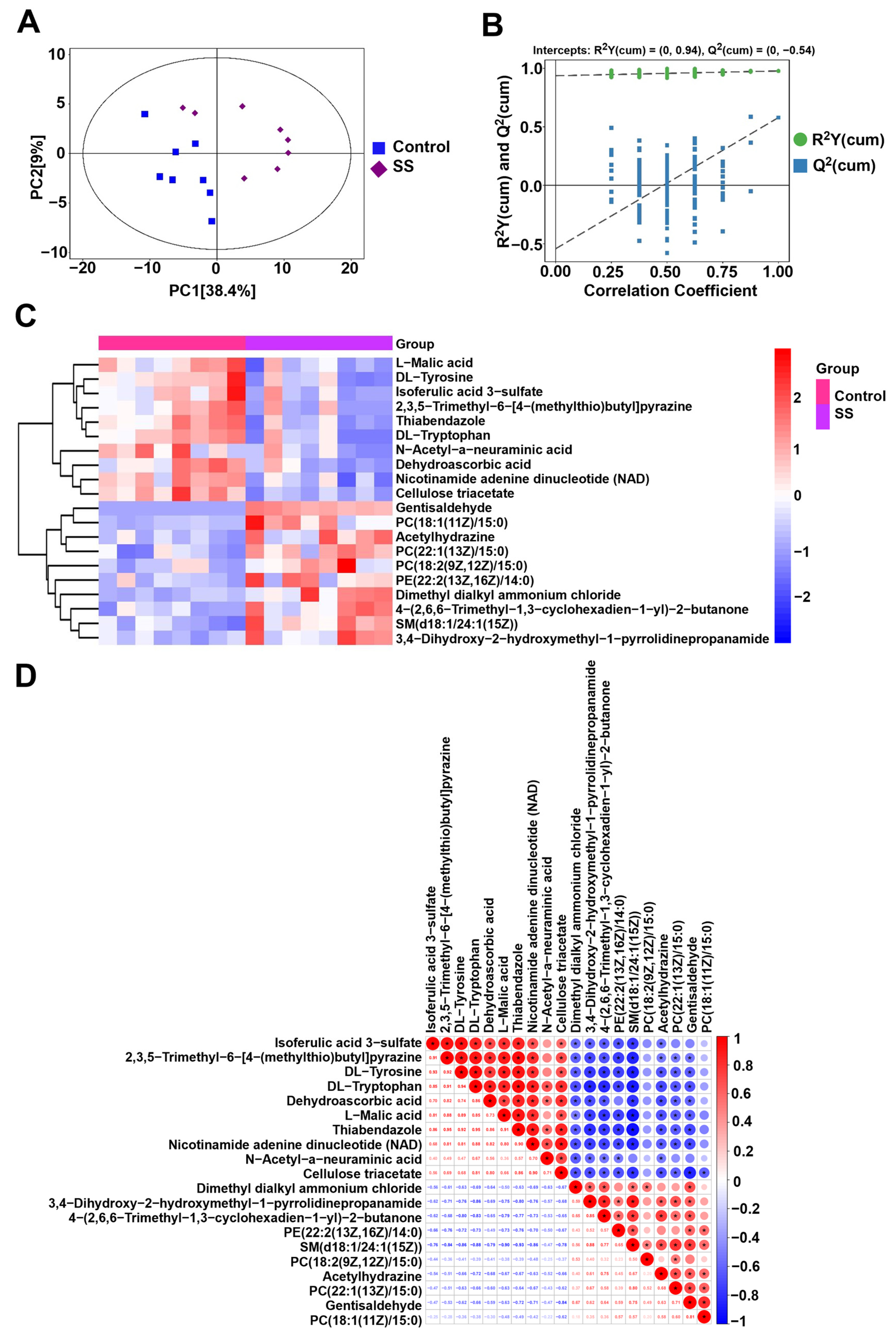
2.7. Metabolomics Analysis
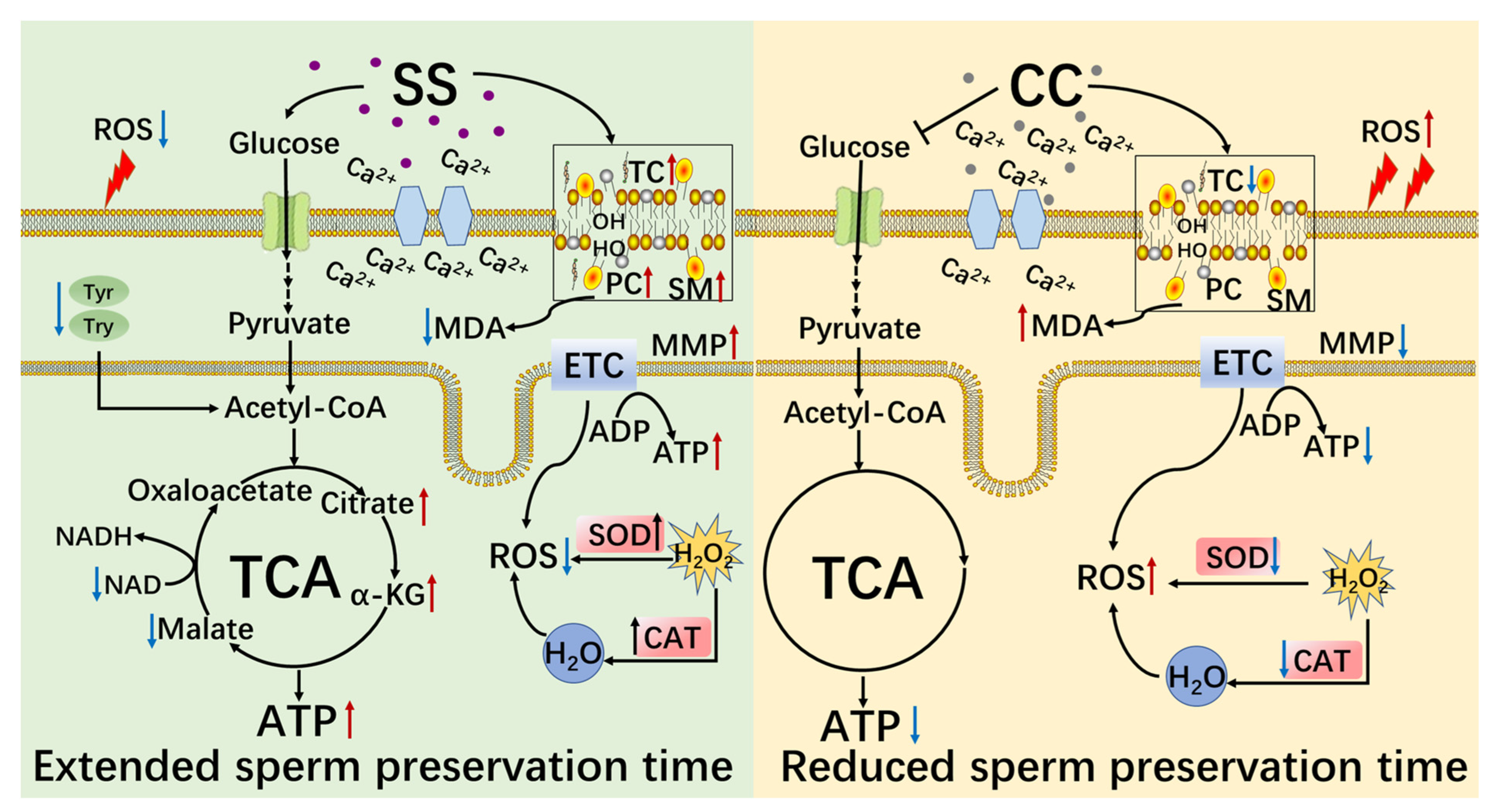
3. Discussion
4. Material and Methods
4.1. Semen Collection
4.2. Preparation of Diluent
4.3. Semen Processing
4.4. Sperm Motility
4.5. Plasma Membrane Integrity
4.6. Acrosome Integrity
4.7. CAT, SOD, TC, MDA, T-AOC, and Ca2+ Levels
4.8. ROS, MMP, and ATP Levels
4.9. Ultrastructural Detection
4.10. Metabolomics Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salimi, A.; Eslami, M.; Farrokhi-Ardabili, F. Influence of trans-ferulic acid on the quality of ram semen upon cold preservation. Vet. Med. Sci. 2023, 9, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- White, I.G. Lipids and calcium uptake of sperm in relation to cold shock and preservation: A review. Reprod. Fertil. Dev. 1993, 5, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Arav, A.; Pearl, M.; Zeron, Y. Does lipid profile explain chilling sensitivity and membrane lipid phase transition of spermatozoa and oocytes? Cryo Lett. 2000, 21, 179–186. [Google Scholar]
- Selvaraju, S.; Raju, P.; Rao, S.B.N.; Raghavendra, S.; Nandi, S.; Dineshkumar, D.; Thayakumar, A.; Parthipan, S.; Ravindra, J.P. Evaluation of maize grain and polyunsaturated fatty acid (PUFA) as energy sources for breeding rams based on hormonal, sperm functional parameters and fertility. Reprod. Fertil. Dev. 2012, 24, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T. Antioxidants and reactive oxygen species in human fertility. Environ. Toxicol. Pharmacol. 2001, 10, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A. Role of Antioxidants in Assisted Reproductive Techniques. World J. Men’s Health 2017, 35, 77–93. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Allai, L.; Benmoula, A.; Maia, M.d.S.; Nasser, B.; El Amiri, B. Supplementation of ram semen extender to improve seminal quality and fertility rate. Anim. Reprod. Sci. 2018, 192, 6–17. [Google Scholar] [CrossRef]
- Aitken, R.J. The Amoroso Lecture. The human spermatozoon—A cell in crisis? J. Reprod. Fertil. 1999, 115, 1–7. [Google Scholar] [CrossRef]
- Al-Mutary, M.G.; Al-Ghadi, M.Q.; Ammari, A.A.; Al-Himadi, A.R.; Al-Jolimeed, A.H.; Arafah, M.W.; Amran, R.A.; Aleissa, M.S.; Swelum, A.A.-A. Effect of different concentrations of resveratrol on the quality and in vitro fertilizing ability of ram semen stored at 5 degrees C for up to 168 h. Theriogenology 2020, 152, 139–146. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Liu, Q.; Wang, L.-Q.; Yang, G.-S.; Hu, J.-H. Effects of glutathione on sperm quality during liquid storage in boars. Anim. Sci. J. 2016, 87, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Madunic, J.; Horvat, L.; Majstorovic, I.; Jodlowska, I.; Antica, M.; Matulic, M. Sodium Salicylate Inhibits Urokinase Activity in MDA MB-231 Breast Cancer Cells. Clin. Breast Cancer 2017, 17, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Madathil, S.K.; Karuppagounder, S.S.; Mohanakumar, K.P. Sodium salicylate protects against rotenone-induced Parkinsonism in rats. Synapse 2013, 67, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Colantoni, A.; de Maria, N.; Caraceni, P.; Bernardi, M.; Floyd, R.A.; Van Thiel, D.H. Prevention of reoxygenation injury by sodium salicylate in isolated-perfused rat liver. Free Radic. Biol. Med. 1998, 25, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vincent, E.E.; Coelho, P.P.; Blagih, J.; Griss, T.; Viollet, B.; Jones, R.G. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene 2015, 34, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Hurtado de Llera, A.; Calle-Guisado, V.; Gonzalez-Fernandez, L.; Garcia-Marin, L.; Julia Bragado, M. AMPK Function in Mammalian Spermatozoa. Int. J. Mol. Sci. 2018, 19, 3293. [Google Scholar] [CrossRef]
- Thi Mong Diep, N.; Froment, P.; Combarnous, Y.; Blesbois, E. AMPK, regulator of sperm energy and functions. Med. Sci. M/S 2016, 32, 491–496. [Google Scholar] [CrossRef]
- Dasgupta, B.; Seibel, W. Compound C/Dorsomorphin: Its Use and Misuse as an AMPK Inhibitor. Methods Mol. Biol. 2018, 1732, 195–202. [Google Scholar] [CrossRef]
- Jin, J.; Mullen, T.D.; Hou, Q.; Bielawski, J.; Bielawska, A.; Zhang, X.; Obeid, L.M.; Hannun, Y.A.; Hsu, Y.-T. AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J. Lipid Res. 2009, 50, 2389–2397. [Google Scholar] [CrossRef]
- Amann, R.; Peskar, B.A. Anti-inflammatory effects of aspirin and sodium salicylate. Eur. J. Pharmacol. 2002, 447, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kotschwar, J.L.; Coetzee, J.F.; Anderson, D.E.; Gehring, R.; KuKanich, B.; Apley, M.D. Analgesic efficacy of sodium salicylate in an amphotericin B-induced bovine synovitis-arthritis model. J. Dairy Sci. 2009, 92, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.; Ghosh, S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Dandapani, M.; Hardie, D.G. AMPK: Mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol. Metab. 2013, 24, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The Ancient Drug Salicylate Directly Activates AMP-Activated Protein Kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K. Mechanisms of active oxygen species reduction by non-steroidal anti-inflammatory drugs. Int. J. Biochem. Cell Biol. 1997, 29, 437–446. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Huang, X.; Shi, M.; Sun, D.; Zhang, Y.; Li, W.; Jin, T.; Feng, J.; Xing, J.; et al. Effects of mitoquinone (MitoQ) supplementation during boar semen cryopreservation on sperm quality, antioxidant status and mitochondrial proteomics. Anim. Reprod. Sci. 2022, 247, 107099. [Google Scholar] [CrossRef]
- Lan, Q.; Xue, L.E.; Cao, J.; Xie, Y.; Xiao, T.; Fang, S. Caffeic Acid Phenethyl Ester (CAPE) Improves Boar Sperm Quality and Antioxidant Capacity in Liquid Preservation (17 degrees C) Linked to AMPK Activity Maintenance. Front. Vet. Sci. 2022, 9, 904886. [Google Scholar] [CrossRef]
- Aurich, C. Factors affecting the plasma membrane function of cooled-stored stallion spermatozoa. Anim. Reprod. Sci. 2005, 89, 65–75. [Google Scholar] [CrossRef]
- Moce, E.; Blanch, E.; Tomas, C.; Graham, J.K. Use of Cholesterol in Sperm Cryopreservation: Present Moment and Perspectives to Future. Reprod. Domest. Anim. 2010, 45, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.E.; Graham, J.K. Effects of cryopreservation procedures on sperm membranes. Theriogenology 1992, 38, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.X.; Adams, G.P.; Zwiefelhofer, E.M.; Rajapaksha, K.; Anzar, M. Cholesterol-cyclodextrin complex as a replacement for egg yolk in bull semen extender: Sperm characteristics post-thawing and in vivo fertility. Anim. Reprod. Sci. 2021, 225, 106691. [Google Scholar] [CrossRef] [PubMed]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Rodriguez-Gil, J.E.; Cruz Gil, M.; Garcia-Marin, L.J.; Julia Bragado, M. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2143–2151. [Google Scholar] [CrossRef]
- Lindemann, C.B.; Lesich, K.A. Functional anatomy of the mammalian sperm flagellum. Cytoskeleton 2016, 73, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Lesich, K.A.; Kelsch, C.B.; Ponichter, K.L.; Dionne, B.J.; Dang, L.; Lindemann, C.B. The Calcium Response of Mouse Sperm Flagella: Role of Calcium Ions in the Regulation of Dynein Activity. Biol. Reprod. 2012, 86, 105. [Google Scholar] [CrossRef] [PubMed]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef]
- Krisfalusi, M.; Miki, K.; Magyar, P.L.; O’Brien, D.A. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol. Reprod. 2006, 75, 270–278. [Google Scholar] [CrossRef]
- Amores-Sanchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, X.; Lv, Y.; Lin, Y.; Wu, D.; Zeng, W. Glutamine protects rabbit spermatozoa against oxidative stress via glutathione synthesis during cryopreservation. Reprod. Fertil. Dev. 2017, 29, 2183–2194. [Google Scholar] [CrossRef]
- Wang, S.; Sun, M.; Wang, N.; Yang, K.; Guo, H.; Wang, J.; Zhang, Y.; Yue, S.; Zhou, J. Effects of L-glutamine on boar sperm quality during liquid storage at 17 degrees C. Anim. Reprod. Sci. 2018, 191, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide Mononucleotide Supplementation Reverses the Declining Quality of Maternally Aged Oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef] [PubMed]
- Di Emidio, G.; Falone, S.; Artini, P.G.; Amicarelli, F.; D’Alessandro, A.M.; Tatone, C. Mitochondrial Sirtuins in Reproduction. Antioxidants 2021, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Hopp, A.-K.; Grueter, P.; Hottiger, M.O. Regulation of Glucose Metabolism by NAD(+) and ADP-Ribosylation. Cells 2019, 8, 890. [Google Scholar] [CrossRef]




| Time (d) | Sodium Salicylate Treatment (μM) | Motility (%) | Plasma Membrane Integrity | Acrosome Integrity |
|---|---|---|---|---|
| 1 | 0 | 89.68 ± 4.53 bc | 0.87 ± 0.03 a | 0.90 ± 0.01 a |
| 10 | 86.16 ± 1.53 bc | 0.85 ± 0.03 a | 0.91 ± 0.03 a | |
| 20 | 90.19 ± 0.80 ab | 0.88 ± 0.02 a | 0.93 ± 0.02 a | |
| 30 | 91.65 ± 3.33 ab | 0.87 ± 0.02 a | 0.92 ± 0.01 a | |
| 50 | 90.75 ± 2.49 ab | 0.87 ± 0.01 a | 0.91 ± 0.01 a | |
| 2 | 0 | 85.32 ± 1.47 b | 0.81 ± 0.04 c | 0.89 ± 0.04 ab |
| 10 | 85.67 ± 1.12 b | 0.84 ± 0.01 bc | 0.91 ± 0.04 ab | |
| 20 | 88.68 ± 1.90 a | 0.88 ± 0.03 a | 0.92 ± 0.02 a | |
| 30 | 89.44 ± 1.50 a | 0.87 ± 0.02 a | 0.89 ± 0.03 a | |
| 50 | 86.88 ± 1.75 ab | 0.83 ± 0.04 bc | 0.87 ± 0.02 b | |
| 4 | 0 | 85.25 ± 1.84 b | 0.78 ± 0.05 b | 0.82 ± 0.02 b |
| 10 | 85.48 ± 2.10 b | 0.83 ± 0.05 ab | 0.87 ± 0.07 ab | |
| 20 | 87.39 ± 2.07 a | 0.86 ± 0.05 a | 0.88 ± 0.04 a | |
| 30 | 88.43 ± 1.75 a | 0.86 ± 0.02 a | 0.88 ± 0.01 ab | |
| 50 | 86.28 ± 1.99 ab | 0.82 ± 0.02 ab | 0.86 ± 0.04 ab | |
| 6 | 0 | 84.13 ± 1.61 b | 0.75 ± 0.03 b | 0.78 ± 0.03 b |
| 10 | 84.80 ± 1.32 b | 0.81 ± 0.08 ab | 0.85 ± 0.03 a | |
| 20 | 86.08 ± 1.75 ab | 0.85 ± 0.03 a | 0.87 ± 0.02 a | |
| 30 | 88.03 ± 1.52 a | 0.85 ± 0.03 a | 0.86 ± 0.02 a | |
| 50 | 84.75 ± 1.35 ab | 0.82 ± 0.02 ab | 0.83 ± 0.02 a | |
| 8 | 0 | 83.01 ± 1.94 b | 0.71 ± 0.14 b | 0.71 ± 0.05 b |
| 10 | 83.13 ± 2.28 b | 0.80 ± 0.02 ab | 0.80 ± 0.03 ab | |
| 20 | 85.55 ± 1.91 a | 0.83 ± 0.05 ab | 0.83 ± 0.06 a | |
| 30 | 87.03 ± 1.12 a | 0.84 ± 0.02 a | 0.82 ± 0.04 a | |
| 50 | 84.59 ± 1.39 b | 0.81 ± 0.04 ab | 0.81 ± 0.05 a | |
| 10 | 0 | 79.07 ± 1.74 c | 0.67 ± 0.03 b | 0.63 ± 0.03 d |
| 10 | 82.00 ± 1.80 b | 0.78 ± 0.03 a | 0.80 ± 0.05 ab | |
| 20 | 85.06 ± 2.06 ab | 0.80 ± 0.05 a | 0.81 ± 0.02 a | |
| 30 | 81.60 ± 1.95 b | 0.79 ± 0.06 a | 0.77 ± 0.04 ab | |
| 50 | 81.26 ± 1.44 bc | 0.77 ± 0.04 a | 0.72 ± 0.04 b | |
| 12 | 0 | 72.79 ± 1.76 c | 0.62 ± 0.08 c | 0.60 ± 0.04 d |
| 10 | 76.26 ± 1.83 b | 0.72 ± 0.02 b | 0.75 ± 0.03 ab | |
| 20 | 80.77 ± 2.56 a | 0.78 ± 0.04 a | 0.79 ± 0.04 a | |
| 30 | 80.49 ± 1.31 a | 0.76 ± 0.04 a | 0.76 ± 0.06 a | |
| 50 | 77.89 ± 2.53 ab | 0.75 ± 0.07 a | 0.69 ± 0.05 c | |
| 14 | 0 | 66.15 ± 1.50 d | 0.56 ± 0.06 b | 0.54 ± 0.06 c |
| 10 | 70.42 ± 1.38 c | 0.72 ± 0.06 a | 0.70 ± 0.16 ab | |
| 20 | 79.25 ± 1.67 a | 0.75 ± 0.11 a | 0.76 ± 0.01 a | |
| 30 | 78.99 ± 1.24 a | 0.73 ± 0.02 a | 0.74 ± 0.04 a | |
| 50 | 75.95 ± 2.06 b | 0.59 ± 0.05 b | 0.67 ± 0.13 b | |
| 16 | 0 | 55.01 ± 3.70 d | 0.52 ± 0.05 c | 0.47 ± 0.02 c |
| 10 | 64.32 ± 1.70 b | 0.64 ± 0.05 a | 0.60 ± 0.09 b | |
| 20 | 71.46 ± 1.80 a | 0.68 ± 0.08 a | 0.71 ± 0.02 a | |
| 30 | 69.28 ± 2.00 a | 0.63 ± 0.11 b | 0.67 ± 0.05 ab | |
| 50 | 60.42 ± 2.11 c | 0.57 ± 0.07 c | 0.63 ± 0.03 b | |
| 18 | 0 | 52.91 ± 1.27 c | 0.47 ± 0.03 c | 0.42 ± 0.05 b |
| 10 | 60.51 ± 2.92 b | 0.58 ± 0.05 a | 0.58 ± 0.06 a | |
| 20 | 68.27 ± 2.34 a | 0.61 ± 0.09 a | 0.66 ± 0.03 a | |
| 30 | 65.18 ± 2.18 b | 0.60 ± 0.07 a | 0.60 ± 0.15 a | |
| 50 | 51.64 ± 2.34 c | 0.56 ± 0.03 b | 0.59 ± 0.11 a | |
| 20 | 0 | 43.41 ± 1.69 e | 0.42 ± 0.04 c | 0.39 ± 0.05 c |
| 10 | 55.75 ± 1.61 c | 0.54 ± 0.06 a | 0.49 ± 0.05 b | |
| 20 | 62.80 ± 2.29 a | 0.59 ± 0.05 a | 0.57 ± 0.05 a | |
| 30 | 59.43 ± 1.50 b | 0.56 ± 0.06 a | 0.54 ± 0.03 a | |
| 50 | 51.33 ± 1.24 d | 0.51 ± 0.07 b | 0.50 ± 0.06 b |
| Time (d) | Compound C Treatment (μM) | Motility (%) | Plasma Membrane Integrity | Acrosome |
|---|---|---|---|---|
| 1 | 0 | 88.33 ± 0.94 d | 0.86 ± 0.02 a | 0.92 ± 0.02 ab |
| 1 | 93.69 ± 0.74 a | 0.90 ± 0.05 a | 0.93 ± 0.01 a | |
| 5 | 90.89 ± 1.56 bc | 0.89 ± 0.02 a | 0.92 ± 0.02 ab | |
| 10 | 91.71 ± 1.31 ab | 0.87 ± 0.04 a | 0.91 ± 0.02 ab | |
| 20 | 88.90 ± 2.39 cd | 0.84 ± 0.02 a | 0.90 ± 0.01 b | |
| 2 | 0 | 87.68 ± 1.57 a | 0.87 ± 0.03 a | 0.91 ± 0.00 a |
| 1 | 90.39 ± 1.57 a | 0.86 ± 0.03 a | 0.91 ± 0.01 a | |
| 5 | 87.14 ± 0.99 a | 0.85 ± 0.04 a | 0.90 ± 0.01 a | |
| 10 | 88.91 ± 1.67 a | 0.85 ± 0.01 a | 0.90 ± 0.01 a | |
| 20 | 87.20 ± 1.22 a | 0.84 ± 0.03 a | 0.90 ± 0.01 a | |
| 3 | 0 | 86.29 ± 2.00 a | 0.85 ± 0.04 a | 0.90 ± 0.01 a |
| 1 | 85.84 ± 2.01 a | 0.85 ± 0.05 a | 0.89 ± 0.01 a | |
| 5 | 85.55 ± 1.11 a | 0.84 ± 0.03 a | 0.88 ± 0.01 a | |
| 10 | 85.43 ± 2.38 a | 0.82 ± 0.03 a | 0.88 ± 0.03 a | |
| 20 | 82.85 ± 0.72 b | 0.81 ± 0.07 a | 0.85 ± 0.01 b | |
| 4 | 0 | 85.64 ± 1.27 a | 0.81 ± 0.01 a | 0.86 ± 0.02 a |
| 1 | 84.88 ± 2.76 a | 0.80 ± 0.01 a | 0.85 ± 0.01 a | |
| 5 | 84.18 ± 1.52 a | 0.79 ± 0.05 a | 0.82 ± 0.02 b | |
| 10 | 82.31 ± 1.96 ab | 0.78 ± 0.03 a | 0.79 ± 0.01 c | |
| 20 | 79.73 ± 2.13 b | 0.76 ± 0.03 ab | 0.76 ± 0.02 d | |
| 5 | 0 | 84.35 ± 1.02 a | 0.79 ± 0.02 a | 0.82 ± 0.01 a |
| 1 | 80.31 ± 1.04 ab | 0.77 ± 0.03 ab | 0.77 ± 0.02 b | |
| 5 | 79.79 ± 5.48 ab | 0.73 ± 0.05 bc | 0.77 ± 0.03 b | |
| 10 | 78.10 ± 2.92 b | 0.73 ± 0.01 bc | 0.76 ± 0.02 bc | |
| 20 | 77.24 ± 1.38 b | 0.71 ± 0.02 c | 0.73 ± 0.01 c | |
| 6 | 0 | 81.66 ± 1.41 a | 0.78 ± 0.02 a | 0.80 ± 0.01 a |
| 1 | 76.08 ± 1.25 b | 0.72 ± 0.01 b | 0.73 ± 0.02 b | |
| 5 | 74.49 ± 2.06 b | 0.69 ± 0.04 bc | 0.71 ± 0.03 b | |
| 10 | 71.30 ± 2.52 c | 0.67 ± 0.04 bc | 0.71 ± 0.03 b | |
| 20 | 69.70 ± 1.46 c | 0.63 ± 0.05 c | 0.66 ± 0.03 c | |
| 7 | 0 | 79.32 ± 1.87 a | 0.77 ± 0.06 a | 0.77 ± 0.04 a |
| 1 | 67.71 ± 1.75 b | 0.66 ± 0.02 b | 0.71 ± 0.03 b | |
| 5 | 65.61 ± 0.93 b | 0.64 ± 0.02 b | 0.67 ± 0.04 bc | |
| 10 | 64.99 ± 3.91 b | 0.63 ± 0.05 b | 0.62 ± 0.01 cd | |
| 20 | 57.76 ± 2.50 c | 0.54 ± 0.01 c | 0.59 ± 0.02 d |
| Pathway | Total | Hits | Raw p | Impact | Hits ID |
|---|---|---|---|---|---|
| Nitrogen metabolism | 9 | 2 | 0.033226 | 0 | L-Glutamine, L-Histidine |
| Histidine metabolism | 14 | 2 | 0.075649 | 0.26619 | L-Histidine, L-Aspartic acid |
| Glyoxylate and dicarboxylate metabolism | 16 | 2 | 0.095705 | 0.33334 | Glycolic acid, citric acid |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 4 | 1 | 0.12599 | 0.5 | L-Phenylalanine |
| Alanine, aspartate and glutamate metabolism | 23 | 2 | 0.17481 | 0.27667 | L-Aspartic acid, L-Glutamine |
| Phenylalanine metabolism | 9 | 1 | 0.26181 | 0.40741 | L-Phenylalanine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Zhang, Z.; Shen, W.; Fu, Y.; Cao, Z.; Liu, Z.; Yang, C.; Sun, S.; Wang, L.; Ling, Y.; et al. Metabolomics Analysis of Sodium Salicylate Improving the Preservation Quality of Ram Sperm. Molecules 2024, 29, 188. https://doi.org/10.3390/molecules29010188
Bai H, Zhang Z, Shen W, Fu Y, Cao Z, Liu Z, Yang C, Sun S, Wang L, Ling Y, et al. Metabolomics Analysis of Sodium Salicylate Improving the Preservation Quality of Ram Sperm. Molecules. 2024; 29(1):188. https://doi.org/10.3390/molecules29010188
Chicago/Turabian StyleBai, Haiyu, Zhiyu Zhang, Wenzheng Shen, Yu Fu, Zhikun Cao, Zibo Liu, Chao Yang, Shixin Sun, Lei Wang, Yinghui Ling, and et al. 2024. "Metabolomics Analysis of Sodium Salicylate Improving the Preservation Quality of Ram Sperm" Molecules 29, no. 1: 188. https://doi.org/10.3390/molecules29010188
APA StyleBai, H., Zhang, Z., Shen, W., Fu, Y., Cao, Z., Liu, Z., Yang, C., Sun, S., Wang, L., Ling, Y., Zhang, Z., & Cao, H. (2024). Metabolomics Analysis of Sodium Salicylate Improving the Preservation Quality of Ram Sperm. Molecules, 29(1), 188. https://doi.org/10.3390/molecules29010188






