Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases
Abstract
:1. Introduction
2. Literature Search for Shionone
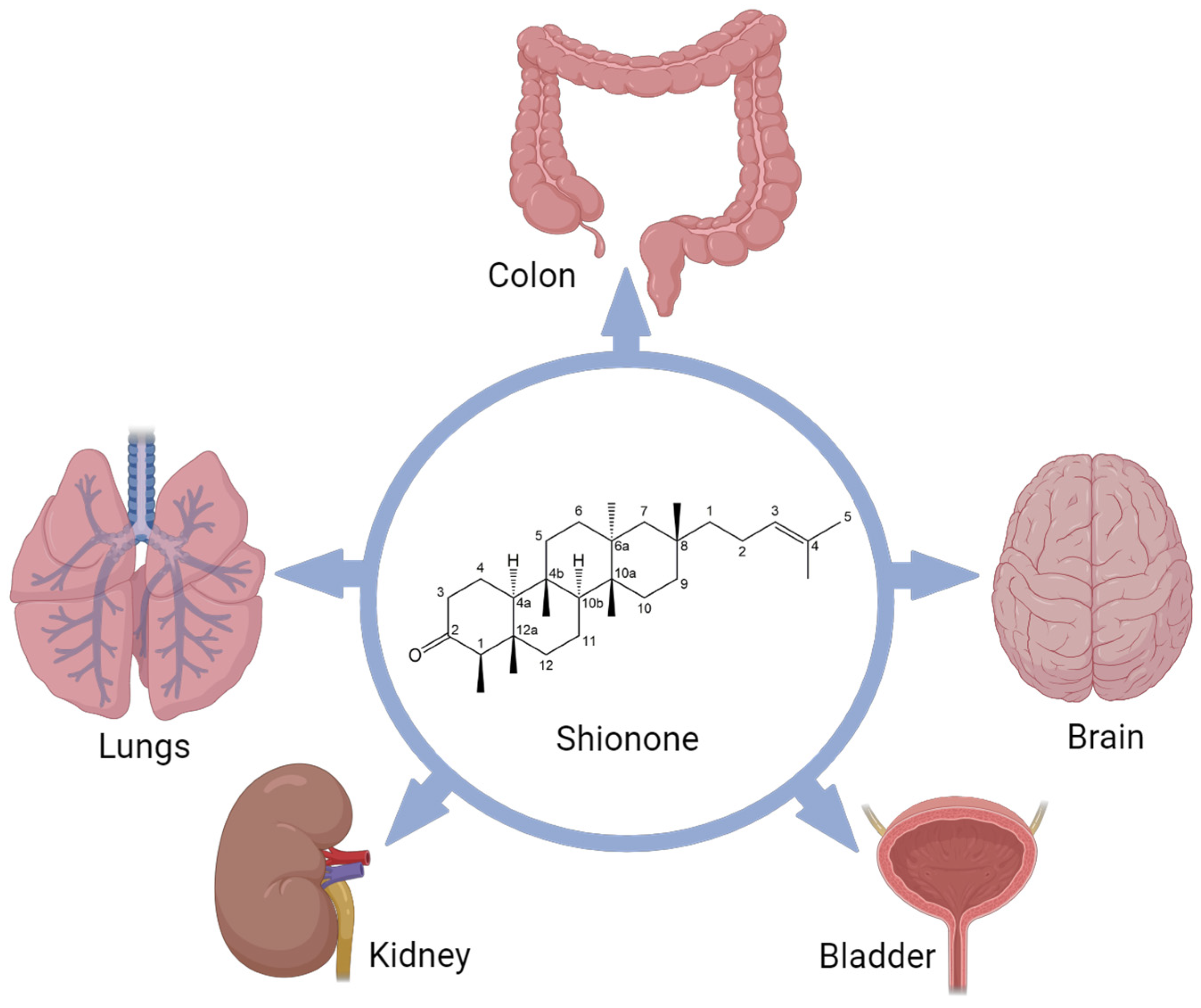
3. Structure and Properties of Shionone
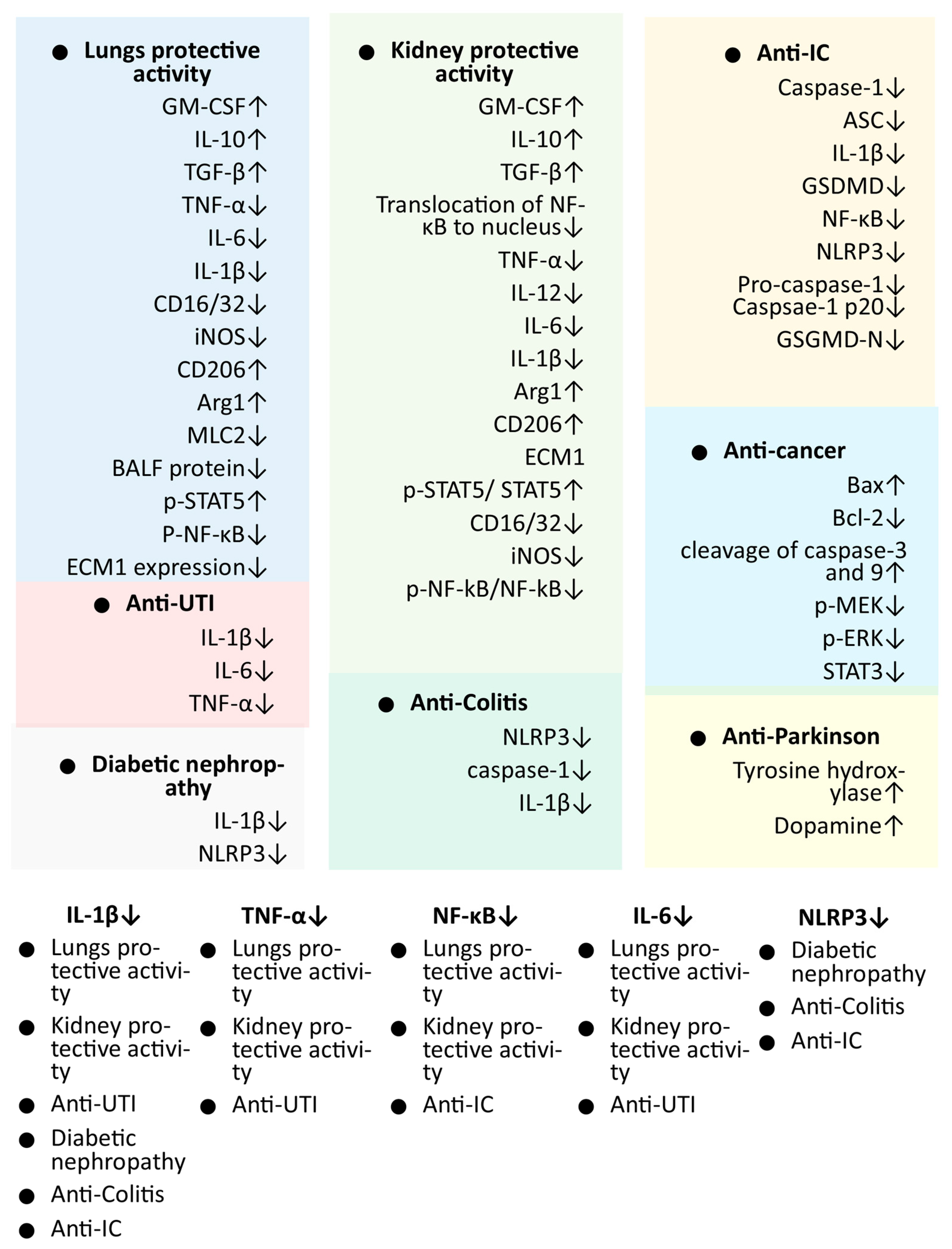
4. Pharmacological Activities of Shionone
4.1. Lung Protective and Anti-COPD Activities of Shionone
4.1.1. In Vitro Lung Protective Activities of Shionone
4.1.2. In Vivo Lung Protective Activities of Shionone
4.1.3. In Silico Docking of Shionone with Pneumolysin
4.2. Kidney Injury Protection through Shionone
4.2.1. In Vitro Kidney Protective Activity of Shionone
4.2.2. In Vivo Kidney Protective Activity of Shionone
4.3. Anti-Cancer Activity of Shionone
| Activity | Dose | Method | Result | References |
|---|---|---|---|---|
| Lung protective and anti-COPD activities | 4–32 μg/mL | Hemolysis test of pneumolysin | Pneumolysin hemolytic activity↓ | [19] |
| WB | Expression of pnemolysin↓ | |||
| A549 cell injury of pneumolysin through live/dead assays | A549 cell injury↓ | |||
| 2, 4, and 8 μg/mL | WB and optical density analysis | Oligomerization of pneumolysin↓ | ||
| 0.5, 1.0, and 2.0 μg/mL | LPS-induced RAW264.7 cells and ELISA | Level of IL-10↑, GM-CSF↑, TGF-β↑, TNF-α↓, IL-6↓, and IL-1β↓ | [20] | |
| LPS-induced RAW264.7 cells and a fluorescence microscope | CD16/32↓, iNOS↓ (M1 polarization indicators), CD206↑, and Arg1↑ (biomarkers of M2 polarization) | |||
| 1 and 10 μM | CSE-stimulated BEAS-2B cell model of MLC2 and WB | MLC2↓ | [21] | |
| Kidney protective activity | 0.5, 1.0, and 2.0 μg/mL | RAW264.7 cells treated with LPS, cell viability by MTT, and immunofluorescence staining for markers | Cell viability↑, level of GM-CSF↑, IL-10↑, IL-12↓, IL-6↓, TGF-β↑, TNF-α↓, and IL-1β↓ | [26] |
| WB | Arg1↑, CD206↑, ECM1, and p-STAT5/STAT5↑ | |||
| LPS-induced RAW264.7 cells and immunofluorescence | Translocation of NF-κB to the nucleus↓ | |||
| Anti-cancer activity | 3.12–100 μM | SK-BR-3 breast cancer cell proliferation studied with a CCK assay | Growth of breast cancer cells↓ (IC50 = 14 μM) | [34] |
| 0, 7, 14, and 28 μM shionone | Nuclear morphology by DAPI staining and fluorescent microscopic examination of the AO/EB-stained cells | Nuclear deformation↑ | ||
| WB analysis | Bcl-2↓, cleavage of caspase-3 and -9↑, and Bax↑ | |||
| Transwell assay for migration and invasion | Migration↓ and invasion↓ | |||
| WB | p-MEK↓, p-ERK↓, and STAT3↓ | |||
| Anti-IC | 2.5, 5, and 10 μg/mL | MTT, Hoechst33342, and PI double staining | Cell viability↑ and PI-positive cell rates↓ | [16] |
| SV-HUC-1 cell | RT-PCR | NLRP3↓, caspase-1↓, ASC↓, IL-1β↓, and GSDMD↓ | ||
| WB and ELISA | NF-κB↓, NLRP3↓, pro-caspase-1↓, caspsae-1 p20↓, and GSDMD and GSGMD-N↓ | |||
| Anti-UTI activity | 5, 10, and 20, μg/mL | Study the bacterial growth in SV-HUC-1 cells through cell smear plate experiments and immunofluorescence | CFU of bacteria↓ | [15] |
| ELISA | TNF-α↓, IL-1β↓, and IL-6↓ |
4.4. Anti-Interstitial Cystitis Activity of Shionone
4.4.1. In Vitro Anti-IC Activities of Shionone
4.4.2. In Vivo Study of the Anti-IC Activity of Shionone
4.5. Activity of Shionone against Urinary Tract Infections
4.5.1. Anti-UTI Activity of Shionone in an In Vitro Study
4.5.2. Anti-UTI Activity of Shionone in an In Vivo Model
4.6. Antioxidant Activity of Shionone
4.7. Expectorant and Antitussive Activities of Shionone
4.8. Anti-Colitis Activity of Shionone
4.9. Anti-Parkinson’s Disease Activity of Shionone
| Activity/Probable Mechanism | Model and Dose | Method | Major Findings | Reference |
|---|---|---|---|---|
| Lung protective activities | Female BALB/c mice and 50 mg/kg orally twice a day | Intranasal infection by S. pneumoniae and histopathology analysis | Structurally intact alveolar tissue↑, inflammatory cell infiltration↓, lung colonies↓, and lung injury↓ | [19] |
| Male ICR mice and 10, 50, and 100 mg/kg intragastrically | CLPS to create the sepsis model | The survival percent↑ | [20] | |
| Histopathology of lung H&E staining | Thickening of alveolar walls↑, infiltration of inflammatory cells↓, pulmonary edema↓, and pulmonary wet/dry ratio↓ | |||
| ELISA and the myeloperoxidase activity assay | Serum level of TNF-α↓, IL-6↓, and IL-1β↓, BALF protein↓, and lung MPO activity↓ | |||
| Flow cytometry | Percentage of cell population neutrophils and macrophages in BALF↓ | |||
| WB and immunohistochemistry staining | CD16/32↓, iNOS↓, CD206↑ and Arg1↑ In ECM1/STAT5/NF-κB, phosphorylation of STAT5↑, ECM1 expression↓, and the phosphorylation of NF-κB↓ and ECM1 expression↓ | |||
| Anti-kidney injury | C57BL/6 mice and 50 and 100 mg/kg | CLPS for acute kidney injury and H&E staining | Survival percentage↑, inflammatory cell infiltration↓, vacuolation↓, blood urea nitrogen↓, and serum creatinine↓ | [26] |
| ELISA | IL-6↓, IL-1b↓, IL-12↓, and TNF-α↓ | |||
| WB | CD16/32↓, iNOS↓, p-NF-kB/NF-kB↓, ECM1↓ CD206↑, Arg1↑, and p-STAT5/STAT5↑ | |||
| Anti-IC activity | Female SD rats and 50 mg/kg 100 mg/kg gavage | IC-induced inflammation and NLRP3 inflammasome activation through cyclophosphamide | Bladder wet weight↓, edema score↓, hemorrhage score↓, and histopathological score↓ | [16] |
| Protein expression in the bladder by WB | Expression of NF-κB↓, GSGMD-N↓, NLRP3↓, ASC↓, pro-caspase-1↓, and cleaved IL-1β↓ | |||
| Immunofluorescence analysis | Expression of NLRP3↓ NLRP3 | |||
| Anti-UTI | Female SD rats and 100 mg/kg and 200 mg/kg through gavage | UPEC solution pushed into the bladder for the animal UTI model and the positive control drug levofloxacin | Congestion↓ and edema↓ in the bladder, infiltration of inflammatory cells↓ in large submucosal and mucosa, and thickening of the bladder↓ | |
| Electron microscopy of superficial cystic tissue | Lysosomes↑ and vesicles↑ | |||
| Bacterial colony counts in bladder homogenate | E. coli in bladder tissue # | |||
| ELISA assay | IL-1β↓, IL-6↓, and TNF-α↓ | |||
| Expectorant and antitussive activity | ICR male mice and 80 mg/kg once daily for 3 consecutive days | Secretion of phenol red in the trachea and parts of the bronchi. NH4Cl (250 mg/kg) as a positive control | Secretion of phenol red↑ (by 11.7% #) | [40] |
| Cough frequency and the latent period were studied. The positive control was pentoxyverine (17.5 mg/kg) | No significant effects on the latent period and the frequency of cough | |||
| Anti-inflammatory activity by the mouse ear welling model | Ear edema↓ (by 11.3% #) | |||
| Anti-colitis activity | BALB/c mice and 25 and 50 mg/kg for two weeks | DSS-induced colitis and H&E stain of the colon tissue | Colonic inflammation ↓ | [43] |
| Immunohistochemistry and the WB assay of the colon tissue | NLRP3↓, caspase-1↓, and IL-1β↓ | |||
| Anti-PD activities | Male 57BL/6J mice and 50 mg/kg/day for 7 consecutive days | Intraperitoneal injection of neurotoxin MPTP | Mouse rod rotation and pole climbing experiments | [46] |
| Morphological analysis of TH expression at the substantia nigra site by immunohistochemistry | TH positive neurons↑ | |||
| Striatum part dopamine content through the HPLC-based method | Increase the dopamine level in the striatum↑ | |||
| Anti-DN | C57BL/6 wild type mice and 25 mg/kg for eight weeks | DN was induced through single nephrectomy and streptozotocin administration | Body weight ↑, kidney index↓, urine volume↓, urine protein↓, serum creatinine↓, serum urea nitrogen↓ | [28] |
| H&E staining | Local fibrosis lesions of glomeruli↓ | |||
| Immunohistochemistry of kidney tissues (glomeruli) | NLRP3↓ and IL-1β↓ |
4.10. Pharmacokinetic Studies of Shionone
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, K.-J.; Liu, Y.-Y.; Wang, D.; Yan, P.-Z.; Lu, D.-C.; Zhao, D.-S. Radix Asteris: Traditional Usage, Phytochemistry and Pharmacology of An Important Traditional Chinese Medicine. Molecules 2022, 27, 5388. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Lee, H.-J. Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life 2023, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, K.H.; Jang, T.; Kang, K.S. (-)-Leucophyllone, a Tirucallane Triterpenoid from Cornus walteri, Enhances Insulin Secretion in INS-1 Cells. Plants 2021, 10, 431. [Google Scholar] [CrossRef]
- Lorenz, H.-M.; Kalden, J.R. Perspectives for TNF-α-targeting therapies. Arthritis Res. Ther. 2002, 4, S17. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Han, X.; Liu, X.H. STAT3: A potential drug target for tumor and inflammation. Curr. Top. Med. Chem. 2019, 19, 1305–1317. [Google Scholar] [CrossRef]
- Menu, P.; Vince, J. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Rani, A.; Murphy, J.J. STAT5 in cancer and immunity. J. Interferon Cytokine Res. 2016, 36, 226–237. [Google Scholar] [CrossRef]
- Ireland, R.E.; Kowalski, C.J.; Tilley, J.W.; Walba, D.M. Total synthesis of terpenes. XX. Total synthesis of (±)-shionone, a tetracyclic triterpene. J. Org. Chem. 1975, 40, 990–1000. [Google Scholar] [CrossRef]
- Ireland, R.E.; Lipinski, C.A.; Kowalski, C.J.; Tilley, J.W.; Walba, D.M. Total synthesis of dl-shionone, a tetracyclic triterpene. J. Am. Chem. Soc. 1974, 96, 3333–3335. [Google Scholar] [CrossRef]
- Sawai, S.; Uchiyama, H.; Mizuno, S.; Aoki, T.; Akashi, T.; Ayabe, S.-I.; Takahashi, T. Molecular characterization of an oxidosqualene cyclase that yields shionone, a unique tetracyclic triterpene ketone of Aster tataricus. FEBS Lett. 2011, 585, 1031–1036. [Google Scholar] [CrossRef]
- Du, J.; Hu, W.; Yang, D.; Meng, F.; Li, Y. Cloning and functional analysis of HMGR gene in Ligularia fischeri. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changsha, China, 18–20 September 2020; p. 012112. [Google Scholar]
- Fu, M.-Q.; Xiao, G.-S.; Xu, Y.-J.; Wu, J.-J.; Chen, Y.-L.; Qiu, S.-X. Chemical Constituents from Roots of Millettia speciosa. Chin. Herb. Med. 2016, 8, 385–389. [Google Scholar] [CrossRef]
- Huang, J.; Huang, N.; Liang, K.; Xie, D.; Liang, K. Technology of Extracting Shionone from Millettia specisoa. CN106916197A, 4 July 2017. [Google Scholar]
- Yin, H.; Zhu, J.; Jiang, Y.; Mao, Y.; Tang, C.; Cao, H.; Huang, Y.; Zhu, H.; Luo, J.; Jin, Q. Shionone Relieves Urinary Tract Infections by Removing Bacteria from Bladder Epithelial Cells. Cell. Microbiol. 2023, 2023, 3201540. [Google Scholar] [CrossRef]
- Wang, X.; Yin, H.; Fan, L.; Zhou, Y.; Tang, X.; Fei, X.; Tang, H.; Peng, J.; Zhang, J.; Xue, Y.; et al. Shionone alleviates NLRP3 inflammasome mediated pyroptosis in interstitial cystitis injury. Int. Immunopharmacol. 2021, 90, 107132. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Vogelmeier, C.F.; Halpin, D.M. Tackling the global burden of lung disease through prevention and early diagnosis. Lancet Respir. Med. 2022, 10, 1013–1015. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-S.; Lee, W.-Y.; Kim, C.-E.; Lee, S. Integrative Approach to Identifying System-Level Mechanisms of Chung-Sang-Bo-Ha-Hwan’s Influence on Respiratory Tract Diseases: A Network Pharmacological Analysis with Experimental Validation. Plants 2023, 12, 3024. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Wang, T.; Lv, H.; Zou, Y.; Hou, X.; Hou, N.; Zhang, P.; Li, H.; Chi, G. Shionone-Targeted Pneumolysin to Ameliorate Acute Lung Injury Induced by Streptococcus pneumoniae In Vivo and In Vitro. Molecules 2022, 27, 6258. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Q.; Jiang, H.; Hu, A.; Xu, L.; Tan, C.; Zhang, B.; Yu, R.; Qiu, Y.; Wang, X.; et al. The Effect of Shionone on Sepsis-Induced Acute Lung Injury by the ECM1/STAT5/NF-κB Pathway. Front. Pharmacol. 2022, 12, 764247. [Google Scholar] [CrossRef]
- Wang, H.; Hou, Y.; Ma, X.; Cui, L.; Bao, Y.; Xie, Y.; Li, S.; Meng, X.; Li, J.; Bai, G. Multi-omics analysis reveals the mechanisms of action and therapeutic regimens of traditional Chinese medicine, Bufei Jianpi granules: Implication for COPD drug discovery. Phytomedicine 2022, 98, 153963. [Google Scholar] [CrossRef]
- Chi, G.; Du, R.; Wang, J.; Feng, J.; Ma, X.; Liu, T.; Liu, Z.; Hou, N.; Liu, S.; Bi, X. Application of Shionone in Preparation of Streptococcus pneumoniae Hemolysin Inhibitor. CN115531392A, 30 December 2022. [Google Scholar]
- Park, S.-J.; Jaiswal, V.; Lee, H.-J. Dietary intake of flavonoids and carotenoids is associated with anti-depressive symptoms: Epidemiological study and in silico—Mechanism analysis. Antioxidants 2021, 11, 53. [Google Scholar] [CrossRef]
- Alam, J.; Jaiswal, V.; Sharma, L. Screening of Antibiotics against β-amyloid as Anti-amyloidogenic Agents: A Drug Repurposing Approach. Curr. Comput.-Aided Drug Des. 2021, 17, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Muntner, P.; Chertow, G.M.; Warnock, D.G. Acute kidney injury and mortality in hospitalized patients. Am. J. Nephrol. 2012, 35, 349–355. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Y.; Zhao, J.; Jiang, H.; Zhu, J.; Yin, H.; Qiu, Y.; Hu, A.; Xu, L.; Song, Y.; et al. Shionone Attenuates Sepsis-Induced Acute Kidney Injury by Regulating Macrophage Polarization via the ECM1/STAT5 Pathway. Front. Med. 2022, 8, 796743. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.-Y.; Nam, G.-Y.; Kim, D.; Oh, Y.S.; Jun, H.-S. Prevention of LPS-Induced Acute Kidney Injury in Mice by Bavachin and Its Potential Mechanisms. Antioxidants 2022, 11, 2096. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Xiao, T. Application of Shionone in Preparation of Medicine for Preventing and Treating Diabetic Nephropathy. CN115990170A, 21 April 2023. [Google Scholar]
- Jaiswal, V.; Lee, H.-J. Antioxidant activity of Urtica dioica: An important property contributing to multiple biological activities. Antioxidants 2022, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, H.Y.; Ha Thi, H.T.; Kim, J.; Lee, Y.J.; Kim, S.J.; Hong, S. Pellino 3 promotes the colitis-associated colorectal cancer through suppression of IRF4-mediated negative regulation of TLR4 signaling. Mol. Oncol. 2023, 17, 2380–2395. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.; Ahmad, A.; Tiwari, R.K.; Ahmad, I.; Alkhathami, A.G.; Alshahrani, M.Y.; Asiri, M.A.; Almeleebia, T.M.; Saeed, M.; Yadav, D.K.; et al. In Vitro Evaluation of Antioxidant, Anticancer, and Anti-Inflammatory Activities of Ethanolic Leaf Extract of Adenium obesum. Front. Pharmacol. 2022, 13, 847534. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Oh, H.S.; Seo, H.J. Association between WHO First-Step Analgesic Use and Risk of Breast Cancer in Women of Working Age. Pharmaceuticals 2023, 16, 323. [Google Scholar] [CrossRef]
- Xu, N.; Hu, J.; Han, K.; Ou, Y.; Ji, T.; Xing, J. Shionone suppresses the growth, migration and invasion of human breast cancer cells via induction of apoptosis and inhibition of MEK/ERK and STAT3 signalling pathways. J. BUON 2020, 25, 1821–1826. [Google Scholar]
- Kim, K.T.; Lee, J.W.; Choe, H.-S. Pilot Study of Cystochon® (Cranberry Extract, Chondroitin Sulfate, and Hyaluronic Acid Complex) in Interstitial Cystitis/Bladder Pain Syndrome. Urogenit. Tract Infect. 2022, 17, 36–41. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhan, J.; Zhang, K.; Chen, H.; Cheng, S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: An analysis of the global burden of disease study 2019. World J. Urol. 2022, 40, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-G.; Jeong, J.-Y.; Yum, S.-H.; Hwang, Y.-J. Inhibitory effects of selected medicinal plants on bacterial growth of Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 7780. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.R.; Won, J.; Han, J.; Chung, W.; Ahn, S.J. Comparison of antimicrobial resistance in patients with obstructive pyelonephritis associated with ureteral stones and uncomplicated pyelonephritis. Medicine 2022, 101, e30376. [Google Scholar] [CrossRef]
- Ng, T.; Liu, F.; Lu, Y.; Cheng, C.; Wang, Z. Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 109–115. [Google Scholar] [CrossRef]
- Yu, P.; Cheng, S.; Xiang, J.; Yu, B.; Zhang, M.; Zhang, C.; Xu, X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015, 164, 328–333. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Yangyang, R.Y.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar]
- Qiao, C. Application of Shionone in Preparation of Medicine for Preventing and Treating Colitis. CN115054607A, 16 September 2022. [Google Scholar]
- Schiess, N.; Cataldi, R.; Okun, M.S.; Fothergill-Misbah, N.; Dorsey, E.R.; Bloem, B.R.; Barretto, M.; Bhidayasiri, R.; Brown, R.; Chishimba, L. Six action steps to address global disparities in Parkinson disease: A World Health Organization priority. JAMA Neurol. 2022, 79, 929–936. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yates, N.J.; Tye, S.J. Inflammatory mechanisms in Parkinson’s disease: From pathogenesis to targeted therapies. Neuroscientist 2022, 28, 485–506. [Google Scholar] [CrossRef]
- Sun, C. Application of Shionone in Preparation of Pharmaceutical Preparation for Treating Parkinson’s Disease. CN114917235A, 19 August 2022. [Google Scholar]
- Yin, D.F.; Zhou, K.; Liu, J.T.; Hu, L.; Liu, Y.; Deng, J.; Wang, S.P.; Xiong, Y.; Zhong, W. Development and validation of an LC/MS/MS method for simultaneous determination of shionone and epi-friedelinol in rat plasma for pharmacokinetic study after oral administration of Aster tataricus extract. Biomed. Chromatogr. 2016, 30, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, S.; Huang, X.; Ma, W.; Xue, Z.; Zhao, L.; Ouyang, H.; He, J. Pharmacokinetic comparison of 15 active compositions in rat plasma after oral administration of raw and honey-processed Aster tataricus extracts. J. Sep. Sci. 2021, 44, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Argollo, M.; Fiorino, G.; Hindryckx, P.; Peyrin-Biroulet, L.; Danese, S. Novel therapeutic targets for inflammatory bowel disease. J. Autoimmun. 2017, 85, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; Di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, M.W.; Kang, J.-H.; Jung, H.J.; Hwang, J.H.; Yang, S.J.; Woo, J.K.; Jeon, Y.; Lee, H.; Yoon, Y.S.; et al. Novel NF-κB reporter mouse for the non-invasive monitoring of inflammatory diseases. Sci. Rep. 2023, 13, 3556. [Google Scholar] [CrossRef]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Lee, H.-J. Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases. Molecules 2024, 29, 189. https://doi.org/10.3390/molecules29010189
Jaiswal V, Lee H-J. Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases. Molecules. 2024; 29(1):189. https://doi.org/10.3390/molecules29010189
Chicago/Turabian StyleJaiswal, Varun, and Hae-Jeung Lee. 2024. "Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases" Molecules 29, no. 1: 189. https://doi.org/10.3390/molecules29010189
APA StyleJaiswal, V., & Lee, H.-J. (2024). Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases. Molecules, 29(1), 189. https://doi.org/10.3390/molecules29010189







