Glutamate’s Effects on the N-Methyl-D-Aspartate (NMDA) Receptor Ion Channel in Alzheimer’s Disease Brain: Challenges for PET Radiotracer Development for Imaging the NMDA Ion Channel
Abstract
1. Introduction
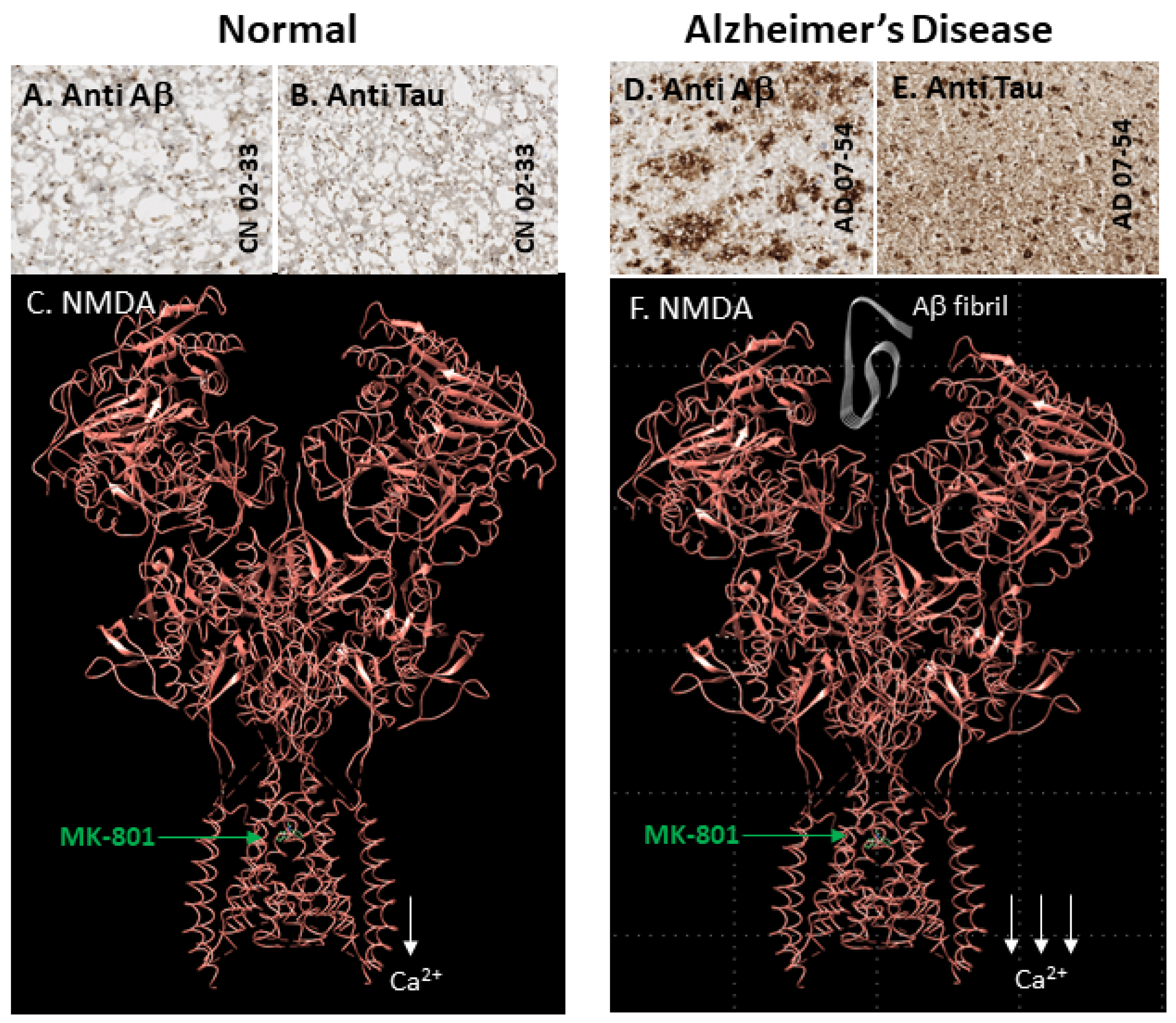
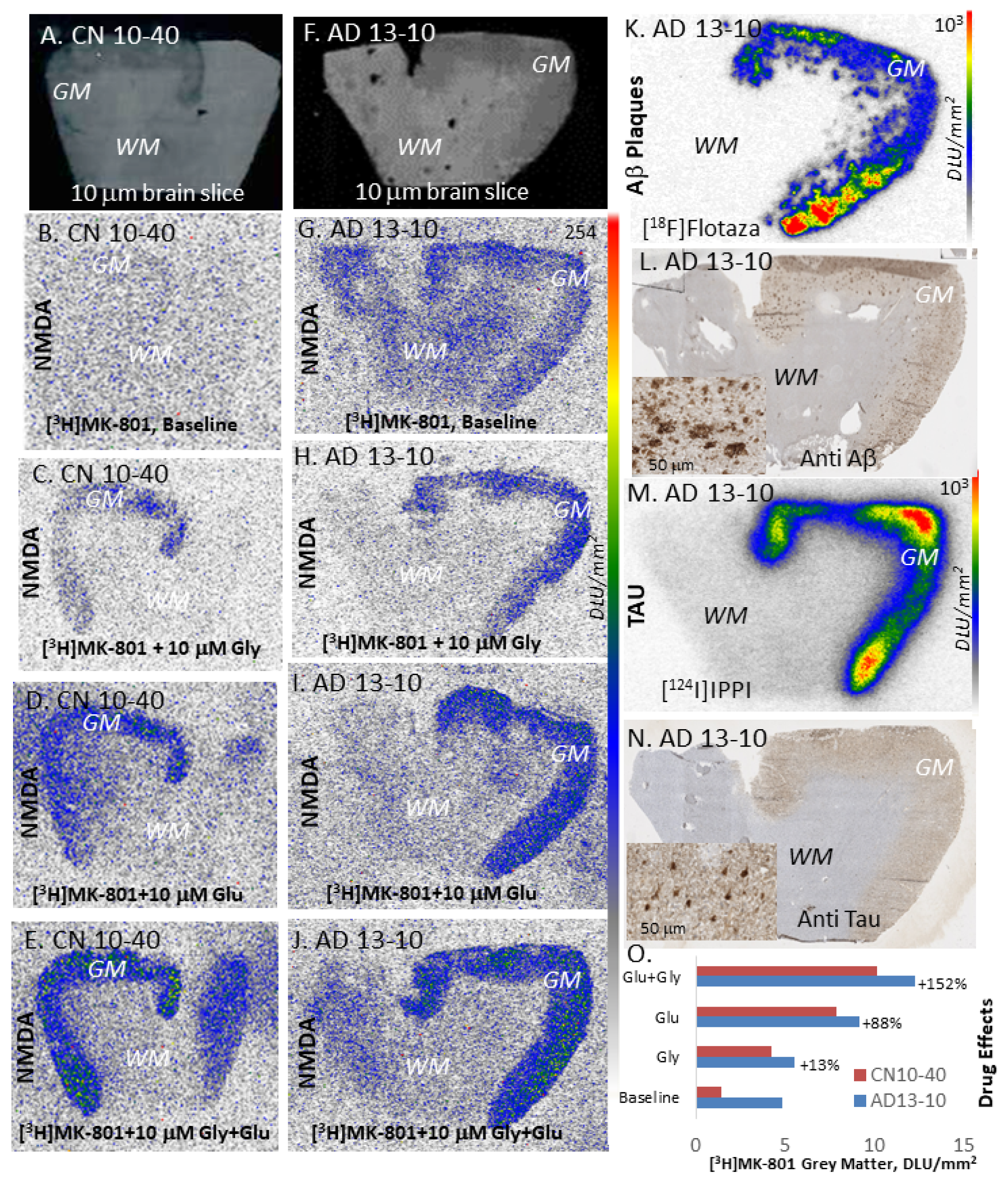
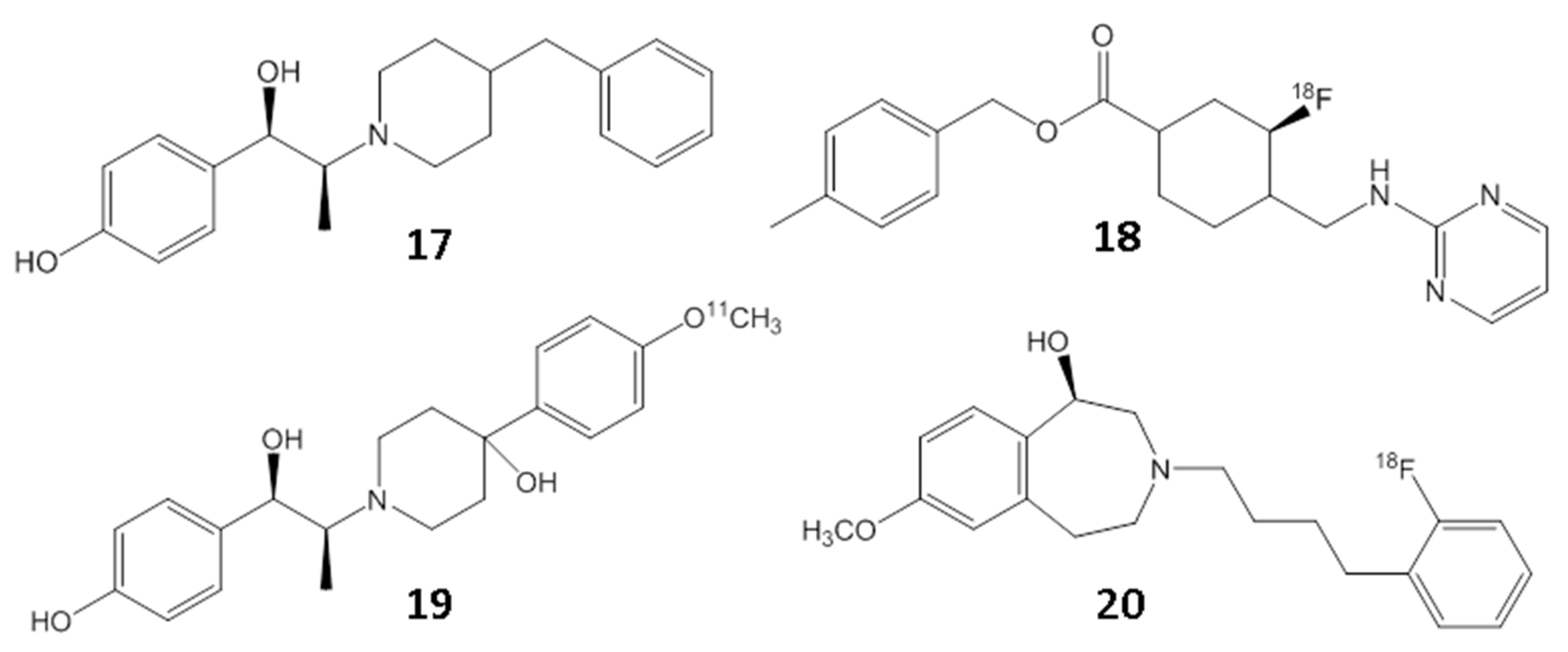
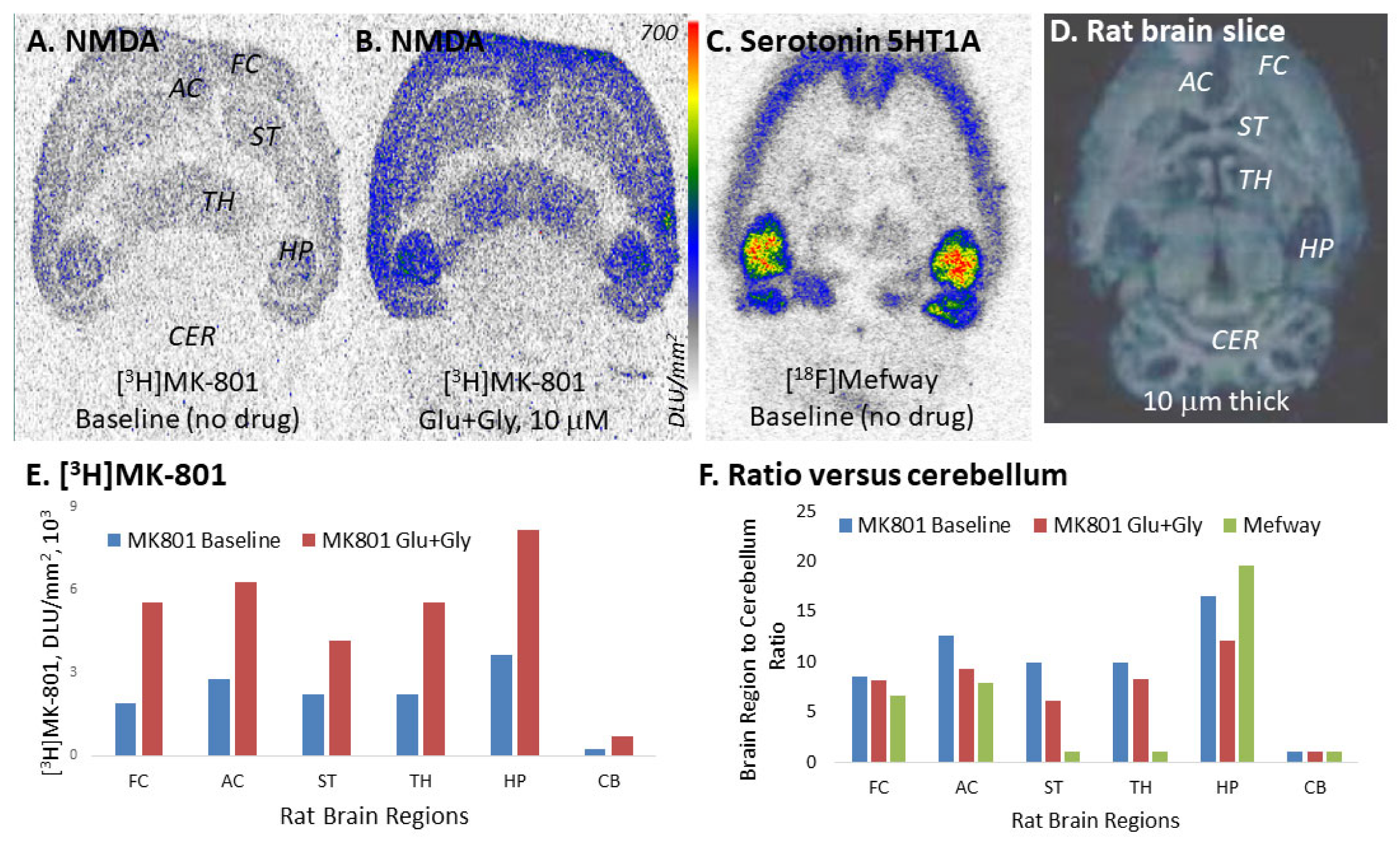
2. Results
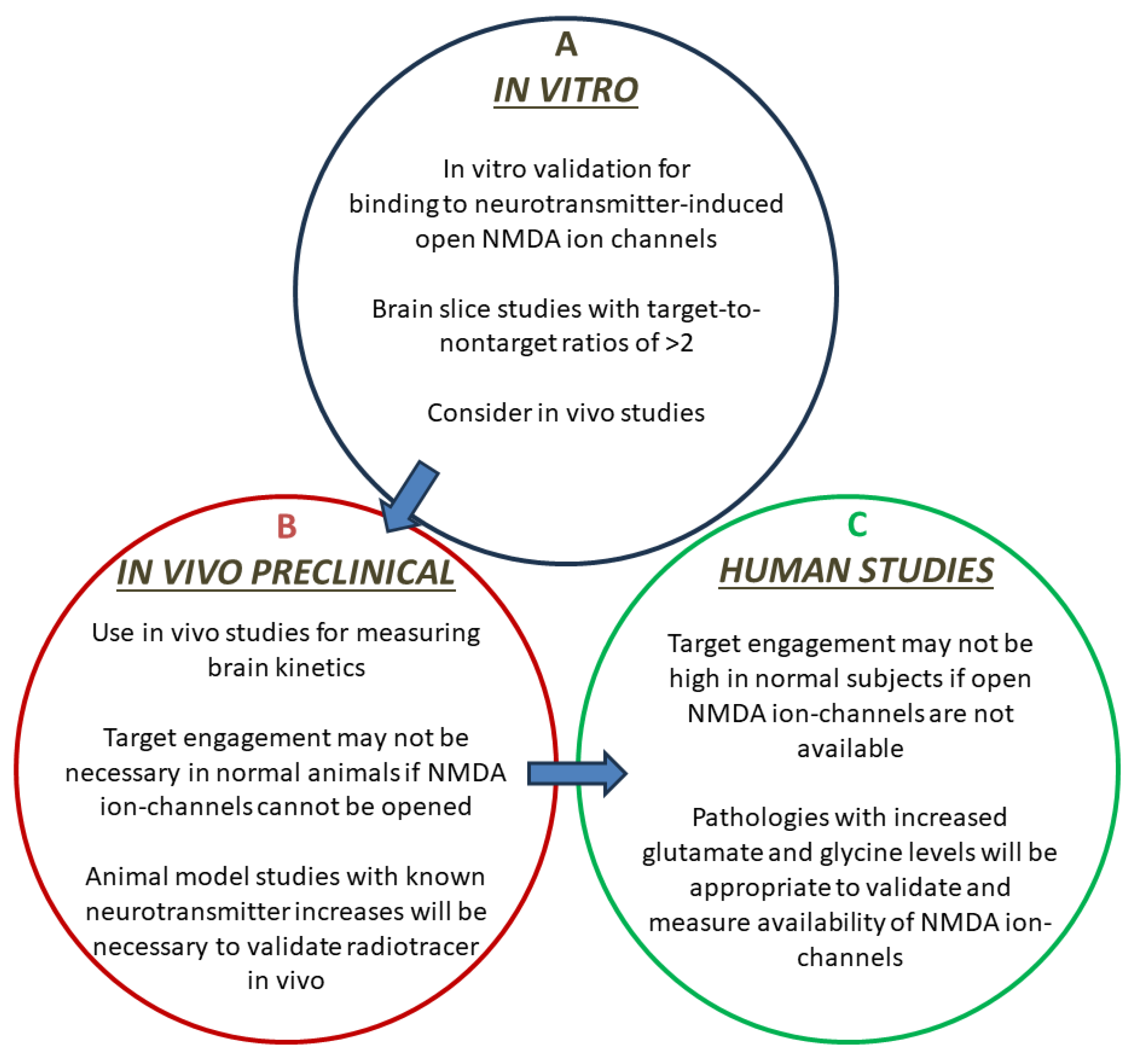
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Post-mortem Human Brain
4.3. Rat Studies
4.4. Aβ Plaque Imaging
4.5. Tau Imaging
4.6. NMDA Ionophore Imaging
4.7. Serotonin 5-HT1A Imaging Using [18F]Mefway
4.8. Molecular Docking
4.9. Immunohistochemistry
4.10. Image Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dejanovic, B.; Sheng, M.; Hanson, J.E. Targeting synapse function and loss for treatment of neurodegenerative disease. Nature Revs. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- Yamakura, T.; Shimoji, K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog. Neurobiol. 1999, 59, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharm. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Rottman, S.M.; Olney, J.W. Excitotoxicity and the NMDA receptor. Trends Neurosci. 1987, 19, 299–302. [Google Scholar] [CrossRef]
- Dingledine, R.; McBain, C.J.; McNamara, J.O. Excitatory amino acid receptors in epilepsy. Trends Pharmacol. Sci. 1990, 11, 334–338. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Niciu, M.J.; Ionescu, D.F.; Richards, E.M.; Zarate, C.A. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J. Neural Transm. 2014, 121, 907–924. [Google Scholar] [CrossRef]
- Wachtel, H.; Turski, L. L-glutamate: A new target in schizophrenia. Trends Pharmacol. Sci. 1990, 11, 219–220. [Google Scholar] [CrossRef]
- Turski, L.; Bressler, K.; Retting, K.J.; Loschman, P.A.; Wachtel, H. Protection of substantia nigra from MPP+ neurotoxicity by NMDA antagonists. Nature 1991, 349, 414–418. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Koerner, J.F. Excitatory amino acid neurotransmission. J. Med. Chem. 1988, 31, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Gliss, T.V. Memories of NMDA receptors and LTP. Trends Neurosci. 1995, 18, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Bresink, I.; Dansyz, W.; Parsons, C.G.; Mutschler, E. Different binding affinities of NMDA receptor channel blockers in various brain regions-Indication of NMDA receptor heterogeneity. Neuropharmacology 1995, 34, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Karakas, E.; Furukawa, H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 2014, 344, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jensen, M.O.; Jogini, V.; Stein, R.A.; Lee, C.H.; Mchaourab, H.S.; Shaw, D.E.; Gouaux, E. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature 2018, 114, 24a–25a. [Google Scholar] [CrossRef]
- Lipton, S.A. Paradigm shift in NMDA receptor antagonist drug development: Molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer’s disease and other neurologic disorders. J. Alzheimer’s Dis. 2004, 6, S61–S74. [Google Scholar] [CrossRef]
- Maragos, W.F.; Penny, J.B.; Young, A.B. Anatomic correlation of NMDA and 3H-TCP-labeled receptors in rat brain. J. Neurosci. 1988, 8, 493–501. [Google Scholar] [CrossRef]
- Cummings, K.A.; Popescu, G.K. Glycine-dependent activation of NMDA receptors. J. Gen. Physiol. 2015, 145, 513–527. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sanchez-Lopez, E. Memantine for the treatment of dementia: A review on its current and future applications. J. Alzheimer’s Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lu, W.; Michel, C.; Goehring, A.; Du, J.; Song, X.; Gouaux, E. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 2014, 511, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, P.; Suwalsky, M.; Jemiola-Rzeminska, M.; Strzalka, K. Studies on the interaction of NMDA receptor antagonist memantine with cell membranes: A mini-review. Chem.-Biol. Interact. 2018, 283, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Feng, J.; Wu, M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 2016, 37, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-H.; Epstein, M.; Michalski, K.; Fine, E.; Biggin, P.C.; Furukawa, H. Structural insights into binding of therapeutic channel blockers in NMDA receptors. Nat. Struct. Mol. Biol. 2022, 29, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.R.; Nigam, A.; Glasgow, N.G.; Narangoda, C.; Phillips, M.B.; Patel, D.S.; Mesbahi-Vasey, S.; Turcu, A.L.; Vazquez, S.; Kurnikova, M.G.; et al. Inhibition of NMDA receptors through membrane-to-channel path. Nat. Commun. 2022, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Bajwa, A.K.; Wooten, D.W.; Hillmer, A.T.; Pan, M.-L.; Pandey, S.K.; Saigal, N.; Christian, B.T. Comparative assessment of 18F-Mefway as a serotonin 5-HT1A receptor PET imaging agent across species-rodents, nonhuman primates and humans. J. Comp. Neurol. 2016, 524, 1457–1471. [Google Scholar] [CrossRef]
- Mukherjee, J.; Lao, P.; Betthauser, T.; Samra, G.K.; Pan, M.L.; Patel, I.H.; Liang, C.; Metherate, R.; Christian, B.T. Human brain imaging of nicotinic acetylcholine α4β2* receptors using [18F]Nifene: Selectivity, functional activity, toxicity, aging effects, gender effects and extrathalamic pathways. J. Comp. Neurol. 2018, 526, 80–96. [Google Scholar] [CrossRef]
- Proctor, A.W.; Wong, E.H.F.; Stratmann, G.C.; Lowe, S.L.; Bowen, D.M. Reduced glycine stimulation of [3H]MK-801 binding in Alzheimer’s disease. J. Neurochem. 1989, 53, 698–704. [Google Scholar] [CrossRef]
- Mouradian, M.M.; Contreras, P.C.; Monahan, J.B.; Chase, T.N. [3H]MK-801 binding in Alzheimer’s disease. Neurosci. Lett. 1988, 93, 225–230. [Google Scholar] [CrossRef]
- Korff, M.; Chaudhary, A.; Li, Y.; Zhou, X.; Zhao, C.; Rong, J.; Chen, J.; Xiao, Z.; Elghazawy, N.H.; Sippl, W.; et al. Synthesis and biological evaluation of enantiomerically pure ®-and (S)-[18F]OF-NB1 for imaging the GluN2B subunit-containing NMDA receptors. J. Med. Chem. 2023. [Google Scholar] [CrossRef]
- Mukherjee, J.; Liang, C.; Patel, K.K.; Lam, P.Q.; Mondal, R. Development and evaluation [125I]IPPI for tau imaging in post-mortem human Alzheimer’s disease brain. Synapse 2021, 74, e22183. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Parsons, C.G. Alzheimer’s disease, b-amyloid, glutamate, NMDA receptors and memantine- searching for the connections. Br. J. Pharmacol. 2012, 167, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Felix, M.R.; Liang, C.; Mukherjee, J. Development and evaluation [18F]Flotaza for Aβ plaque imaging in post-mortem Alzheimer’s disease brain. Bioorg. Med. Chem. Lett. 2021, 46, 128164. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Sandhu, Y.K.; Kamalia, V.M.; Delaney, B.A.; Syed, A.U.; Nguyen, G.A.H.; Moran, T.R.; Limpengco, R.R.; Liang, C.; Mukherjee, J. Measurement of Ab amyloid and Tau in postmortem human Alzheimer’s disease brain by immunohistochemistry analysis using QuPath and autoradiography using [18F]flotaza, [125I]IBETA and [124/125I]IPPI. Biomedicines 2023, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Cowburn, R.F.; Wiehager, B.; Trief, E.; LiLi, M.; Sundstrom, E. Effects of beta-amyloid-(25-35) peptides on radioligand binding to excitatory amino acid receptors and voltage-dependent calcium channels: Evidence for a selective affinity for the glutamate and glycine recognition sites of the NMDA receptor. Neurochem. Res. 1997, 22, 1437–1442. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stoffler, A.F.; Schmitt, S.; Ferris, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Eng. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Fuchigami, T.; Nakayama, M.; Magata, Y. Development of radioligands for in vivo imaging of NMDA receptors. In PET and SPECT of Neurobiological Systems; Dierckx, R.A.J.O., Otte, A., de Vries, E.F.J., van Waarde, A., Luiten, P.G.M., Eds.; Springer: New York, NY, USA, 2014; pp. 513–559. [Google Scholar]
- Ouyang, X.-H.; Mukherjee, J.; Yang, Z.Y. Syntheses, radiosyntheses and biological evaluation of thienylcyclohexyl piperidine derivatives as potential radiotracers for the N-methyl-D-aspartate receptor-linked calcium ionophore. Nucl. Med. Biol. 1996, 23, 315–324. [Google Scholar] [CrossRef]
- Brown, D.R.; Wyper, D.J.; Owens, J.; Patterson, J.; Kelly, R.C.; Hunter, R.; McCulloch, J. 123I-iodoMK-801, A SPECT agent for imaging the pattern and extent of glutamate (NMDA) receptor activation in Alzhimer’s disease. J. Psychiatr. Res. 1997, 31, 605–617. [Google Scholar] [CrossRef]
- Sihver, S.; Sihver, W.; Andersson, Y.; Murata, T.; Bergström, M.; Onoe, H.; Matsumura, K.; Tsukada, H.; Oreland, L.; Långström, B.; et al. In vitro and in vivo characterization of (+)-3-[11C]cyano-dizocilpine. J. Neural Transm. 1998, 105, 117–131. [Google Scholar]
- Asselin, M.C.; Hammers, A.; Turton, D.; Osman, S.; Koepp, M.; Brooks, D. Initial kinetic analysis of the in vivo binding of the putative NMDA receptor ligand [C-11]CNS 5161 in humans. Neuroimage 2004, 22 (Suppl. S2), T137–T138. [Google Scholar]
- Owens, J.; Wyper, D.J.; Patterson, J.; Brown, D.R.P.; Elliott, A.T.; Teasdale, G.M.; McCulloch, J. First SPET images of glutamate (NMDA) receptor activation in vivo in cerebral ischaemia. Nucl. Med. Commun. 1997, 18, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Murphy, S.N.; Hartwig, W.; Miller, R.J.; Cooper, M.D. N-(3-F-18 Fluoropropyl) MK-801: A PET tracer for in-vivo studies of the N-methyl-D-aspartate receptors. J Nucl. Med. 1988, 29, 932. [Google Scholar]
- Ametamey, S.M.; Bruehlmeier, M.; Kneifel, S.; Kokic, M.; Honer, M.; Arigoni, M.; Buck, A.; Burger, C.; Samnick, S.; Quack, G.; et al. PET studies of 18F-memantine in healthy volunteers. Nucl. Med. Biol. 2002, 29, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Haradahira, T.; Ito, H.; Fujimura, Y.; Seki, C.; Ikoma, Y.; Maeda, J.; Arakawa, R.; Takano, A.; Takahashi, H.; et al. Measurement of glycine binding site of NMDA receptors in living human brain using 4-acetoxy derivative of L-703,717, 4-acetoxy-7-chloro-3-[3-(4-[11C]methoxybenzyl)phenyl]-2-(1H)-quinoline (AcL703) with positron emission tomography. Synapse 2007, 61, 795–800. [Google Scholar] [CrossRef] [PubMed]
- McGinnity, C.J.; Hammers, A.; Riano Barros, D.A.; Luthra, S.K.; Jones, P.A.; Trigg, W.; Micallef, C.; Symms, M.R.; Brooks, D.J.; Koepp, M.J.; et al. Initial evaluation of 18F-GE-179, a putative PET tracer for activated N-methyl-D-aspartate receptors. J. Nucl. Med. 2014, 55, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Schoenberger, M.; Schroeder, F.A.; Placzek, M.S.; Carter, R.L.; Rosen, B.R.; Hooker, J.M.; Sander, C.Y. In vivo [18F]GE-179 brain signal does not show NMDA specific modulation with drug challenge in rodents and nonhuman primates. ACS Chem. Neurosci. 2018, 9, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Golla, S.S.V.; Klein, P.J.; Bakker, J.; Schuit, R.C.; Christaans, J.A.M.; Geest, L.V.; Kooijman, E.J.M.; Oropeza-Seguias, G.M.; Langermans, J.A.M.; Leysen, J.E.; et al. Preclinical evaluation of 18F-PK-209, a new PET ligand for imaging the ion-channel site of NMDA receptors. Nucl. Med. Biol. 2015, 42, 205–212. [Google Scholar] [CrossRef]
- Borza, I.; Bozó, É.; Barta-Szalai, G.; Kiss, C.; Tárkányi, G.; Demeter, Á.; Gáti, T.; Háda, V.; Kolok, S.; Gere, A.; et al. Selective NR1/2B N-methyl-D-aspartate receptor antagonists among indole-2-carboxamides and benzimidazole-2-carboxamides. J. Med. Chem. 2007, 50, 901–914. [Google Scholar] [CrossRef]
- Christiaans, J.A.M.; Klein, P.J.; Metaxas, A.; Koojiman, E.J.M.; Schuit, R.C.; Leysen, J.E.; Lammertsma, A.A.; van Berckel, B.N.M.; Windhorst, A.D. Synthesis and preclinical evaluation of carbon-11 labelled N-((5-(4-fluoro-2-[11C]methoxyphenyl)pyridine-3-yl)methyl)cyclopentanamine as a PET tracer for NR2B subunit-containing NMDA receptors. Nucl. Med. Biol. 2014, 41, 670–680. [Google Scholar] [CrossRef]
- Haider, A.; Iten, I.; Ahmed, H.; Muller-Herder, A.; Gruber, S.; Kramer, S.D.; Keller, C.; Schibli, R.; Wunsch, B.; Mu, L.; et al. Identification and preclinical evaluation of a radiofluorinated benzazepine derivative for imaging the GluN2B subunit of the ionotropic NMDA receptor. J. Nucl Med. 2018, 60, 259–266. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Anderson, K.J. Age-related changes in [3H]MK-801 binding in the Fischer 344 rat brain. Neurobiol. Aging 1998, 19, 259–265. [Google Scholar] [CrossRef]
- Ulas, J.; Brunner, L.C.; Geddes, J.W.; Choe, W.; Cotman, C.W. N-methyl-D-aspartate receptor complex in the hippocampus of elderly, normal individuals and those with Alzheimer’s disease. Neuroscience 1992, 49, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Nguyen, G.A.H.; Danh, T.B.; Sandhu, A.K.; Melkonyan, L.L.; Syed, A.U.; Mukherjee, J. Abnormal [18F]Nifene binding in transgenic 5xFAD mouse model of Alzheimer’s disease: In vivo PET/CT imaging studies of α4β2* nicotinic acetylcholinergic receptors and in vitro correlations with Ab plaques. Synapse 2023, 77, e22265. [Google Scholar] [CrossRef] [PubMed]
- Campoy, A.-D.T.; Liang, C.; Ladwa, R.M.; Patel, K.K.; Patel, I.H.; Mukherjee, J. [18F]Nifene PET/CT imaging in mice models: Improved methods and preliminary studies of α4β2* nicotinic acetylcholinergic receptors in transgenic A53T mouse model of α-synucleinopathy and post-mortem human Parkinson’s disease. Molecules 2021, 26, 7360. [Google Scholar] [CrossRef]
- Hanson, J.E.; Pare, J.-F.; Deng, L.; Smith, Y.; Zhou, Q. Altered GluN2B NMDA receptor function and synaptic plasticity during early pathology in the PS2APP mouse model of Alzheimer’s disease. Neurobiol. Dis. 2015, 74, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memnatine for Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimers Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Chrem Méndez, P.A.; Surace, E.I.; Bérgamo, Y.; Calandri, I.L.; Vázquez, S.; Sevlever, G.E.; Allegri, R.F. Biomarkers for Alzheimers disease. Where we stand and where we are headed. Medicina 2019, 79, 546–551. [Google Scholar]
- Syed, A.U.; Liang, C.; Patel, K.K.; Mondal, R.; Kamalia, V.M.; Moran, T.R.; Ahmed, S.T.; Mukherjee, J. Comparison of Monoamine oxidase-A, Ab plaques, Tau and Translocator protein in postmortem human Alzheimer’s disease brain. Int. J. Mol. Sci. 2023, 24, 10808. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Serrano, G.; Shill, H.A.; Walker, D.G.; Lue, L.; Roher, A.E.; Dugger, B.N.; Maarouf, C.; et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015, 35, 354–389. [Google Scholar] [CrossRef]
- Saigal, N.; Bajwa, A.K.; Faheem, S.S.; Coleman, R.; Pandey, S.K.; Constantinescu, C.C.; Fong, V.; Mukherjee, J. Evaluation of serotonin 5HT1A receptors in rodent models using 18F-mefway PET. Synapse 2013, 67, 596–608. [Google Scholar] [CrossRef][Green Version]
- Nguyen, G.A.H.; Liang, C.; Mukherjee, J. [124I]IBETA, a new Ab amyloid plaque PET imaging agent for Alzheimer’s disease. Molecules 2022, 27, 4552. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Ametamey, S.M. Imaging the glutamate receptor subtypes—Much achieved, and still much to do. Drug Discov. Today 2017, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, D.E. Intracellular glutamate signalling in the nervous system and beyond. ACS Chem. Neurosci. 2010, 1, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, I.D.; Westbrook, G.L.; Mayer, M.L. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J. Neurosci. 1988, 8, 3733–3741. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Murphy, S.N.; Miller, R.J. 3H-Labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is ehanced by glycine. Proc. Natl. Acad. Sci. USA 1987, 84, 7744–7748. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.M.; Ghazaryan, N.; Gonzaga, N.L.; Paclibar, C.G.; Biju, A.P.; Liang, C.; Mukherjee, J. Glutamate’s Effects on the N-Methyl-D-Aspartate (NMDA) Receptor Ion Channel in Alzheimer’s Disease Brain: Challenges for PET Radiotracer Development for Imaging the NMDA Ion Channel. Molecules 2024, 29, 20. https://doi.org/10.3390/molecules29010020
Shah NM, Ghazaryan N, Gonzaga NL, Paclibar CG, Biju AP, Liang C, Mukherjee J. Glutamate’s Effects on the N-Methyl-D-Aspartate (NMDA) Receptor Ion Channel in Alzheimer’s Disease Brain: Challenges for PET Radiotracer Development for Imaging the NMDA Ion Channel. Molecules. 2024; 29(1):20. https://doi.org/10.3390/molecules29010020
Chicago/Turabian StyleShah, Nehal M., Nane Ghazaryan, Noresa L. Gonzaga, Cayz G. Paclibar, Agnes P. Biju, Christopher Liang, and Jogeshwar Mukherjee. 2024. "Glutamate’s Effects on the N-Methyl-D-Aspartate (NMDA) Receptor Ion Channel in Alzheimer’s Disease Brain: Challenges for PET Radiotracer Development for Imaging the NMDA Ion Channel" Molecules 29, no. 1: 20. https://doi.org/10.3390/molecules29010020
APA StyleShah, N. M., Ghazaryan, N., Gonzaga, N. L., Paclibar, C. G., Biju, A. P., Liang, C., & Mukherjee, J. (2024). Glutamate’s Effects on the N-Methyl-D-Aspartate (NMDA) Receptor Ion Channel in Alzheimer’s Disease Brain: Challenges for PET Radiotracer Development for Imaging the NMDA Ion Channel. Molecules, 29(1), 20. https://doi.org/10.3390/molecules29010020






