Enhanced Polarization Properties of Holographic Storage Materials Based on RGO Size Effect
Abstract
:1. Introduction
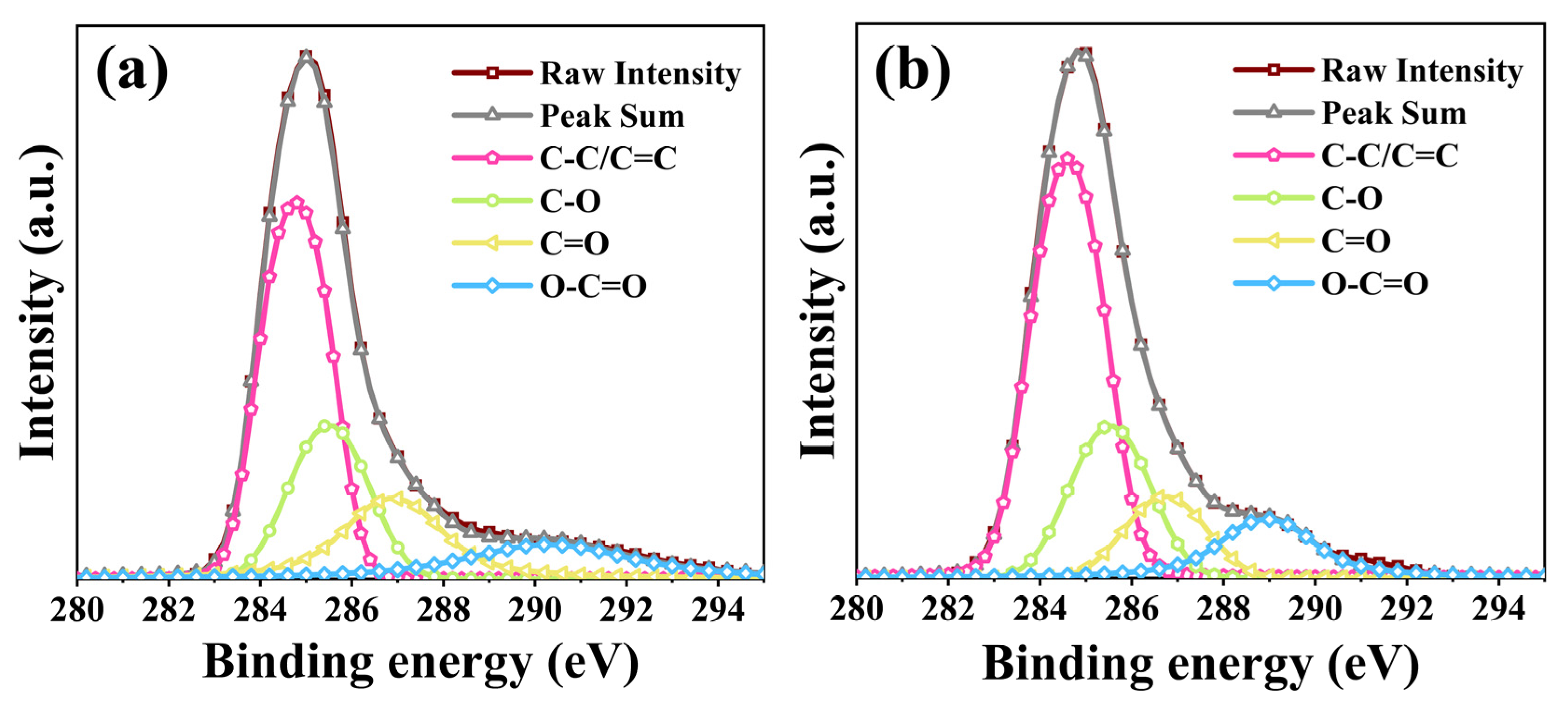
2. Results
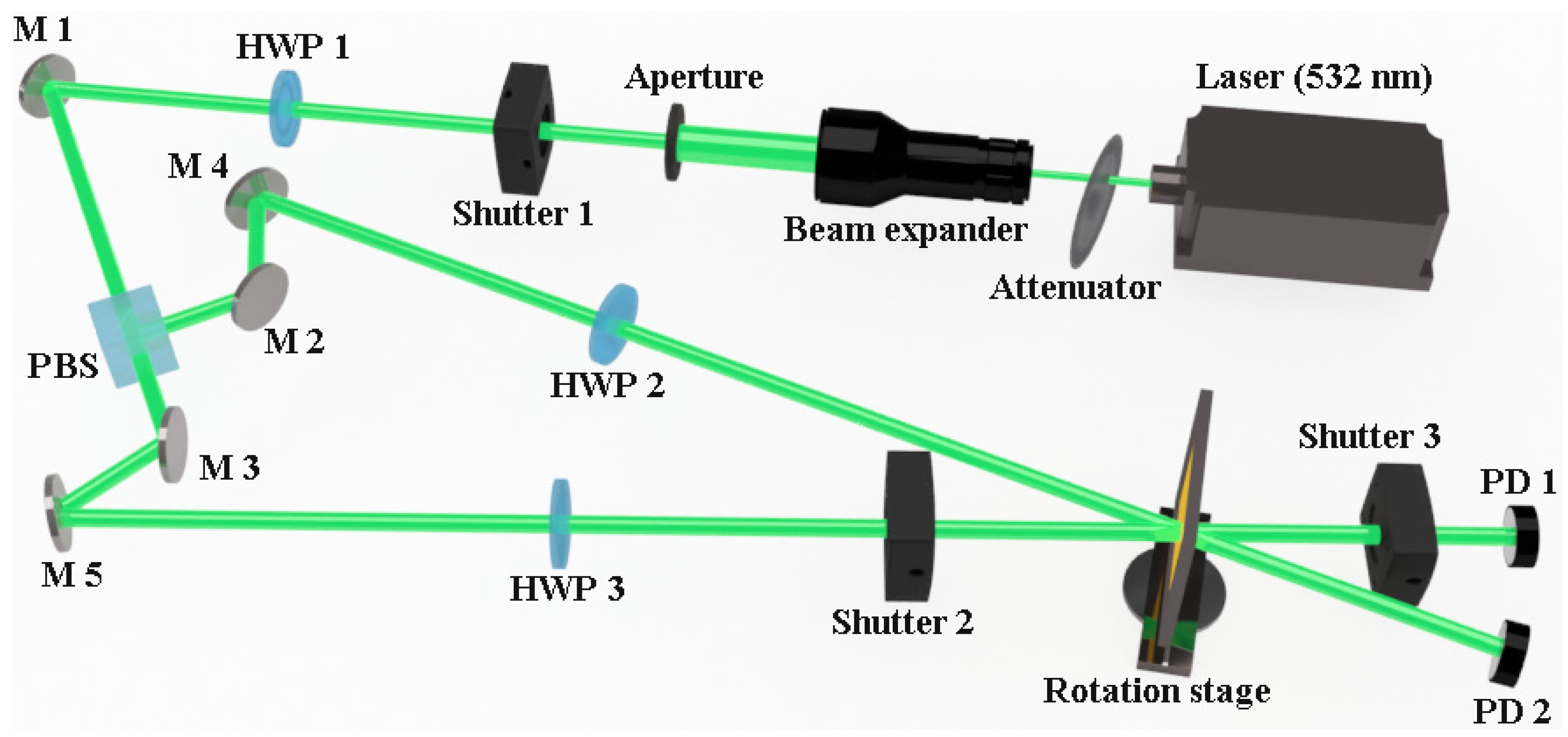
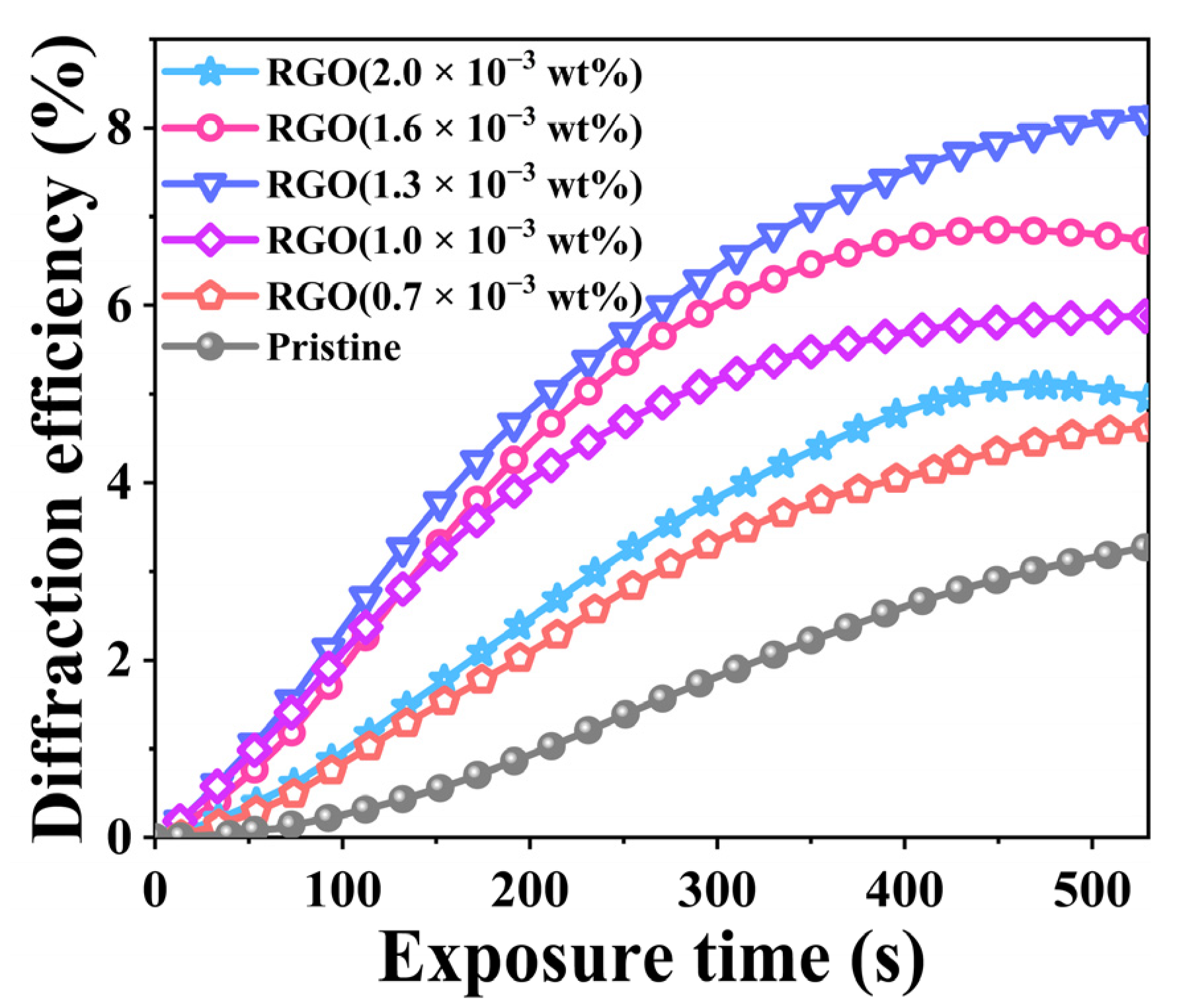
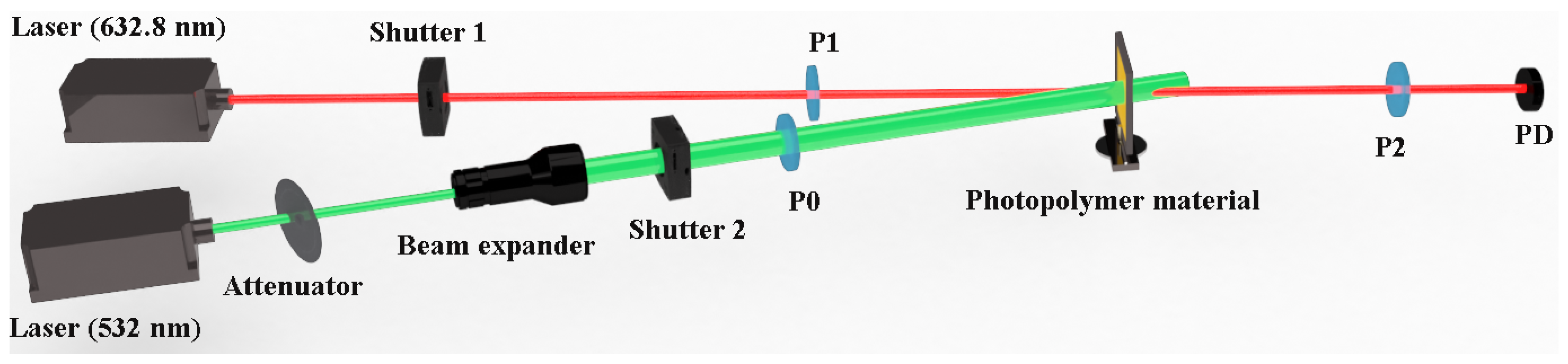
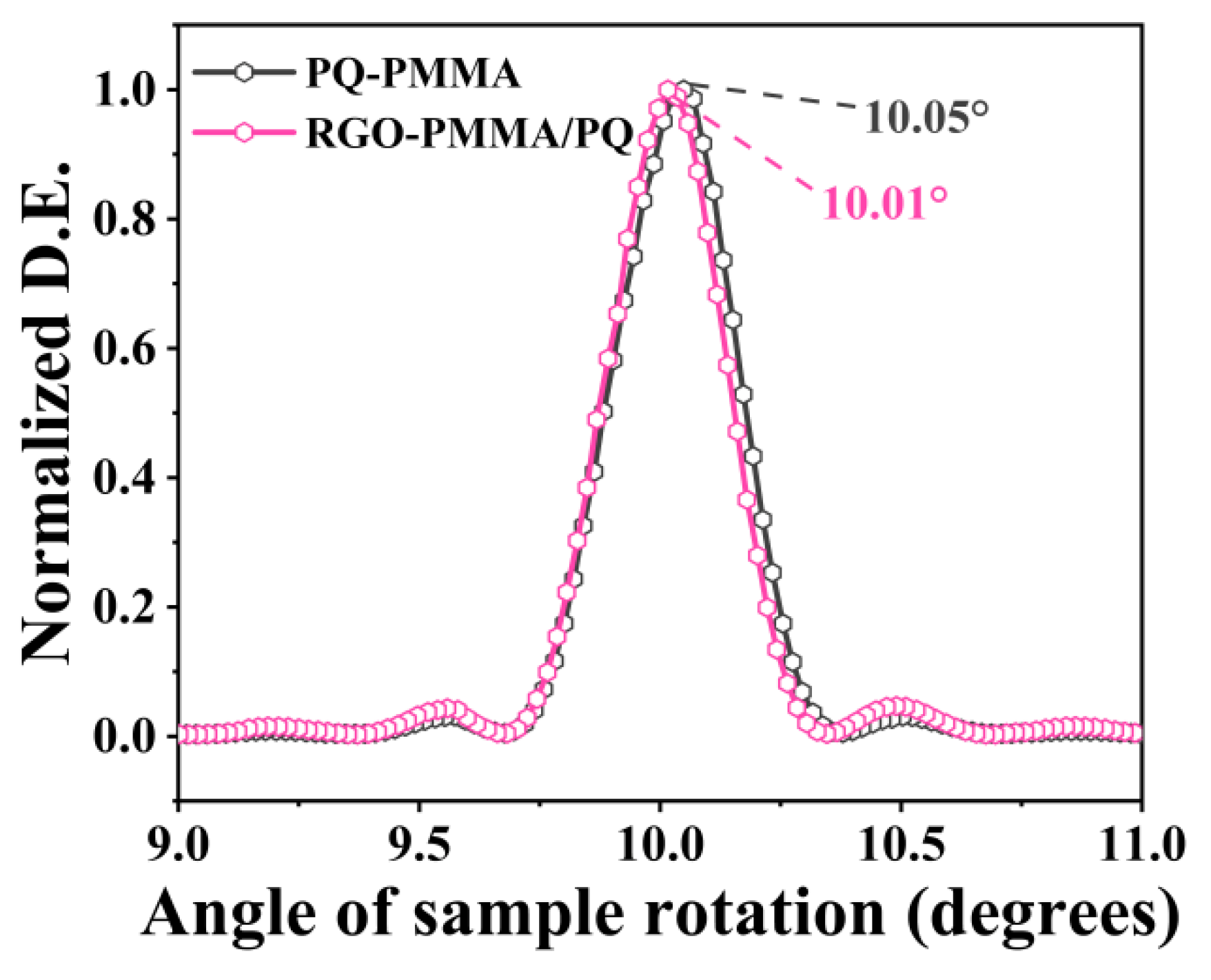
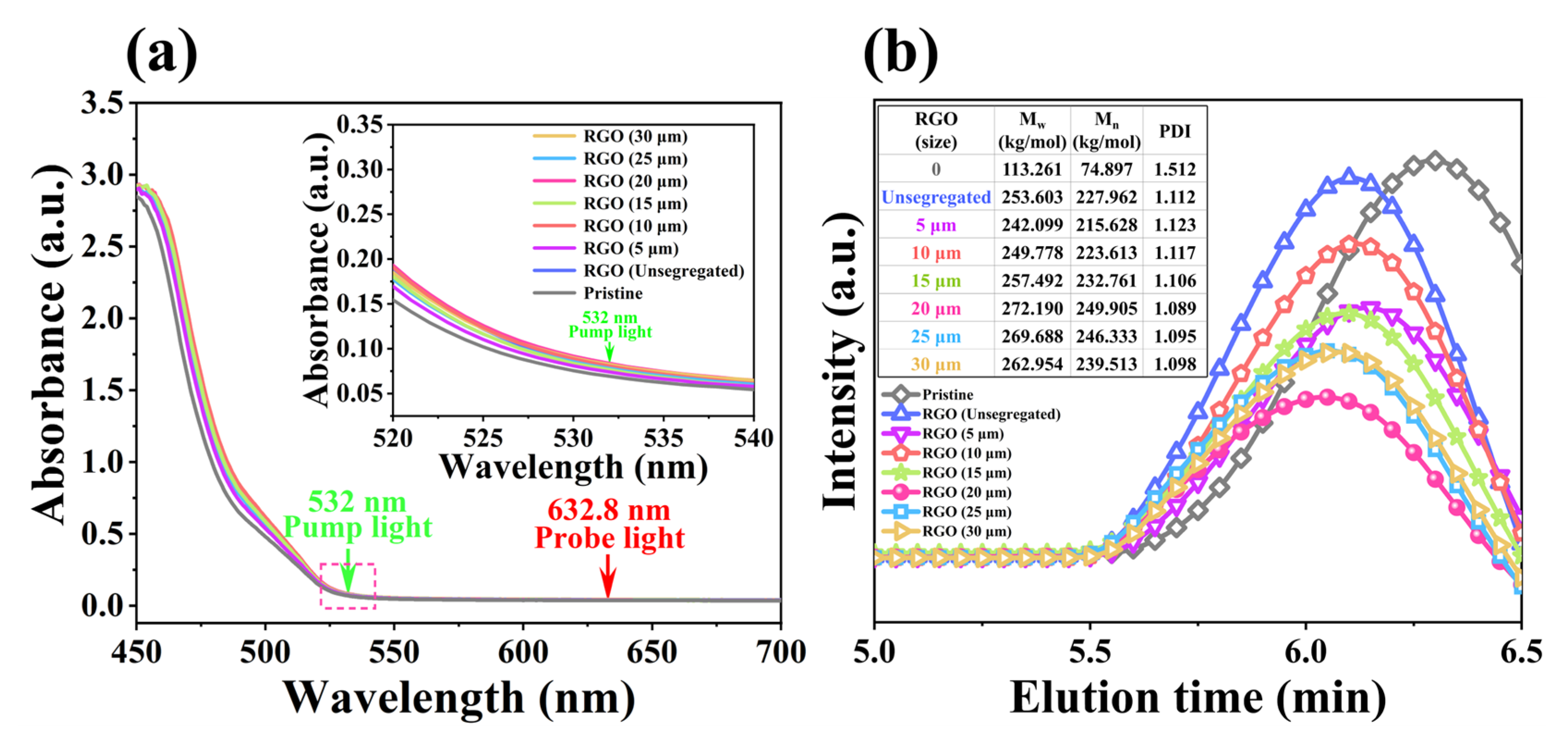
2.1. Holographic Characteristics
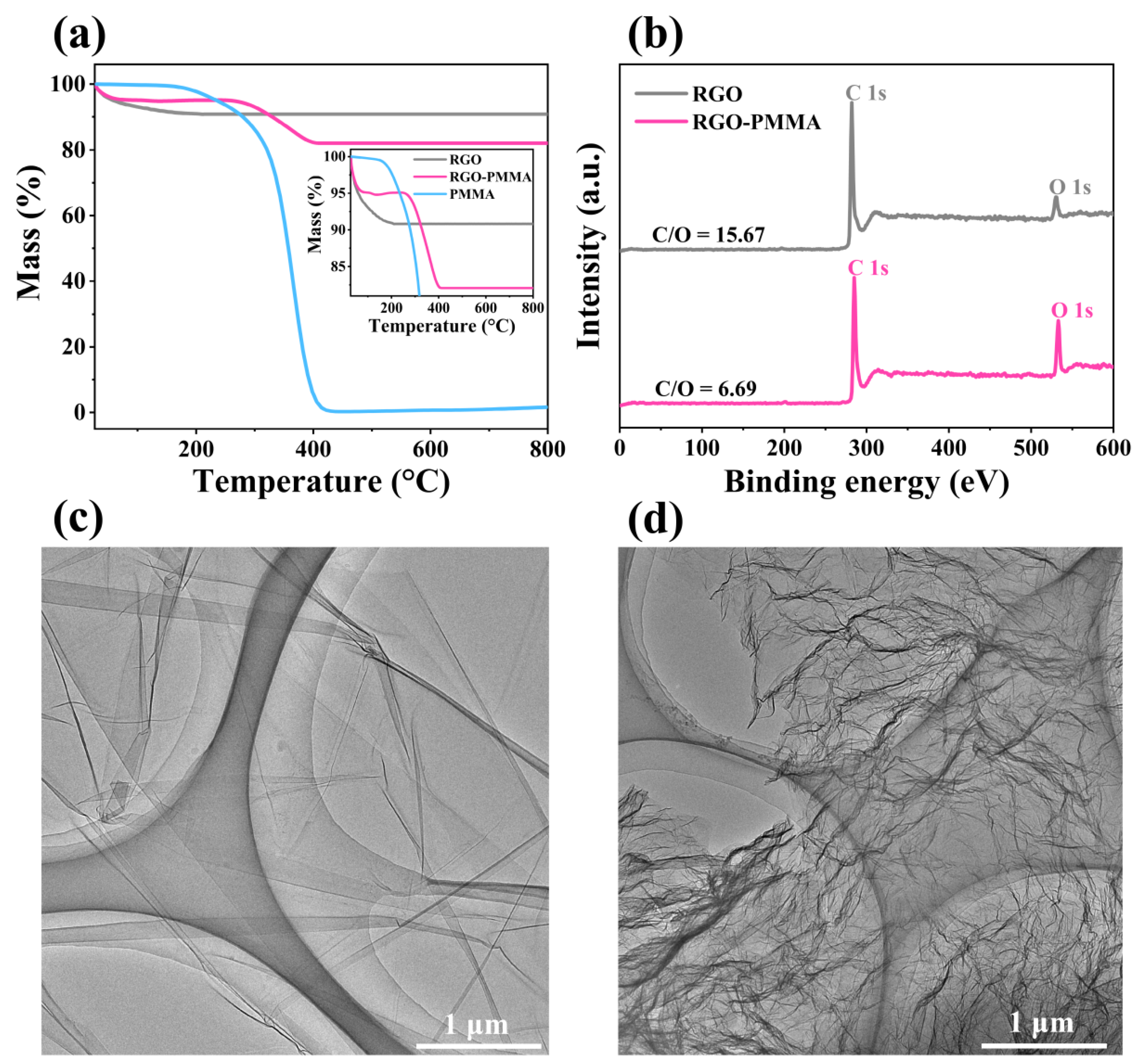
2.2. Stability Study of RGO-PMMA/PQ Photopolymer
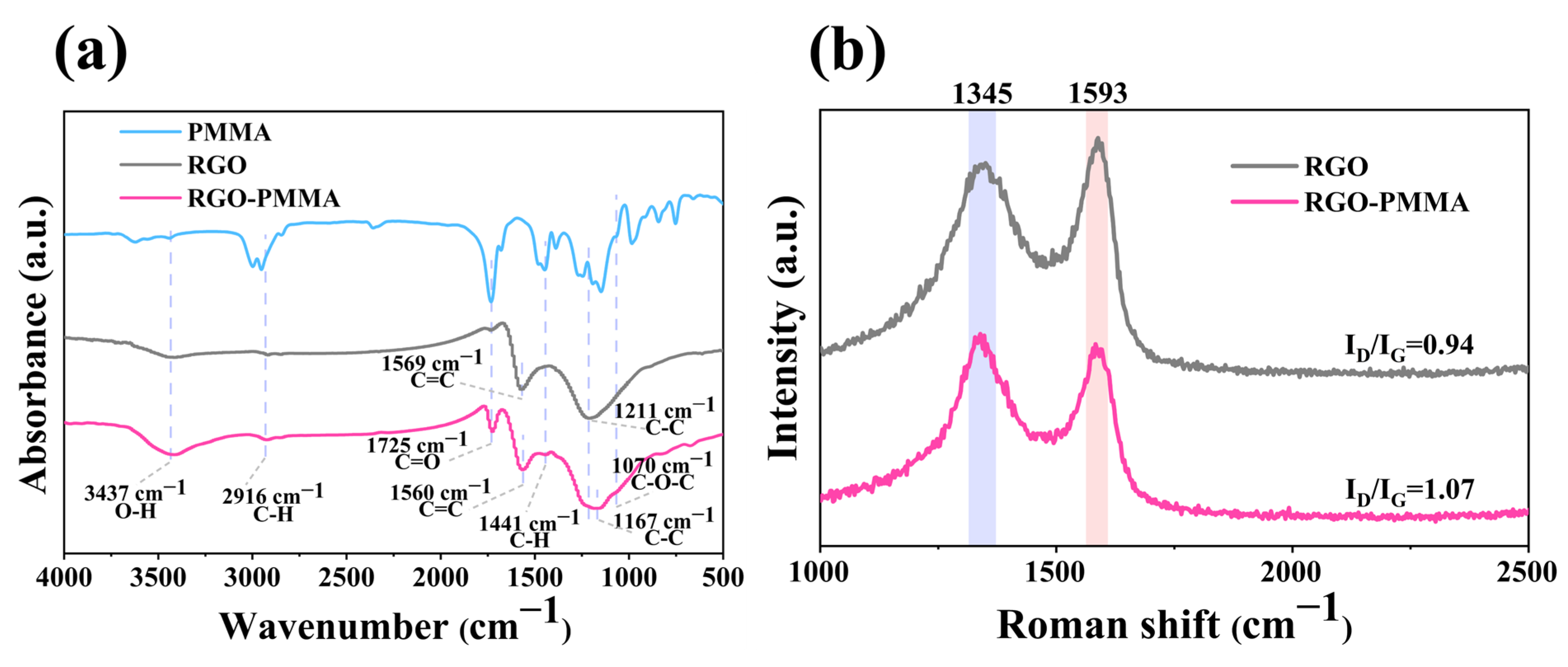
3. Reaction Mechanism
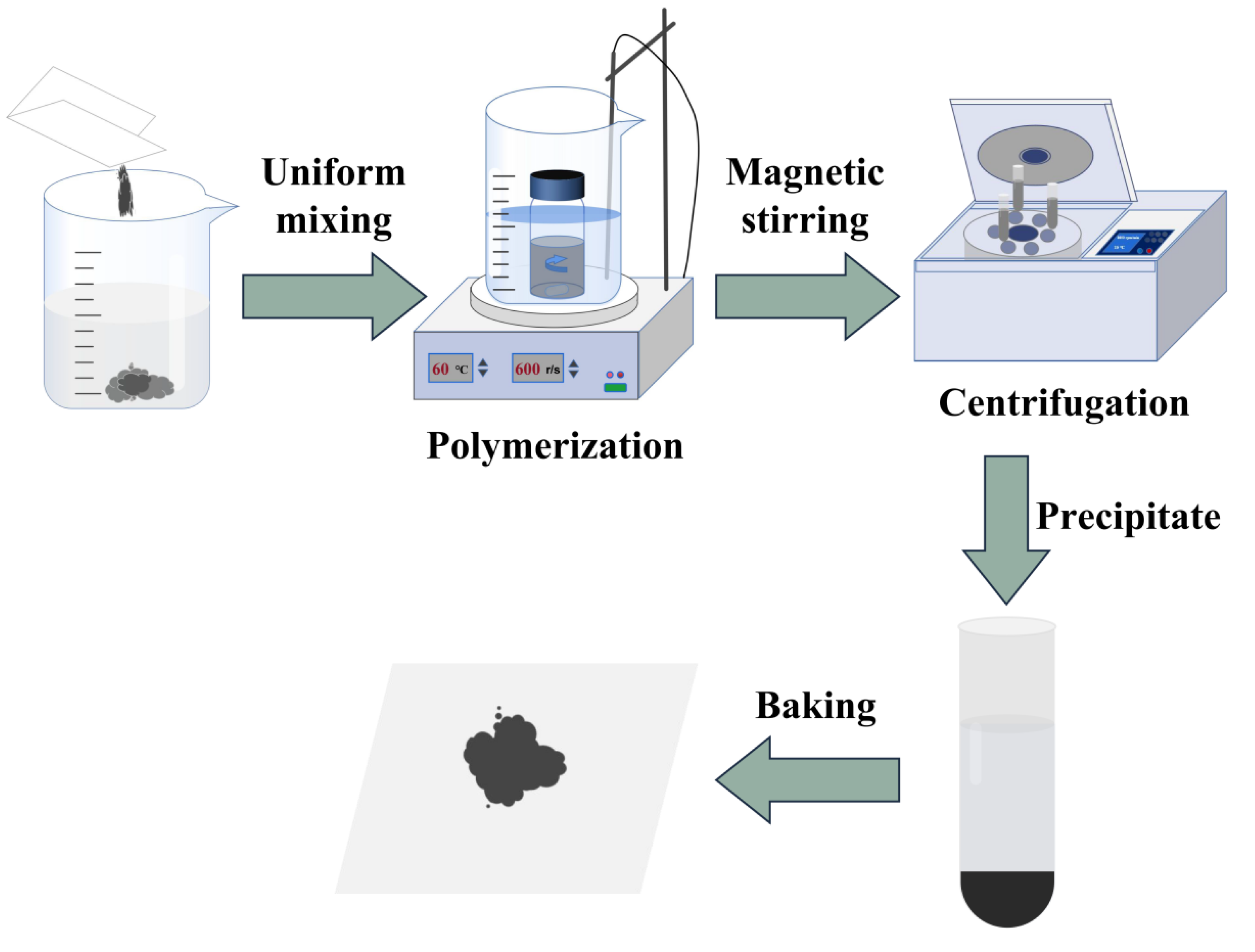
4. Material Preparation
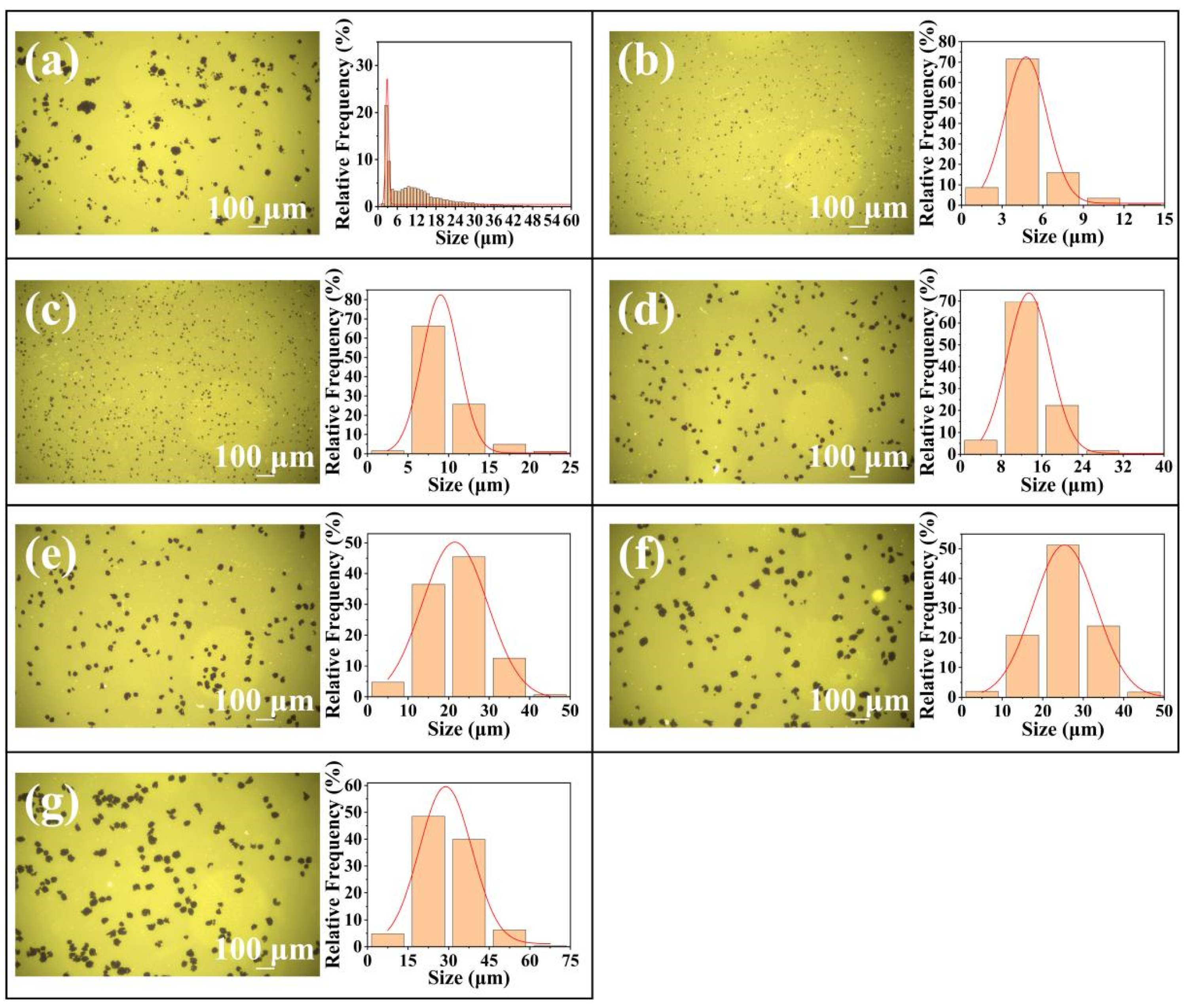
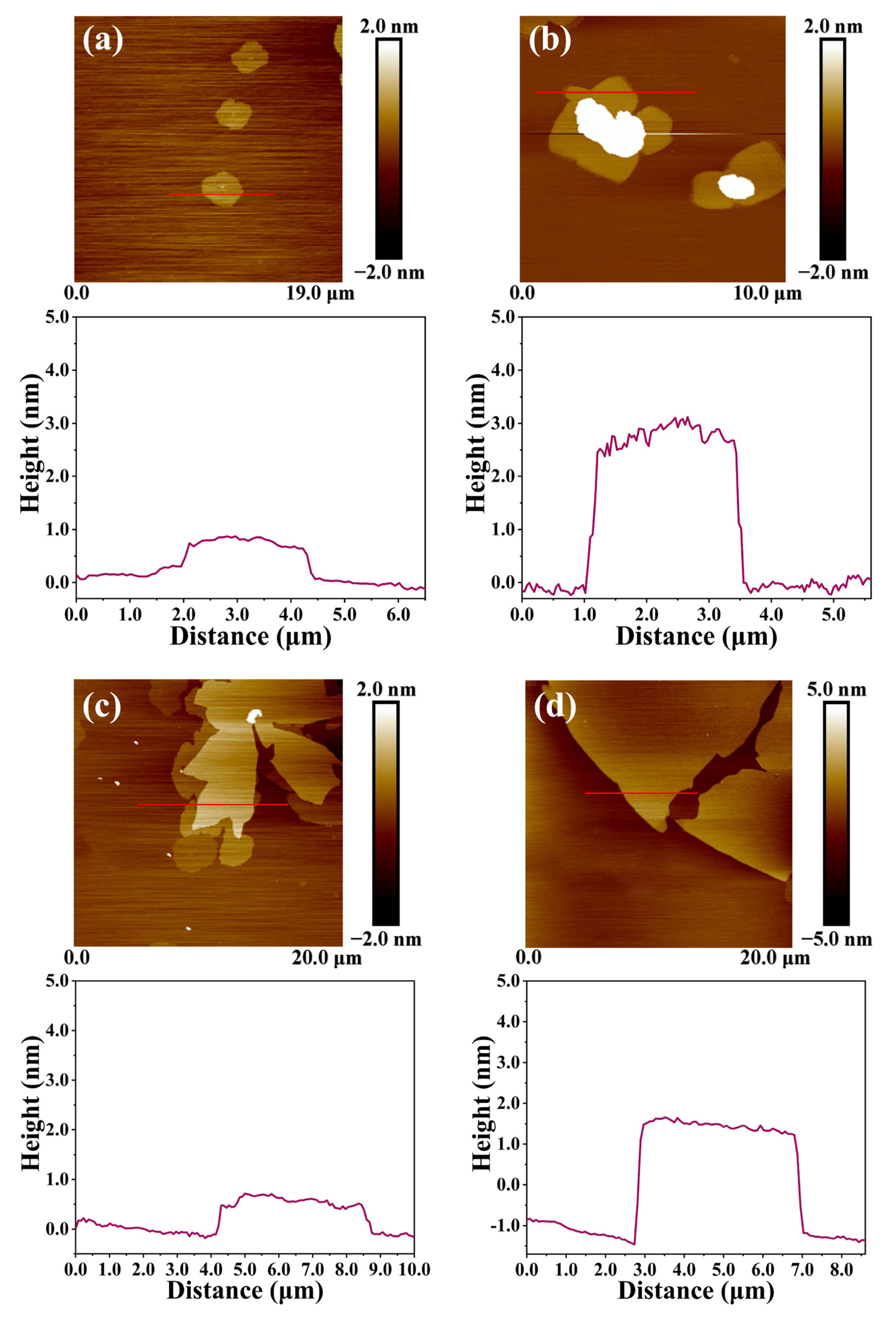
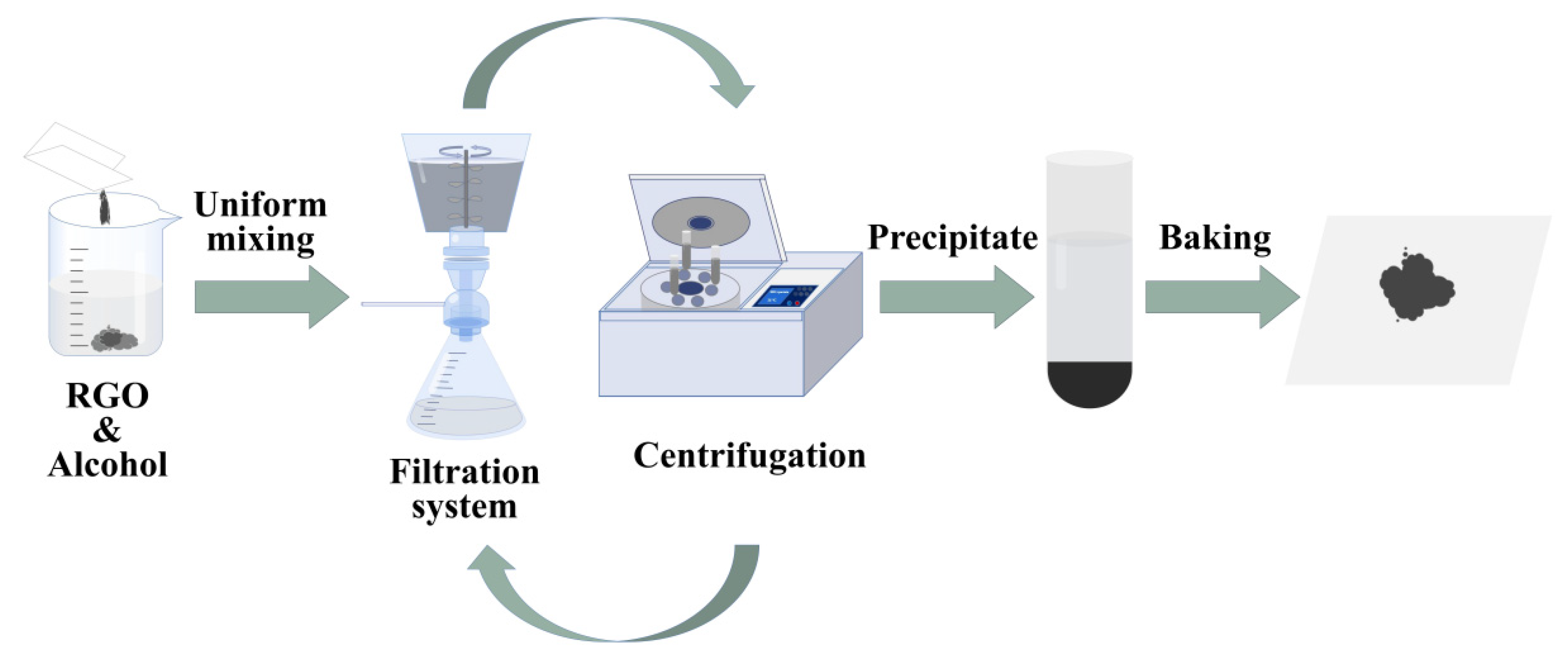
4.1. Preparation of RGO with Different Lateral Dimensions
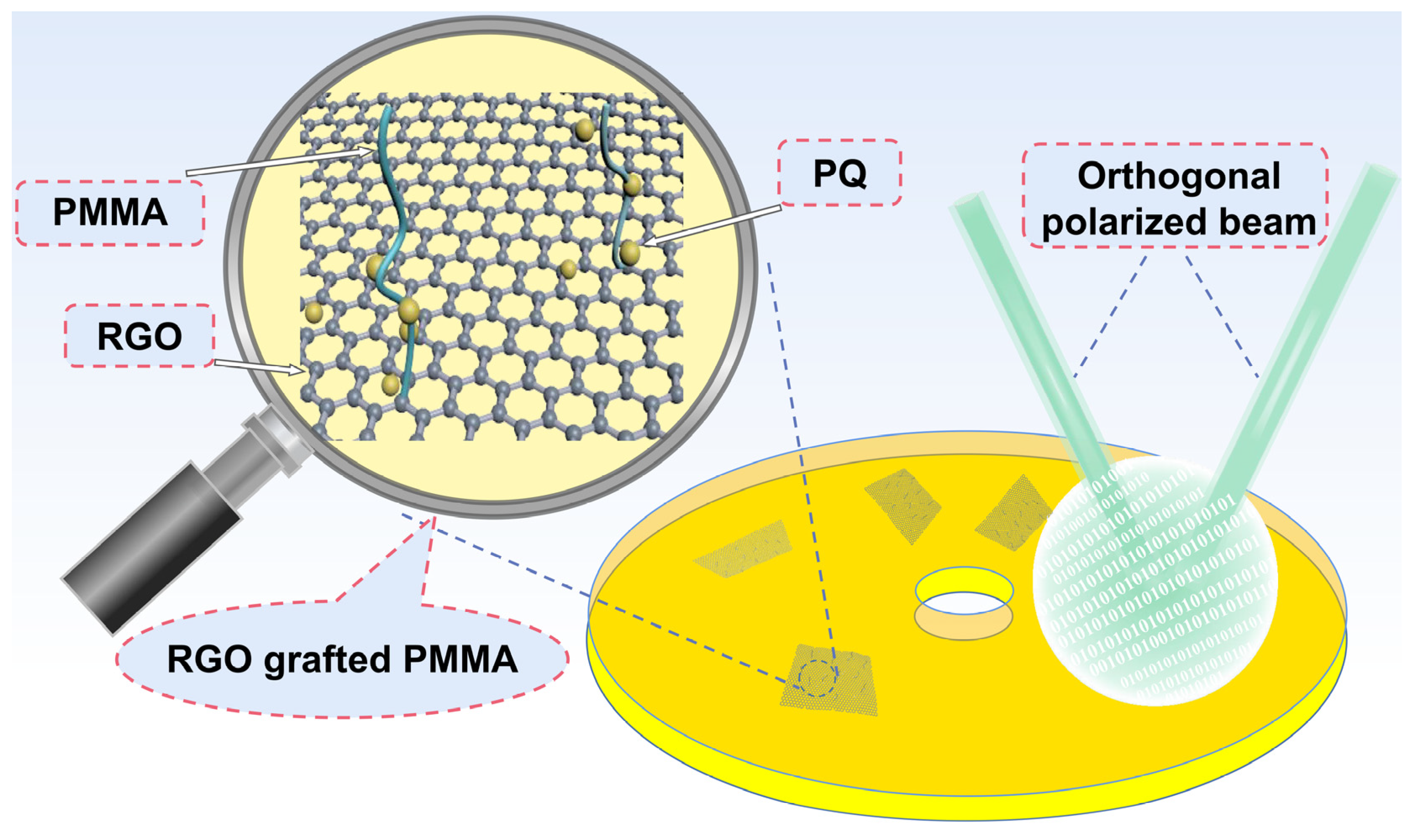
4.2. Preparation of RGO-PMMA Polymers
4.3. Preparation of the RGO-PMMA/PQ Photopolymer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, M.; Li, X.; Cao, Y. Optical storage arrays: A perspective for future big data storage. Light. Sci. Appl. 2014, 3, e177. [Google Scholar] [CrossRef]
- Almoro, P.; Hanson, S. Wavefront sensing using speckles with fringe compensation. Opt. Express 2008, 16, 7608–7618. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, Q.; Lamon, S. Nanomaterials for optical data storage. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Ogiwara, A.; Watanabe, M.; Ito, Y. Tolerance of holographic polymer-dispersed liquid crystal memory for gamma-ray irradiation. Appl. Opt. 2017, 56, 4854–4860. [Google Scholar] [CrossRef] [PubMed]
- Moothanchery, M.; Bavigadda, V.; Pramanik, M.; Toal, V.; Naydenova, I. Application of phase shifting electronic speckle pattern interferometry in studies of photoinduced shrinkage of photopolymer layers. Opt. Express 2017, 25, 9647–9653. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Escuti, M.; Van Heesch, C.; Bastiaansen, C.; Broer, D.; Loos, J.; Nussbaumer, R. TiO2 nanoparticle-photopolymer composites for volume holographic recording. Adv. Funct. Mater. 2005, 15, 1623–1629. [Google Scholar] [CrossRef]
- Lin, X.; Liu, J.; Hao, J.; Wang, K.; Zhang, Y.; Li, H.; Horimai, H.; Tan, X. Collinear holographic datastorage technologies. Opto-Electron. Adv. 2020, 3, 19000401. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Wang, L.; Liao, W.; Dong, B.; Xu, C.; Zhu, C.; Ang, K.; Qiu, C.; Lee, C. Zero-bias mid-infrared graphene photodetectors with bulk photoresponse and calibration-free polarization detection. Nat. Commun. 2020, 11, 6404. [Google Scholar] [CrossRef]
- Veniaminov, A.; Mahilny, U. Holographic polymer materials with diffusion development: Principles, Arrangement, Investigation, and Applications. Opt. Spectrosc. 2013, 115, 906–930. [Google Scholar] [CrossRef]
- Hsiao, Y.; Whang, W.; Lin, S. Analyses on physical mechanism of holographic recording in phenanthrenequinone-doped poly(methyl methacrylate) hybrid materials. Opt. Eng. 2004, 43, 1993–2002. [Google Scholar] [CrossRef]
- Day, D.; Gu, M.; Smallridge, A. Rewritable 3D bit optical data storage in a PMMA-based photorefractive polymer. Adv. Mater. 2001, 13, 1005–1007. [Google Scholar] [CrossRef]
- Tomita, Y.; Hata, E.; Momose, K.; Takayama, S.; Liu, X.; Chikama, K.; Klepp, J.; Pruner, C.; Fally, M. Photopolymerizable nanocomposite photonic materials and their holographic applications in light and neutron optics. J. Mod. Opt. 2016, 63, S1–S31. [Google Scholar] [CrossRef] [PubMed]
- Manukhin, B.; Chivilikhin, S.; Schelkanova, I.; Andreeva, N.; Materikina, D.; Andreeva, O. Reversible and irreversible alterations of the optical thickness of PQ/PMMA volume recording media samples. Part I: Experiment. Appl. Opt. 2017, 56, 7351–7357. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, L.; Jian, J.; Ma, H.; Wang, D.; Zhang, X. Single-wall carbon nanotube promoted allylic homopolymerization for holographic patterning. Carbon 2020, 157, 64–69. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, F.; Tan, X. SiO2 NPs-PQ/PMMA photopolymer material doped with a high-concentration photosensitizer for holographic storage. Polymer 2020, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, P.; Huang, Z.; Wang, J.; Song, H.; Chen, X.; Lin, X.; Wu, T.; Tan, X. Significant enhancement of the polarization holographic performance of photopolymeric materials by introducing graphene oxide. ACS Appl. Mater. Interfaces 2021, 13, 27500–27512. [Google Scholar] [CrossRef]
- Cai, L.; Yu, G. Recent advances in growth and modification of graphene-based energy materials: From chemical vapor deposition to reduction of graphene oxide. Small Methods 2019, 3, 1900071. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Du, W.; Jiang, X.; Zhu, L. From graphite to graphene: Direct liquid-phase exfoliation of graphite to produce single-and few-layered pristine graphene. J. Mater. Chem. A 2013, 1, 10592–10606. [Google Scholar] [CrossRef]
- Xin, G.; Yao, T.; Sun, H.; Scott, S.; Shao, D.; Wang, G.; Lian, J. Highly thermally conductive and mechanically strong graphene fibers. Science 2015, 349, 1083–1087. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, H.; Mao, D.; Du, Y.; Hua, H.; Zhao, W.; Zhao, J. Graphene-empowered dynamic metasurfaces and metadevices. Opto-Electron. Adv. 2022, 5, 200098. [Google Scholar] [CrossRef]
- Luo, F.; Fan, Y.; Peng, G.; Xu, S.; Yang, Y.; Yuan, K.; Liu, J.; Ma, W.; Xu, W.; Zhu, Z.; et al. Graphene thermal emitter with enhanced joule heating and localized light emission in air. ACS Photonics 2019, 6, 2117–2125. [Google Scholar] [CrossRef]
- Chang, L.; Fei, Y.; Hu, Y. Structurally and chemically engineered graphene for capacitive deionization. J. Mater. Chem. A 2021, 9, 1429–1455. [Google Scholar] [CrossRef]
- Ghosh, A.; Sharma, A.; Chizhik, A.; Isbaner, S.; Ruhlandt, D.; Tsukanov, R.; Gregor, I.; Karedla, N.; Enderlein, J. Graphene-based metal-induced energy transfer for sub-nanometre optical localization. Nat. Photonics 2019, 13, 860–865. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Wang, H.; Hao, C.; Lin, H.; Wang, Y.; Lan, T.; Qiu, C.; Jia, B. Generation of super-resolved optical needle and multifocal array using graphene oxide metalenses. Opto-Electron. Adv. 2021, 4, 200031. [Google Scholar] [CrossRef]
- Choi, J.; Kim, D.; Ryu, K.; Lee, H.; Jeong, H.; Shin, C.; Kim, J.; Kim, B. Functionalized graphene sheet/polyurethane nanocomposites: Effect of particle size on physical properties. Macromol. Res. 2011, 19, 809–814. [Google Scholar] [CrossRef]
- Qi, J.; Benipal, N.; Wang, H.; Chadderdon, D.; Jiang, Y.; Wei, W.; Hu, Y.; Li, W. Metal-catalyst-free carbohydrazide fuel cells with three-dimensional graphene anodes. ChemSusChem 2015, 8, 1147–1150. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Shen, W.; Sun, S.; Li, Y. Monolayer of close-packed Pt nanocrystals on a reduced graphene oxide (RGO) nanosheet and its enhanced catalytic performance towards methanol electrooxidation. RSC Adv. 2015, 5, 46017–46025. [Google Scholar] [CrossRef]
- Yin, J.; Wang, H.; Peng, H.; Tan, Z.; Liao, L.; Lin, L.; Sun, X.; Koh, A.; Chen, Y.; Peng, H.; et al. Selectively enhanced photocurrent generation in twisted bilayer graphene with van hove singularity. Nat. Commun. 2016, 7, 10699. [Google Scholar] [CrossRef]
- Asakawa, K.; Sugimoto, Y.; Nakamura, S. Silicon photonics for telecom and data-com applications. Opto-Electron. Adv. 2020, 3, 10200011. [Google Scholar] [CrossRef]
- Tessonnier, J.; Barteau, M. Dispersion of alkyl-chain-functionalized reduced graphene oxide sheets in nonpolar solvents. Langmuir 2012, 28, 6691–6697. [Google Scholar] [CrossRef] [PubMed]
- Ayán-Varela, M.; Paredes, J.; Villar-Rodil, S.; Rozada, R.; Martínez-Alonso, A.; Tascón, J. A quantitative analysis of the dispersion behavior of reduced graphene oxide in solvents. Carbon 2014, 75, 390–400. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.; An, S.; Li, X.; Ruoff, R. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano. Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Stylianakis, M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.; Kabiri, S.; Auyoong, Y.; Tran, D.; Losic, D. Tuning the multifunctional surface chemistry of reduced graphene oxide via combined elemental doping and chemical modifications. ACS Omega 2019, 4, 19787–19798. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Jung, H. Porphyrin functionalized graphene sheets in aqueous suspensions: From the preparation of graphene sheets to highly conductive graphene films. J. Phys. Chem. C 2010, 114, 8227–8234. [Google Scholar] [CrossRef]
- Chen, H.; Wu, M.; Li, C. Structural integrity versus lateral size: Enhancing graphene-based film materials by reducing planar defects rather than flake boundary. Carbon 2018, 139, 216–225. [Google Scholar] [CrossRef]
- Lin, J.; Li, P.; Liu, Y.; Wang, Z.; Wang, Y.; Ming, X.; Cao, C.; Xu, Z. The Origin of the sheet size predicament in graphene macroscopic papers. ACS Nano 2021, 15, 2134–4832. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Functional gels based on chemically modified graphenes. Adv. Mater. 2014, 26, 3992–4012. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, L.; Yao, Y.; Peng, H. Graphene membranes for multi-dimensional electron microscopy imaging: Preparation, application and prospect. Adv. Funct. Mater. 2022, 32, 2202502. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, X.; Chen, J.; Zhao, J. Large-size graphene microsheets as a protective layer for transparent conductive silver nanowire film heaters. Carbon 2014, 69, 437–443. [Google Scholar] [CrossRef]
- Chen, C.; Lee, S.; Deshpande, V.; Lee, G.; Lekas, M.; Shepard, K.; Hone, J. Graphene mechanical oscillators with tunable frequency. Nat. Nanotechnol. 2013, 8, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Jung, G.; Kang, M.; Buehler, M. The mechanics and design of a lightweight three-dimensional graphene assembly. Sci. Adv. 2017, 3, e1601536. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, X.; Qi, P.; Wu, C.; Huang, L.; Xu, X.; Huang, Z.; Zhu, L.; Zhang, Y.; Lin, X.; et al. Linear polarization holography. Opto-Electron. Sci. 2022, 1, 210009. [Google Scholar] [CrossRef]
- Liu, P.; Sun, X.; Wang, L. Polarization holographic characteristics of Ti/PMMA polymers by linearly polarized exposure. Opt. Mater. 2020, 107, 109992. [Google Scholar] [CrossRef]
- Kogelink, H. Coupled wave theory for thick hologram gratings. Bell Syst. Technol. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Ye, T.; Wang, J.; Liu, J.; Qi, P.; Zheng, S.; Yang, Y.; Lin, X.; Huang, Z.; Tan, X. Scalar vortex beams produced by Pancharatnam-Berry phase optical elements that utilize polarization holography. Opt. Lett. 2023, 48, 4105–4108. [Google Scholar] [CrossRef]
- An, X.; Psaltis, D. Experimental characterization of an angle-multiplexed holographic memory. Opt. Lett. 1995, 20, 1913–1915. [Google Scholar] [CrossRef]
- Vojtsek, P.; Kveton, M.; Richter, I. Complex method for angular-spectral analysis of volume phase diffraction gratings recorded in photopolymers. J. Eur. Opt. Soc. 2016, 11, 16009. [Google Scholar] [CrossRef]
- Peng, H.; Wang, C.; Xi, W.; Kowalsk, B.A.; Gong, T.; Xie, X.; Wang, W.; Nair, D.P.; McLeod, R.R.; Bowman, C.N. Facile image patterning via sequential thiol-Michael-thiol-yne click reactions. Chem. Mater. 2014, 26, 6819–6826. [Google Scholar] [CrossRef]
- Voylov, D.; Saito, T.; Lokitz, B.; Uhrig, D.; Wang, Y.; Agapov, A.; Holt, A.; Bocharova, V.; Kisliuk, A.; Sokolov, A.P. Graphene oxide as a radical initiator: Free radical and controlled radical polymerization of sodium 4-vinylbenzenesulfonate with graphene oxide. ACS Macro Lett. 2016, 5, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.; Binder, W. Graphene as initiator/catalyst in polymerization chemistry. Prog. Polym. Sci. 2017, 67, 48–76. [Google Scholar]
- Wu, Y.; Jia, P.; Xu, L.; Chen, Z.; Xiao, L.; Sun, J.; Zhang, J.; Huang, Y.; Bielawski, C.; Geng, J. Tuning the surface properties of graphene oxide by surface-initiated polymerization of epoxides: An efficient method for enhancing gas separation. ACS Appl. Mater. Interfaces 2017, 9, 4998–5005. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Yu, Y. Binding energy and work function of organic electrode materials phenanthraquinone, pyromellitic dianhydride and their derivatives adsorbed on graphene. ACS Appl. Mater. Interfaces 2014, 6, 16267–16275. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Jia, N.; Li, C.; Shi, G. Size fractionation of graphene oxide sheets via filtration through track-etched membranes. Adv. Mater. 2015, 27, 3654–3660. [Google Scholar] [CrossRef]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.; Berner, N.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z.; et al. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef]
- Lin, S.; Hsu, K.; Chen, W.; Whang, W. Phenanthrenequinone-doped poly (methyl methacrylate) photopolymer bulk for volume holographic data storage. Opt. Lett. 2000, 25, 451–453. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hu, P.; Ye, T.; Li, J.; Li, J.; Chen, M.; Zhang, Z.; Lin, X.; Tan, X. Enhanced Polarization Properties of Holographic Storage Materials Based on RGO Size Effect. Molecules 2024, 29, 214. https://doi.org/10.3390/molecules29010214
Liu J, Hu P, Ye T, Li J, Li J, Chen M, Zhang Z, Lin X, Tan X. Enhanced Polarization Properties of Holographic Storage Materials Based on RGO Size Effect. Molecules. 2024; 29(1):214. https://doi.org/10.3390/molecules29010214
Chicago/Turabian StyleLiu, Jie, Po Hu, Tian Ye, Jianan Li, Jinhong Li, Mingyong Chen, Zuoyu Zhang, Xiao Lin, and Xiaodi Tan. 2024. "Enhanced Polarization Properties of Holographic Storage Materials Based on RGO Size Effect" Molecules 29, no. 1: 214. https://doi.org/10.3390/molecules29010214
APA StyleLiu, J., Hu, P., Ye, T., Li, J., Li, J., Chen, M., Zhang, Z., Lin, X., & Tan, X. (2024). Enhanced Polarization Properties of Holographic Storage Materials Based on RGO Size Effect. Molecules, 29(1), 214. https://doi.org/10.3390/molecules29010214





