Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments
Abstract
:1. Introduction
2. Biosensors
3. Electrochemical Biosensor Detection of Heavy Metals
3.1. Mercury

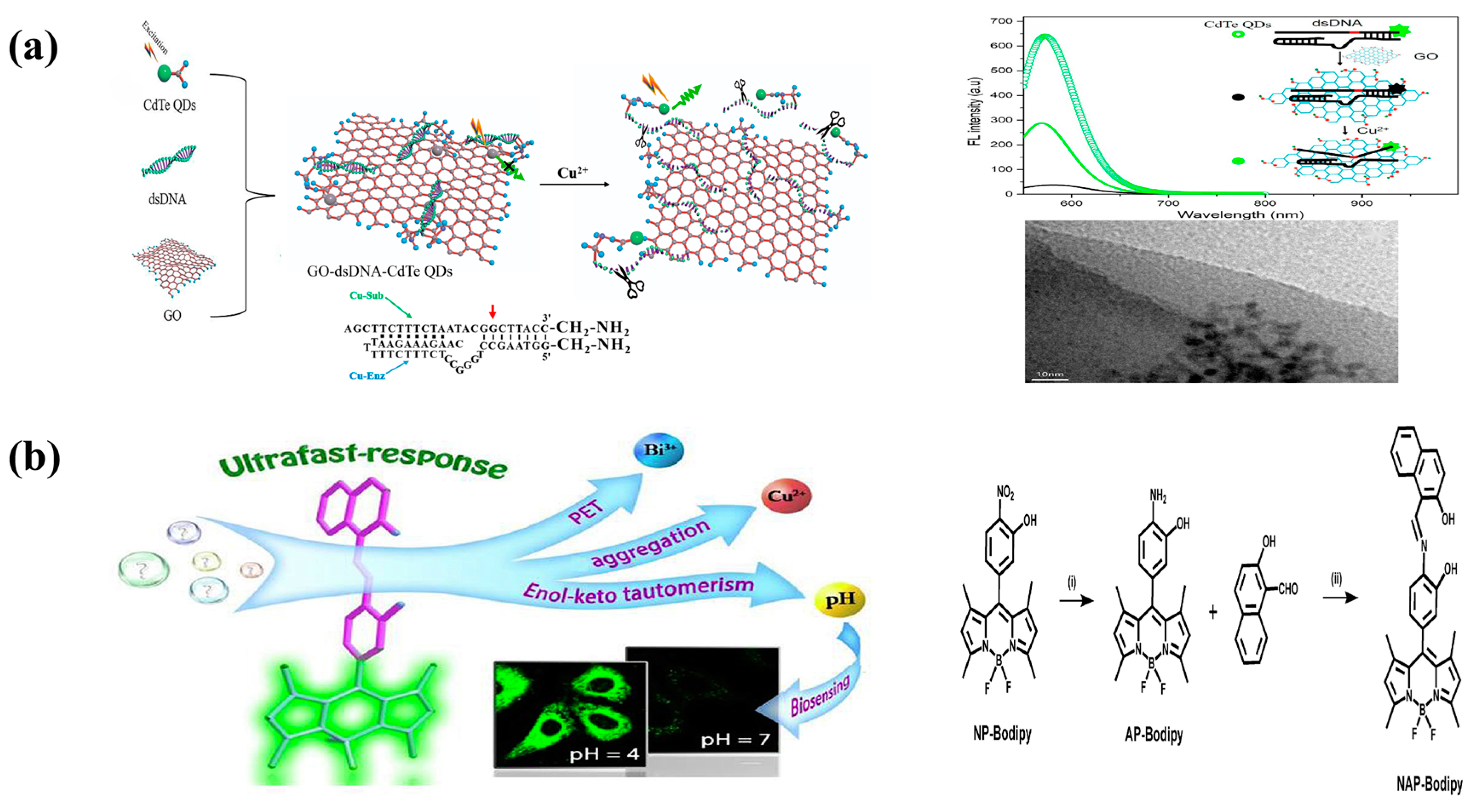
3.2. Copper
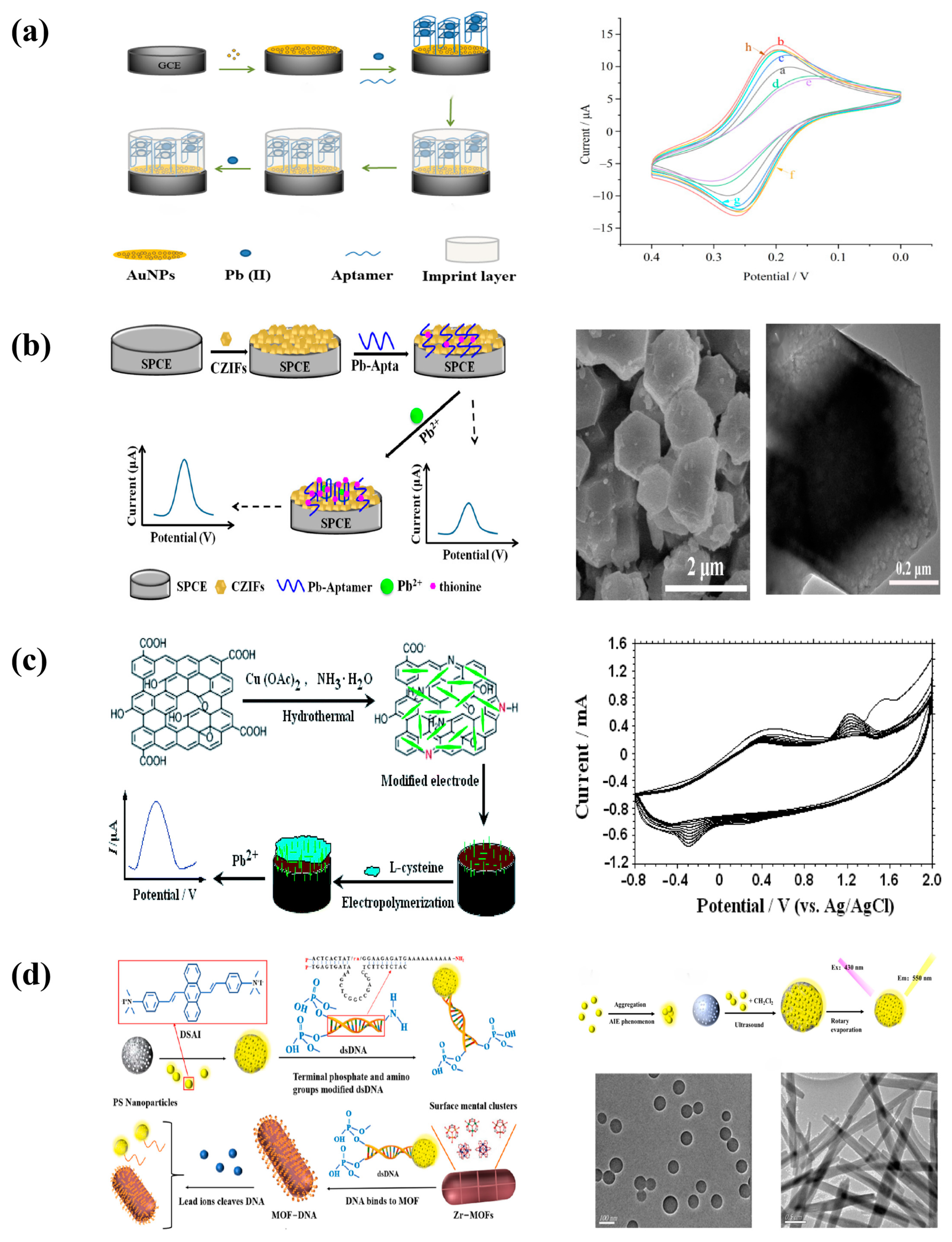
3.3. Lead
3.4. Cadmium

3.5. Chromium
3.6. Zinc
4. Optical Biosensor Detection of Heavy Metals
4.1. Mercury
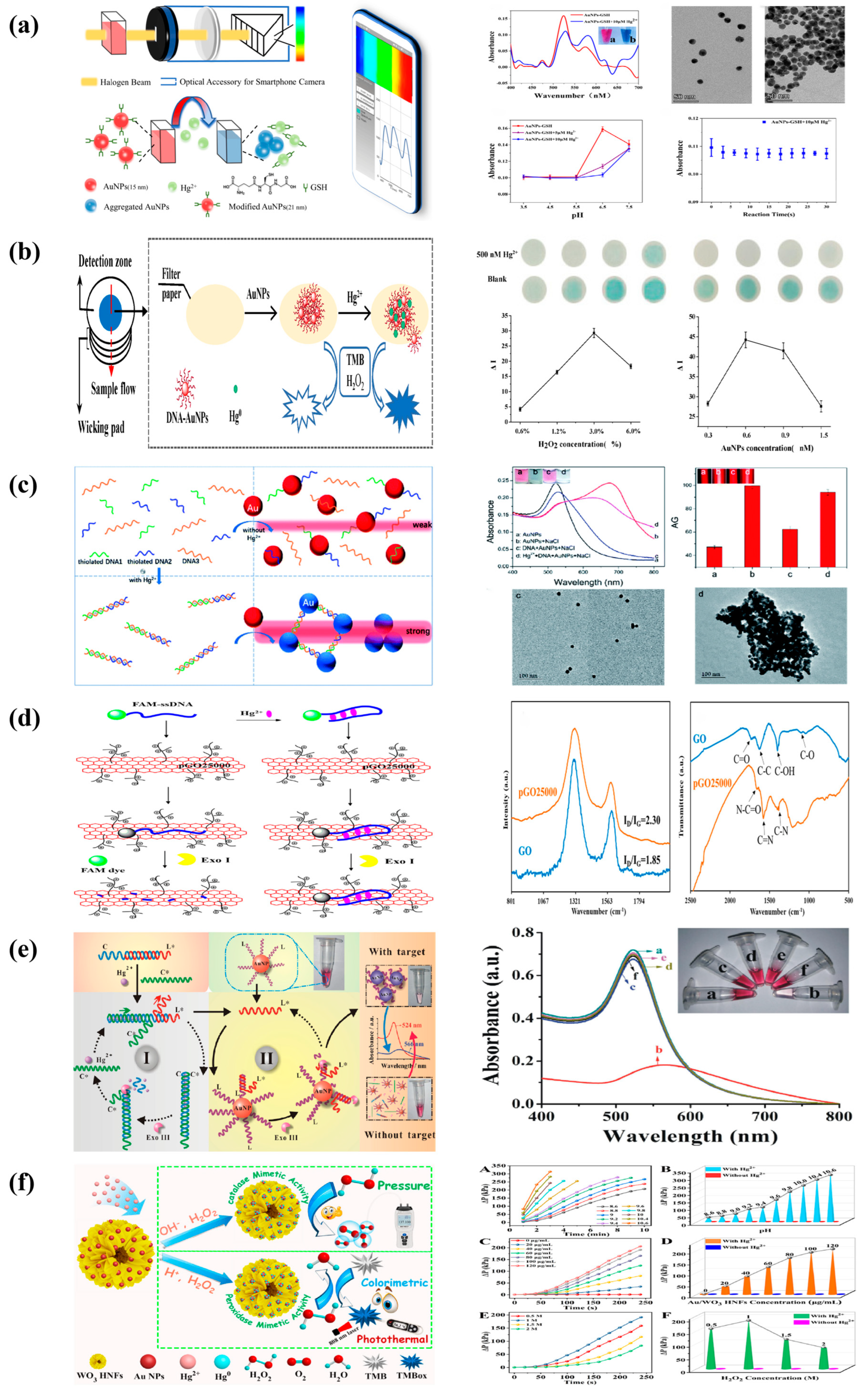
4.2. Copper
4.3. Lead
4.4. Cadmium
4.5. Chromium
5. Conclusions
6. Current Limitations and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Renedo, O.; Alonso-Lomillo, M.A.; Arcos-Martínez, M.J. Determination of metals based on electrochemical biosensors. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1042–1073. [Google Scholar] [CrossRef]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Israa; Wu, N. Nanostructured sensors for detection of heavy metals: A review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Hussain, B.; Abbas, Y.; Ali, H.; Zafar, M.; Ali, S.; Ashraf, M.N.; Zehra, Q.; Esinoza, S.T.L.; Valderrama, J.R.D. Metal and metalloids speciation, fractionation, bioavailability, and transfer toward plants. In Metals Metalloids Soil Plant Water Systems; Academic Press: Cambridge, MA, USA, 2022; pp. 29–50. [Google Scholar]
- Bodo, M.; Balloni, S.; Lumare, E.; Bacci, M.; Calvitti, M.; Dell’Omo, M.; Murgia, N.; Marinucci, L. Effects of sub-toxic cadmium concentrations on bone gene expression program: Results of an in vitro study. Toxicol. In Vitro 2010, 24, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lin, L.; Rong, M.; Chen, X. A cross-reactive sensor array for the fluorescence qualitative analysis of heavy metal ions. Talanta 2014, 129, 296–302. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Ali Khan, K.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ.-Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, P.; Liang, Y.; Chen, W.; Li, L.; Wang, H.; Yu, Z.; Liu, Y.; Zhang, X. Rapid electrochemical quantification of trace Hg2+ using a hairpin DNA probe and quantum dot modified screen-printed gold electrodes. RSC Adv. 2022, 12, 13448–13455. [Google Scholar] [CrossRef]
- Centers for Disease Control (US). Preventing Lead Poisoning in Young Children: A Statement; The Centers: Cleveland, OH, USA, 1991. [Google Scholar]
- Garza, A.; Vega, R.; Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 2006, 12, RA57. [Google Scholar]
- Turner, A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019, 657, 1409–1418. [Google Scholar] [CrossRef]
- Arain, M.B.; Ali, I.; Yilmaz, E.; Soylak, M. Nanomaterial’s based chromium speciation in environmental samples: A review. TrAC Trends Anal. Chem. 2018, 103, 44–55. [Google Scholar] [CrossRef]
- Jin, W.; Yan, K. Recent advances in electrochemical detection of toxic Cr (VI). RSC Adv. 2015, 5, 37440–37450. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Aarthy, M.; Rajesh, T.; Thirunavoukkarasu, M. Critical review on microbial fuel cells for concomitant reduction of hexavalent chromium and bioelectricity generation. J. Chem. Technol. Biotechnol. 2020, 95, 1298–1307. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, S.K.; Bhardwaj, N.; Bhardwaj, N.; Paul, A.K.; Kumar, P.; Kim, K.-H.; Deep, A. Progress in the biosensing techniques for trace-level heavy metals. Biotechnol. Adv. 2016, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-M.; Yang, H.; Sun, P.; Gong, J.; Wu, Q.; Wu, J. A review of biosensor development research. China High-Tech. 2022, 120, 118–120. [Google Scholar]
- Youxue, W.; Meijiao, W.; Yachen, T.; Zheng, W.; Liang, G.; Cheng, L.; Shuiqin, F.; Qing, L. Advances in biosensor research for Salmonella detection. Food Sci. 2021, 42, 339–345. [Google Scholar]
- Ma, L.-P.; Mao, B.; Liu, B.; Li, G.; Han, G.; Liu, G. Current status and development trend of biosensor applications. Sens. Microsyst. 2009, 28, 1–4. [Google Scholar]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 1–17. [Google Scholar] [CrossRef]
- Durkalec, M.; Szkoda, J.; Kolacz, R.; Opalinski, Z.; Zmudzki, J. Bioaccumulation of lead, cadmium and mercury in roe deer and wild boars from areas with different levels of toxic metal pollution. Int. J. Environ. Res. 2015, 9, 205–212. [Google Scholar]
- Evans, E.H.; Day, J.A.; Palmer, C.D.; Price, W.J.; Smith, C.M.M.; Tyson, J.F. Atomic spectrometry update. Advances in atomic emission, absorption and fluorescence spectrometry, and related techniques. J. Anal. Atomic Spectrom. 2005, 20, 562–590. [Google Scholar] [CrossRef]
- Montes-Bayón, M.; DeNicola, K.; Caruso, J.A. Liquid chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2003, 1000, 457–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Adeloju, S.B. Coupling of non-selective adsorption with selective elution for novel in-line separation and detection of cadmium by vapour generation atomic absorption spectrometry. Talanta 2015, 137, 148–155. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A. Electrochemical sensors and devices for heavy metals assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef]
- Sadak, O. Chemical sensing of heavy metals in water. In Advanced Sensor Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 565–591. [Google Scholar]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar]
- Gibi, C.; Liu, C.H.; Barton, S.C.; Wu, J.J. Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals. Nanomaterials 2022, 12, 3930. [Google Scholar] [CrossRef]
- Xie, X. Research on Electrochemical Biosensors Based on Metal Nanomaterials and Biomagnification Technology. Master’s Thesis, Southwest University, Shenzhen, China, 2019. [Google Scholar]
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J.P. Screen-printed carbon based biosensors and their applications in agri-food safety. TrAC Trends Anal. Chem. 2020, 127, 115898. [Google Scholar] [CrossRef]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Adrian, C.; Roy, P.; John, H. Recent advances in the fabrication and application of screen-printed electrochemical (bio) sensors based on carbon materials for biomedical, agri-food and environmental analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef]
- Brisset, H.; Briand, J.F.; Barry-Martinet, R.; Duong, R.; Hy, T.; Pierre, F.; Frédéric, G.; Philippe, L.; Christine, B. 96X screen-printed gold electrode platform to evaluate electroactive polymers as marine antifouling coatings. Anal. Chem. 2018, 90, 4978–4981. [Google Scholar] [CrossRef]
- Zribi, R.; Maalej, R.; Messina, E.; Gillibert, R.; Neri, G. Exfoliated 2D-MoS2 nanosheets on carbon and gold screen printed electrodes for enzyme-free electrochemical sensing of tyrosine. Sens. Actuators B Chem. 2020, 303, 127229. [Google Scholar] [CrossRef]
- Motia, S.; Bouchikhi, B.; Llobet, E.; El Bari, N. Synthesis and characterization of a highly sensitive and selective electrochemical sensor based on molecularly imprinted polymer with gold nanoparticles modified screen-printed electrode for glycerol determination in wastewater. Talanta 2020, 216, 120953. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew. Chem. 2004, 116, 4400–4402. [Google Scholar] [CrossRef]
- Liu, T.; Chu, Z.; Jin, W. Electrochemical mercury biosensors based on advanced nanomaterials. J. Mater. Chem. B 2019, 7, 3620–3632. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.; Bhagat, P.R.; Chidambaram, K. Biopolymer composites with high dielectric performance: Interface engineering. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar]
- Frost, S.J.; Mawad, D.; Higgins, M.J.; Ruprai, H.; Lauto, A. Gecko-inspired chitosan adhesive for tissue repair. NPG Asia Mater. 2016, 8, e280. [Google Scholar] [CrossRef]
- Do, J.S.; Lin, K.H. Kinetics of urease inhibition-based amperometric biosensors for mercury and lead ions detection. J. Taiwan Inst. Chem. Eng. 2016, 63, 25–32. [Google Scholar] [CrossRef]
- Luo, Y.C.; Do, J.S. Urea biosensor based on PANi (urease)-Nafion®/Au composite electrode. Biosens. Bioelectron. 2004, 20, 15–23. [Google Scholar] [CrossRef]
- Saenchoopa, A.; Klangphukhiew, S.; Somsub, R.; Talodthaisong, C.; Patramanon, R.; Daduang, J.; Daduang, S.; Kulchat, S. A disposable electrochemical biosensor based on screen-printed carbon electrodes modified with silver nanowires/hpmc/chitosan/urease for the detection of mercury (ii) in water. Biosensors 2021, 11, 351. [Google Scholar] [CrossRef]
- Cao, X.; Yue, L.; Lian, F.; Wang, C.; Cheng, B.; Lv, J.; Wang, Z. CuO nanoparticles doping recovered the photocatalytic antialgal activity of graphitic carbon nitride. J. Hazard. Mater. 2021, 403, 123621. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P. The edge-epitaxial growth of yellow g-C3N4 on red g-C3N4 nanosheets with superior photocatalytic activities. Chem. Commun. 2021, 57, 3119–3122. [Google Scholar] [CrossRef]
- Qian, X.; Meng, X.; Sun, J.; Jiang, L.; Zhu, J. Salt-assisted synthesis of 3D porous g-C3N4 as a bifunctional photo-and electrocatalyst. ACS Appl. Mater. Interfaces 2019, 11, 27226–27232. [Google Scholar] [CrossRef]
- Wu, J.; Tian, L.; Duan, H.; Cheng, Y.; Shi, L. Unveiling the working mechanism of g-C3N4 as a protection layer for lithium-and sodium-metal anode. ACS Appl. Mater. Interfaces 2021, 13, 46821–46829. [Google Scholar] [CrossRef]
- Jin, Y.; Kang, Q.; Guo, X.; Zhang, B.; Shen, D.; Zou, G. Electrochemical-signal-amplification strategy for an electrochemiluminescence immunoassay with g-C3N4 as tags. Anal. Chem. 2018, 90, 12930–12936. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Zeng, G.; Zou, C.; Xue, W.; Gong, X.; Yan, X.; Chen, S.; Wang, W.; Cheng, M. Megamerger in photocatalytic field: 2D g-C3N4 nanosheets serve as support of 0D nanomaterials for improving photocatalytic performance. Appl. Catal. B Environ. 2019, 240, 153–173. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Z.; Wang, T.; Zhang, M.; Ding, C.; Guo, Y.; Jiang, D.; Wang, K. Ternary Z-scheme heterojunction of Bi SPR-promoted BiVO4/g-C3N4 with effectively boosted photoelectrochemical activity for constructing oxytetracycline aptasensor. Biosens. Bioelectron. 2020, 166, 112453. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Li, P.; Chen, X.; Wang, H. Near-infrared light-driven photoelectrochemical sensor for mercury (II) detection using bead-chain-like Ag@Ag2S nanocomposites. Chem. Eng. J. 2021, 409, 128154. [Google Scholar] [CrossRef]
- Xiao, F.X.; Liu, B. Plasmon-Dictated Photo-Electrochemical Water Splitting for Solar-to-Chemical Energy Conversion: Current Status and Future Perspectives. Adv. Mater. Interfaces 2018, 5, 1701098. [Google Scholar] [CrossRef]
- Wang, D.; Ding, Z.; Zhou, H.; Chen, L.; Feng, X. Au Nanoparticle-Decorated TiO2 Nanowires for Surface Plasmon Resonance-Based Photoelectrochemical Bioassays with a Solid–Liquid–Air Triphase Interface. ACS Appl. Nano Mater. 2021, 4, 9401–9408. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, D.; Zhang, M.; Zhang, Z.; Wang, K. High-performance photoelectrochemical aptasensor for enrofloxacin based on Bi-doped ultrathin polymeric carbon nitride nanocomposites with SPR effect and carbon vacancies. Sens. Actuators B Chem. 2020, 316, 128142. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; An, S.; Yan, Z. Au NP-Decorated g-C3N4-Based Photoelectochemical Biosensor for Sensitive Mercury Ions Analysis. ACS Omega 2022, 7, 19622–19630. [Google Scholar] [CrossRef]
- Hasanjani, H.R.A.; Zarei, K. An electrochemical sensor for attomolar determination of mercury (II) using DNA/poly-L-methionine-gold nanoparticles/pencil graphite electrode. Biosens. Bioelectron. 2019, 128, 1–8. [Google Scholar] [CrossRef]
- Ma, J. Preparation of Gold Nanobiosensor and Its Application in Heavy Metal Detection. Master’s Thesis, Anhui University, Hefei, China, 2021. [Google Scholar]
- Narouei, F.H.; Livernois, L.; Andreescu, D.; Andreescu, S. Highly sensitive mercury detection using electroactive gold-decorated polymer nanofibers. Sens. Actuators B Chem. 2021, 329, 129267. [Google Scholar] [CrossRef]
- Atapour, M.; Amoabediny, G.; Ahmadzadeh-Raji, M. Integrated optical and electrochemical detection of Cu2+ ions in water using a sandwich amino acid–gold nanoparticle-based nano-biosensor consisting of a transparent-conductive platform. RSC Adv. 2019, 9, 8882–8893. [Google Scholar] [CrossRef]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Chen, J.R.; Hui, F. A fluorescent nanosensor based on graphene quantum dots–aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens. Bioelectron. 2015, 68, 225–231. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, H.; Zhang, M.G.; Song, J.; Nie, L.; Wang, S.; Niu, G.; Huang, P.; Lu, G.; Chen, X. An aptamer-targeting photoresponsive drug delivery system using “off–on” graphene oxide wrapped mesoporous silica nanoparticles. Nanoscale 2015, 7, 6304–6310. [Google Scholar] [CrossRef]
- Yang, D.; Liu, X.; Zhou, Y.; Lin, L.; Lin, T. Aptamer-based biosensors for detection of lead (ii) ion: A review. Anal. Methods 2017, 9, 1976–1990. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, X.; Peng, K.; Cao, H.; Yuan, M.; Ye, T.; Wu, X.; Yin, F.; Yu, J.; Hao, L.; et al. A novel aptamer-imprinted polymer-based electrochemical biosensor for the detection of lead in aquatic products. Molecules 2022, 28, 196. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, D.; Liu, Y.; Zhan, X.; Lu, Y.; Zhou, P.; Zhang, D. An electrochemical aptasensor for Pb2+ detection based on metal–organic-framework-derived hybrid carbon. Biosensors 2020, 11, 1. [Google Scholar] [CrossRef]
- Yang, S.; Liu, P.; Wang, Y.; Guo, Z.; Qu, L. Electrochemical sensor using poly-(l-cysteine) functionalized CuO nanoneedles/N-doped reduced graphene oxide for detection of lead ions. RSC Adv. 2020, 10, 18526–18532. [Google Scholar] [CrossRef]
- Gao, L.; Deng, Y.; Liu, H.; Solomon, K.; Zhang, B.; Cai, H. Detection of Pb2+ in tea using aptamer labeled with AIEgen nanospheres based on MOFs sensors. Biosensors 2022, 12, 745. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chen, S.Y.; Liaw, H.W. Immobilization of lactate dehydrogenase within multiwalled carbon nanotube-chitosan nanocomposite for application to lactate biosensors. Sens. Actuators B Chem. 2007, 125, 474–481. [Google Scholar] [CrossRef]
- Hamdy, M.E.; Del Carlo, M.; Hussein, H.A.; Salah, T.A.; El-Dee, A.H.; Emara, M.M.; Pezzoni, G.; Compagnone, D. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J. Nanobiotechnol. 2018, 16, 48. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A novel glucose biosensor based on immobilization of glucose oxidase in chitosan on a glassy carbon electrode modified with gold–platinum alloy nanoparticles/multiwall carbon nanotubes. Anal. Biochem. 2007, 369, 71–79. [Google Scholar] [CrossRef]
- Rabai, S.; Teniou, A.; Catanante, G.; Benounis, M.; Marty, J.-L.; Rhouati, A. Fabrication of AuNPs/MWCNTS/chitosan nanocomposite for the electrochemical aptasensing of cadmium in water. Sensors 2021, 22, 105. [Google Scholar] [CrossRef]
- Attaallah, R.; Amine, A. An ultrasensitive and selective determination of cadmium ions at ppt level using an enzymic membrane with colorimetric and electrochemical detection. Biosensors 2022, 12, 310. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Ding, J.; Hayat, K.; Yang, X.; Zhan, X.; Zhang, D.; Lu, Y.; Zhou, P. Label-free and sensitive determination of cadmium ions using a Ti-modified Co3O4-based electrochemical aptasensor. Biosensors 2020, 10, 195. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, B.; Fan, L.; Liu, H.; Sun, L. Efficient BiVO4 photoanodes by postsynthetic treatment: Remarkable improvements in photoelectrochemical performance from facile borate modification. Angew. Chem. 2019, 131, 19203–19209. [Google Scholar] [CrossRef]
- Lu, H.; Andrei, V.; Jenkinson, K.J.; Regoutz, A.; Li, N.; Creissen, C.E.; Wheatley, A.E.H.; Hao, H.; Reisner, E.; Wright, D.S.; et al. Single-Source Bismuth (Transition Metal) Polyoxovanadate Precursors for the Scalable Synthesis of Doped BiVO4 Photoanodes. Adv. Mater. 2018, 30, 1804033. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Chansaenpak, K.; Channei, D. Evaluating the photocatalytic efficiency of the BiVO4/rGO photocatalyst. Sci. Rep. 2019, 9, 16091. [Google Scholar] [CrossRef]
- Wu, W.; Tan, Z.; Chen, X.; Chen, X.; Cheng, L.; Wu, H.; Li, P.; Zhang, Z. Carnation-like morphology of BiVO4-7 enables sensitive photoelectrochemical determination of Cr(VI) in the food and environment. Biosensors 2022, 12, 130. [Google Scholar] [CrossRef]
- Juang, Y.J.; Li, W.S.; Chen, P.S. Fabrication of microfluidic paper-based analytical devices by filtration-assisted screen printing. J. Taiwan Inst. Chem. Eng. 2017, 80, 71–75. [Google Scholar] [CrossRef]
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2016, 76, 180–194. [Google Scholar] [CrossRef]
- Dabhade, A.; Jayaraman, S.; Paramasivan, B. Development of glucose oxidase-chitosan immobilized paper biosensor using screen-printed electrode for amperometric detection of Cr (VI) in water. 3 Biotech 2021, 11, 183. [Google Scholar] [CrossRef]
- Bowers, M.T.; Liu, D.; Wyttenbach, T. Interaction of divalent metal ions with the hormone oxytocin: Hormone receptor binding. J. Am. Chem. Soc. 2008, 130, 1–19. [Google Scholar]
- Liu, D.; Seuthe, A.B.; Ehrler, O.T.; Zhang, X.; Wyttenbach, T.; Hsu, J.F.; Bowers, M.T. Oxytocin-receptor binding: Why divalent metals are essential. J. Am. Chem. Soc. 2005, 127, 2024–2025. [Google Scholar] [CrossRef]
- Attia, J.; Nir, S.; Mervinetsky, E.; Balogh, D.; Gitlin-Domagalska, A.; Alshanski, I.; Reches, M.; Hurevich, M.; Yitzchaik, S. Non-covalently embedded oxytocin in alkanethiol monolayer as Zn2+ selective biosensor. Sci. Rep. 2021, 11, 7051. [Google Scholar] [CrossRef]
- Khan, A.U.; Zhao, S.; Liu, G. Key parameter controlling the sensitivity of plasmonic metal nanoparticles: Aspect ratio. J. Phys. Chem. C 2016, 120, 19353–19364. [Google Scholar] [CrossRef]
- Hou, J.; Li, M.; Song, Y. Recent advances in photonic crystal sensors. Sci. Sin. Chim. 2016, 46, 1080–1092. [Google Scholar]
- Lee, B. Review of the present status of optical fiber sensors. Opt. Fiber Technol. 2003, 9, 57–79. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Hirsch, T.; Wolfbeis, O.S. Detection of biotin–avidin affinity binding by exploiting a self-referenced system composed of upconverting luminescent nanoparticles and gold nanoparticles. J. Nanopart. Res. 2011, 13, 4603–4611. [Google Scholar] [CrossRef]
- Srivastava, P.; Razi, S.S.; Ali, R.; Gupta, R.C.; Yadav, S.S.; Narayan, G.; Misra, A. Selective naked-eye detection of Hg2+ through an efficient turn-on photoinduced electron transfer fluorescent probe and its real applications. Anal. Chem. 2014, 86, 8693–8699. [Google Scholar] [CrossRef]
- Razi, S.S.; Ali, R.; Gupta, R.C.; Dwivedi, S.K.; Sharma, G.; Koch, B.; Misra, A. Phenyl-end-capped-thiophene (PT type) based ICT fluorescent probe (D–π–A) for detection of Hg2+ and Cu2+ ions: Live cell imaging and logic operation at molecular level. J. Photochem. Photobiol. A Chem. 2016, 324, 106–116. [Google Scholar] [CrossRef]
- Srivastava, P.; Shahid, M.; Misra, A. Protein assisted fluorescence enhancement of a dansyl containing fluorescent reagent: Detection of Hg+ ion in aqueous medium. Org. Biomol. Chem. 2011, 9, 5051–5055. [Google Scholar] [CrossRef]
- Kim, I.; Lee, N.E.; Jeong, Y.J.; Chung, Y.H.; Cho, B.K.; Lee, E. Micellar and vesicular nanoassemblies of triazole-based amphiphilic probes triggered by mercury (II) ions in a 100% aqueous medium. Chem. Commun. 2014, 50, 14006–14009. [Google Scholar] [CrossRef]
- Zong, L.; Wang, C.; Song, Y.; Xie, Y.; Zhang, P.; Peng, Q.; Li, Q.; Li, Z. A dual-function probe based on naphthalene diimide for fluorescent recognition of Hg2+ and colorimetric detection of Cu2+. Sens. Actuators B Chem. 2017, 252, 1105–1111. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, G.; Chen, J.; Kong, L.; Yang, L.; Bi, H.; Yang, J. An AIE active probe for specific sensing of Hg2+ based on linear conjugated bis-Schiff base. Sens. Actuators B Chem. 2016, 229, 338–346. [Google Scholar] [CrossRef]
- Aliberti, A.; Vaiano, P.; Caporale, A.; Consales, M.; Ruvo, M.; Cusano, A. Fluorescent chemosensors for Hg2+ detection in aqueous environment. Sens. Actuators B Chem. 2017, 247, 727–735. [Google Scholar] [CrossRef]
- Zhong, K.; Zhou, X.; Hou, R.; Zhou, P.; Hou, S.; Bian, Y.; Zhang, G.; Tang, L.; Shang, X. A water-soluble highly sensitive and selective fluorescent sensor for Hg2+ based on 2-(2-(8-hydroxyquinolin)-yl) benzimidazole via ligand-to-metal charge transfer (LMCT). RSC Adv. 2014, 4, 16612–16617. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Ma, J.S.; Yang, G. Ratiometric Zn2+ sensor and strategy for Hg2+ selective recognition by central metal ion replacement. Inorg. Chem. 2006, 45, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.K.; John, S.A. Fluorogenic mercury ion sensor based on pyrene-amino mercapto thiadiazole unit. J. Hazard. Mater. 2018, 343, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Youn, N.J.; Park, S.M.; Chang, S.K. Diametrically disubstituted cyclam derivative having Hg2+-selective fluoroionophoric behaviors. J. Org. Chem. 2005, 70, 2394–2397. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, S.W.; Kim, S.H.; Kang, C.; Kim, J.S. Nanomolar Hg (II) detection using Nile blue chemodosimeter in biological media. Org. Lett. 2009, 11, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wu, M.; Li, Y.; Chen, Y.; Su, J. New fluoro-and chromogenic chemosensors for the dual-channel detection of Hg2+ and F−. Tetrahedron Lett. 2014, 55, 4711–4715. [Google Scholar] [CrossRef]
- Song, K.C.; Kim, J.S.; Park, S.M.; Chung, K.C.; Ahn, S.; Chang, S.K. Fluorogenic Hg2+-selective chemodosimeter derived from 8-hydroxyquinoline. Org. Lett. 2006, 8, 3413–3416. [Google Scholar] [CrossRef]
- Angupillai, S.; Hwang, J.Y.; Lee, J.Y.; Rao, B.A.; Son, Y.A. Efficient rhodamine-thiosemicarbazide-based colorimetric/fluorescent ‘turn-on’ chemodosimeters for the detection of Hg2+ in aqueous samples. Sens. Actuators B Chem. 2015, 214, 101–110. [Google Scholar] [CrossRef]
- Madhu, S.; Kumar, S.; Chatterjee, T.; Ravikanth, M. Synthesis, X-ray structure, spectral and electrochemical properties of a β-meso covalently linked BODIPY–Ru (II) dipyrrin complex. New J. Chem. 2014, 38, 5551–5558. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, T.; Ou, Z.; Cai, W.; Yang, G.; Li, Y.; Xu, M.; Li, Q. Highly sensitive and selective turn-on fluorescent chemosensors for Hg2+ based on thioacetal modified pyrene. Talanta 2018, 178, 663–669. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, R.; Wang, Y.Z.; Qiu, F.Z.; Feng, Y.; Tang, X.L.; Bai, D.; Zhang, G.L.; Liu, W.S. A selective turn-on fluorescent sensor for Hg (II) in living cells and tissues. Sens. Actuators B Chem. 2018, 255, 3479–3487. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, H.; Zhong, K.; Gao, X.; Li, J. An aggregation-induced emission-based fluorescence turn-on probe for Hg2+ and its application to detect Hg2+ in food samples. RSC Adv. 2019, 9, 23316–23323. [Google Scholar] [CrossRef]
- Fan, M.; Pan, Z.; Wang, C.; Guo, Y.; Sun, J.; Liu, M.; Peng, B.; Wu, J.; Fang, Y. Quantitative visual detection of mercury ions with ratiometric fluorescent test paper sensor. Front. Chem. 2022, 10, 859379. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Ghannay, S.; Messaoudi, S.; Alminderej, F.M.; Aouadi, K.; Saleh, S.M. A Reversible Optical Sensor Film for Mercury Ions Discrimination Based on Isoxazolidine Derivative and Exhibiting pH Sensing. Biosensors 2022, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sarkar, S.; Katranidis, A.; Bhattacharya, J. Development of a cell-free optical biosensor for detection of a broad range of mercury contaminants in water: A plasmid DNA-based approach. ACS Omega 2019, 4, 9480–9487. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, H.; Shin, W.H.; Oh, J.M.; Koo, S.M.; Kim, Y.; Lee, T.; Yu, B.J.; Park, C. Development of colorimetric whole-cell biosensor for detection of heavy metals in environment for public health. Int. J. Environ. Res. Public Health 2021, 18, 12721. [Google Scholar] [CrossRef] [PubMed]
- De Acha, N.; Elosúa, C.; Arregui, F.J. Development of an aptamer based luminescent optical fiber sensor for the continuous monitoring of Hg2+ in aqueous media. Sensors 2020, 20, 2372. [Google Scholar] [CrossRef]
- Huang, D.; Liu, X.; Lai, C.; Qin, L.; Zhang, C.; Yi, H.; Zeng, G.; Li, B.; Deng, R.; Liu, S.; et al. Colorimetric determination of mercury (II) using gold nanoparticles and double ligand exchange. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Tsogas, G.Z.; Kappi, F.A.; Vlessidis, A.G.; Giokas, D.L. Recent advances in nanomaterial probes for optical biothiol sensing: A review. Anal. Lett. 2018, 51, 443–468. [Google Scholar] [CrossRef]
- Yoon, S.J.; Nam, Y.S.; Lee, Y.; Oh, I.H.; Lee, K. A dual colorimetric probe for rapid environmental monitoring of Hg2+ and As3+ using gold nanoparticles functionalized with d-penicillamine. RSC Adv. 2021, 11, 5456–5465. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yin, H.; Wei, R.; Wang, W. Facile colorimetric detection of Hg2+ based on anti-aggregation of silver nanoparticles. Biosens. Bioelectron. 2014, 57, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Tolessa, T.; Tan, Z.Q.; Yin, Y.G.; Liu, J.F. Single-drop gold nanoparticles for headspace microextraction and colorimetric assay of mercury (II) in environmental waters. Talanta 2018, 176, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Jiao, L.; Cao, F.; Liu, X.; Zhou, Y.; Yang, C.; Gao, Z.; Zhang, M.; Lin, P.; Han, Y.; et al. A Real-Time Detection Method of Hg2+ in Drinking Water via Portable Biosensor: Using a Smartphone as a Low-Cost Micro-Spectrometer to Read the Colorimetric Signals. Biosensors 2022, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.X.; Zheng, R.; Peng, C.F.; Wei, X.L. DNA–Gold Nanozyme-modified paper device for enhanced colorimetric detection of mercury ions. Biosensors 2020, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.; Qiang, H.; Li, Y.; Sun, J.; Hu, L.; Chen, Z. A sensitive Hg (II) colorimetric sensor based on synergistic catalytic effect of gold nanoparticles and Hg. Sens. Actuators B Chem. 2016, 229, 686–691. [Google Scholar] [CrossRef]
- Peng, C.F.; Pan, N.; Xie, Z.J.; Wu, L.L. Highly sensitive and selective colorimetric detection of Hg2+ based on the separation of Hg2+ and formation of catalytic DNA–gold nanoparticles. Anal. Methods 2016, 8, 1021–1025. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Mo, X.; Gao, Q.; Deng, Y.; Hu, M.; Zou, J.; Nie, J.; Zhang, Y. On-site, rapid and visual method for nanomolar Hg2+ detection based on the thymine–Hg–thymine triggered “double” aggregation of Au nanoparticles enhancing the Tyndall effect. RSC Adv. 2021, 11, 36859–36865. [Google Scholar] [CrossRef]
- Gao, L.; Lv, Q.; Xia, N.; Lin, Y.; Lin, F.; Han, B. Detection of mercury ion with high sensitivity and selectivity using a DNA/graphene oxide hybrid immobilized on glass slides. Biosensors 2021, 11, 300. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.; Jin, X.; Wu, Z.; Chen, X.; Qiu, J. Function of Graphene Oxide as the “Nanoquencher” for Hg2+ Detection Using an Exonuclease I-Assisted Biosensor. Int. J. Mol. Sci. 2022, 23, 6326. [Google Scholar] [CrossRef] [PubMed]
- Song, X. Research on Biosensor Based on DNA Isothermal Signal Amplification for Detection of Heavy Metal Ions in Water Environment. Master’s Thesis, Jinan University, Guangzhou, China, 2019. [Google Scholar]
- Zhi, L.; Zhang, S.; Li, M.; Tu, J.; Lu, X. Achieving ultrasensitive point-of-care assay for mercury ions with a triple-mode strategy based on the mercury-triggered dual-enzyme mimetic activities of Au/WO3 hierarchical hollow nanoflowers. ACS Appl. Mater. Interfaces 2022, 14, 9442–9453. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xu, B.; Li, T.; Huang, J.; Bai, W. A “turn-on” fluorescence copper biosensor based on DNA cleavage-dependent graphene oxide-dsDNA-Cdte quantum dots complex. Sensors 2018, 18, 2605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, H.; Wang, F.; Zhao, Y.; Yin, H.; Chen, Y.; Zhang, J.; Jiang, J. An ultrafast BODIPY single molecular sensor for multi-analytes (acid/base/Cu2+/Bi3+) with different sensing mechanism. Dyes Pigment. 2019, 165, 279–286. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Sun, Y.; Xu, Z.; Lin, H.; Lin, Q.; Zhang, C. Nanoclusters prepared from a silver/gold alloy as a fluorescent probe for selective and sensitive determination of lead (II). Microchim. Acta 2015, 182, 695–701. [Google Scholar] [CrossRef]
- Xu, H.; Xu, P.; Gao, S.; Zhang, S.; Zhao, X.; Fan, C.; Zuo, X. Highly sensitive recognition of Pb2+ using Pb2+ triggered exonuclease aided DNA recycling. Biosens. Bioelectron. 2013, 47, 520–523. [Google Scholar] [CrossRef]
- Li, W.; Hou, X.M.; Wang, P.Y.; Xi, X.G.; Li, M. Direct measurement of sequential folding pathway and energy landscape of human telomeric G-quadruplex structures. J. Am. Chem. Soc. 2013, 135, 6423–6426. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Y.; Deng, S.; Wu, C.; Deng, R.; He, G.; Zhou, M.; Zhong, K.; Gao, H. Metal-induced G-quadruplex polymorphism for ratiometric and label-free detection of lead pollution in tea. Food Chem. 2021, 343, 128425. [Google Scholar] [CrossRef]
- Nicoludis, J.M.; Barrett, S.P.; Mergny, J.L.; Yatsunyk, L.A. Interaction of human telomeric DNA with N-methyl mesoporphyrin IX. Nucleic Acids Res. 2012, 40, 5432–5447. [Google Scholar] [CrossRef]
- Li, T.; Wang, E.; Dong, S. Potassium—Lead-switched G-quadruplexes: A new class of DNA logic gates. J. Am. Chem. Soc. 2009, 131, 15082–15083. [Google Scholar] [CrossRef]
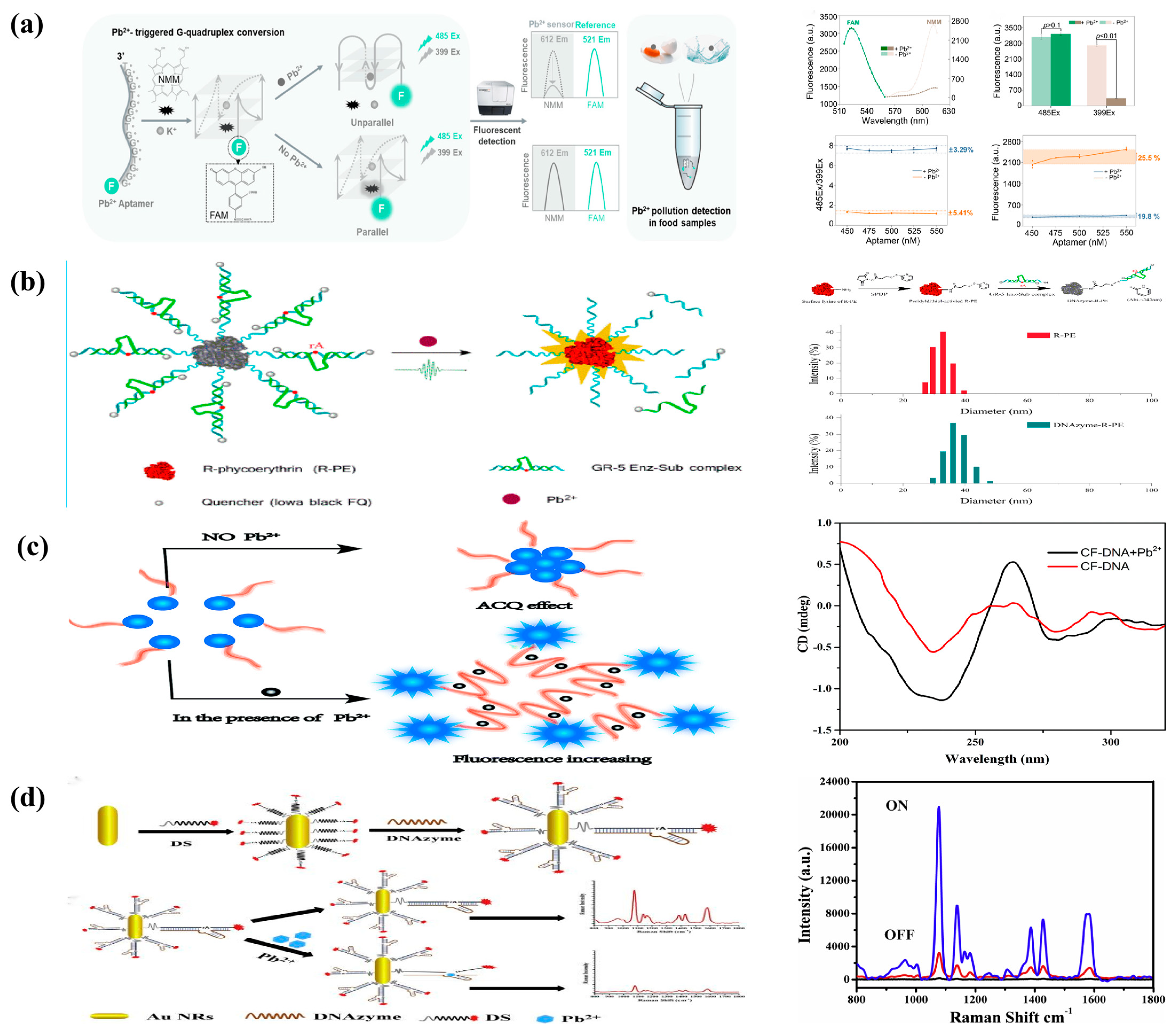
- Liu, Y.; Yang, H.; Wan, R.; Khan, M.R.; Wang, N.; Busquets, R.; Deng, R.; He, Q.; Zhao, Z. Ratiometric G-quadruplex assay for robust lead detection in food samples. Biosensors 2021, 11, 274. [Google Scholar] [CrossRef]
- Wu, J.; Lu, Y.; Ren, N.; Jia, M.; Wang, R.; Zhang, J. DNAzyme-functionalized R-phycoerythrin as a cost-effective and environment-friendly fluorescent biosensor for aqueous Pb2+ detection. Sensors 2019, 19, 2732. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, Y.; Feng, Z.; Liu, F. A highly sensitive and selective fluorescent probe without quencher for detection of Pb2+ ions based on aggregation-caused quenching phenomenon. RSC Adv. 2018, 8, 38929–38934. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, A.; Zuo, F.; Hussain, H.M.J.; Khan, R. A “turn-off” SERS aptasensor based DNAzyme-gold nanorod for ultrasensitive lead ion detection. Anal. Chim. Acta X 2019, 2, 100020. [Google Scholar] [CrossRef] [PubMed]
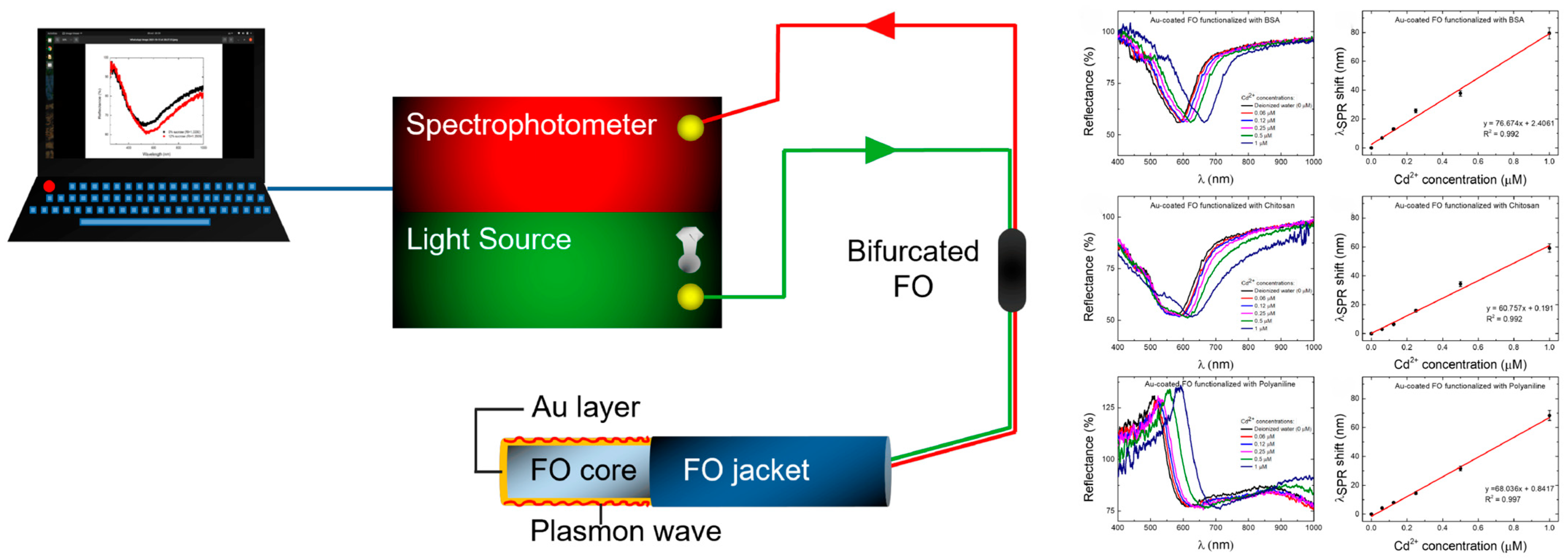
- Şolomonea, B.G.; Jinga, L.I.; Antohe, V.A.; Socol, G.; Antohe, I. Cadmium ions’ trace-level detection using a portable fiber optic—Surface plasmon resonance sensor. Biosensors 2022, 12, 573. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, X.; Wei, H.; Zhao, L.; Yao, B.; Zhang, C.; Zhou, J.; Liu, J.; Yang, S. Solid-Phase Synthesis of Red Fluorescent Carbon Dots for the Dual-Mode Detection of Hexavalent Chromium and Cell Imaging. Biosensors 2022, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ou, X.; Cheng, Y.; Zhai, T.; Liu, B.; Lou, X.; Xia, F. Coordination-induced structural changes of DNA-based optical and electrochemical sensors for metal ions detection. Dalton Trans. 2019, 48, 5879–5891. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Togashi, H.; Tashiro, M.; Yamaguchi, H.; Oda, S.; Kudo, M.; Tanaka, Y.; Kondo, Y.; Sawa, R.; Fujimoto, T.; et al. MercuryII-mediated formation of thymine− HgII− thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef]
- Gellert, M.; Lipsett, M.N.; Davies, D.R. Helix formation by guanylic acid. Proc. Natl. Acad. Sci. USA 1962, 48, 2013–2018. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Ga, L.; Wang, Y.; Ai, J. Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments. Molecules 2024, 29, 34. https://doi.org/10.3390/molecules29010034
Wu B, Ga L, Wang Y, Ai J. Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments. Molecules. 2024; 29(1):34. https://doi.org/10.3390/molecules29010034
Chicago/Turabian StyleWu, Bin, Lu Ga, Yong Wang, and Jun Ai. 2024. "Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments" Molecules 29, no. 1: 34. https://doi.org/10.3390/molecules29010034
APA StyleWu, B., Ga, L., Wang, Y., & Ai, J. (2024). Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments. Molecules, 29(1), 34. https://doi.org/10.3390/molecules29010034








