Investigating the Potential of Ghee Precursor-Derived Carbon Nano Onions for Enhancing Interfacial Bonding in Thermoplastic Composites
Abstract
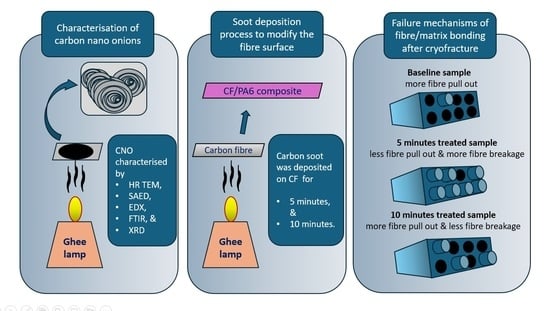
:1. Introduction
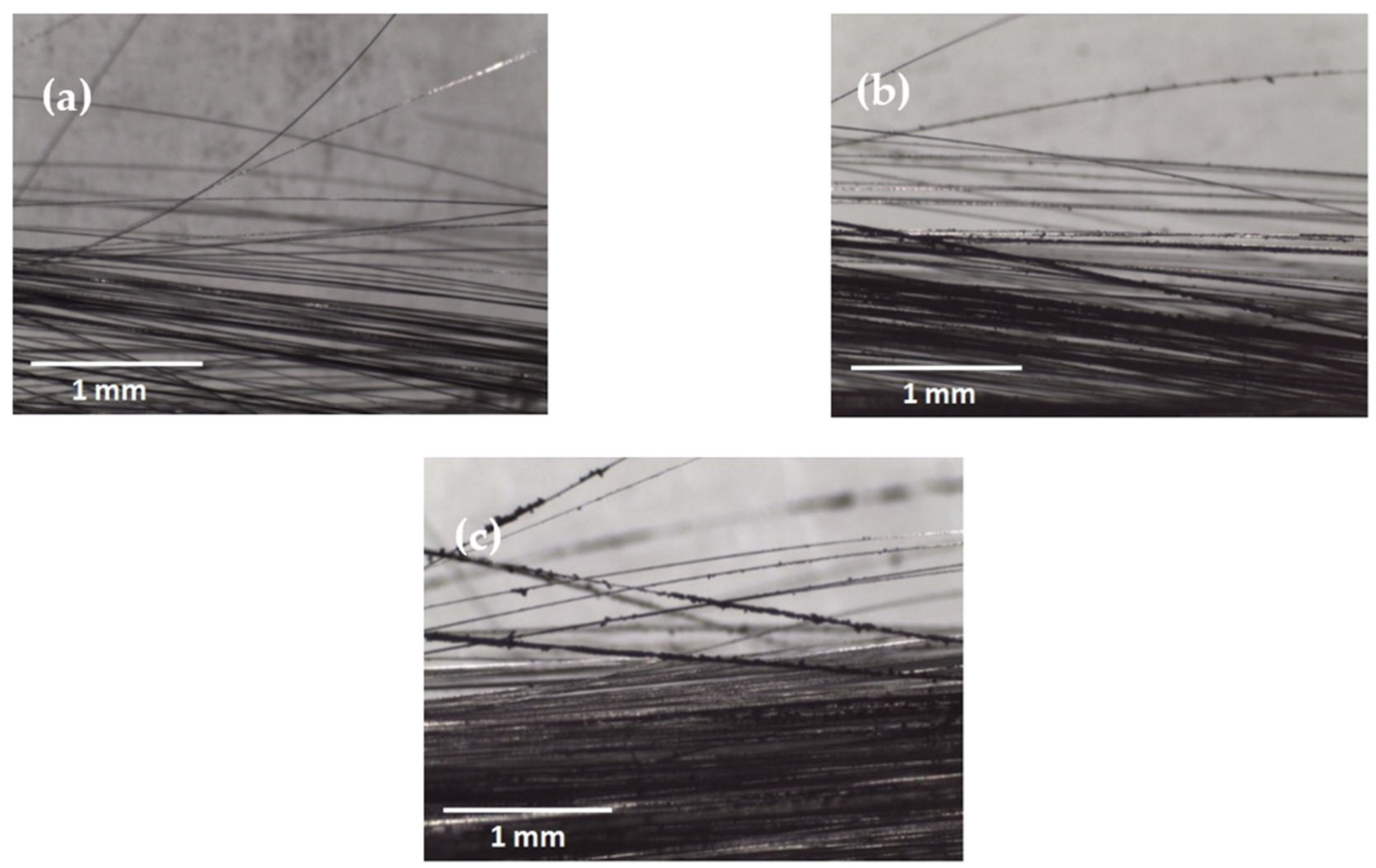
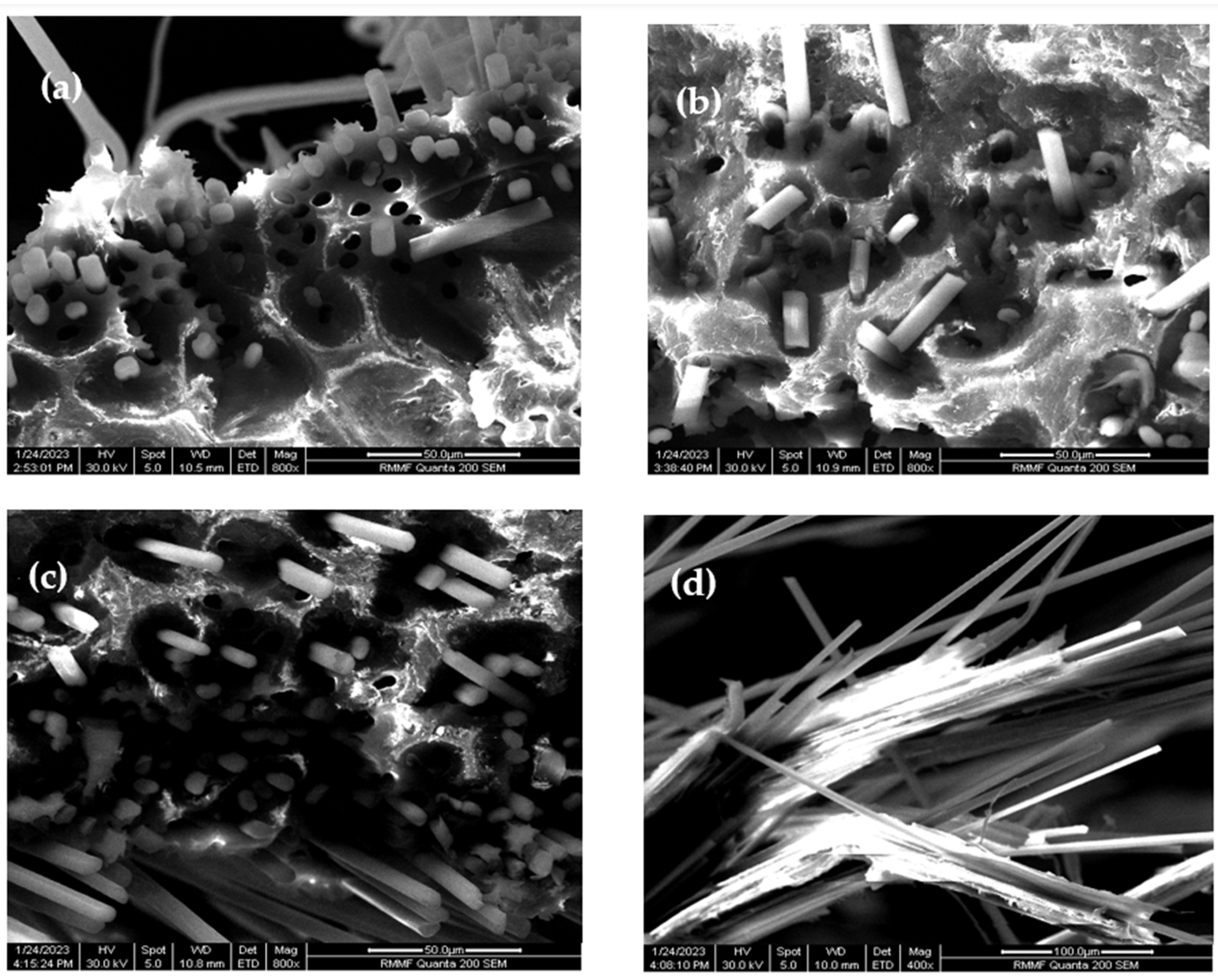
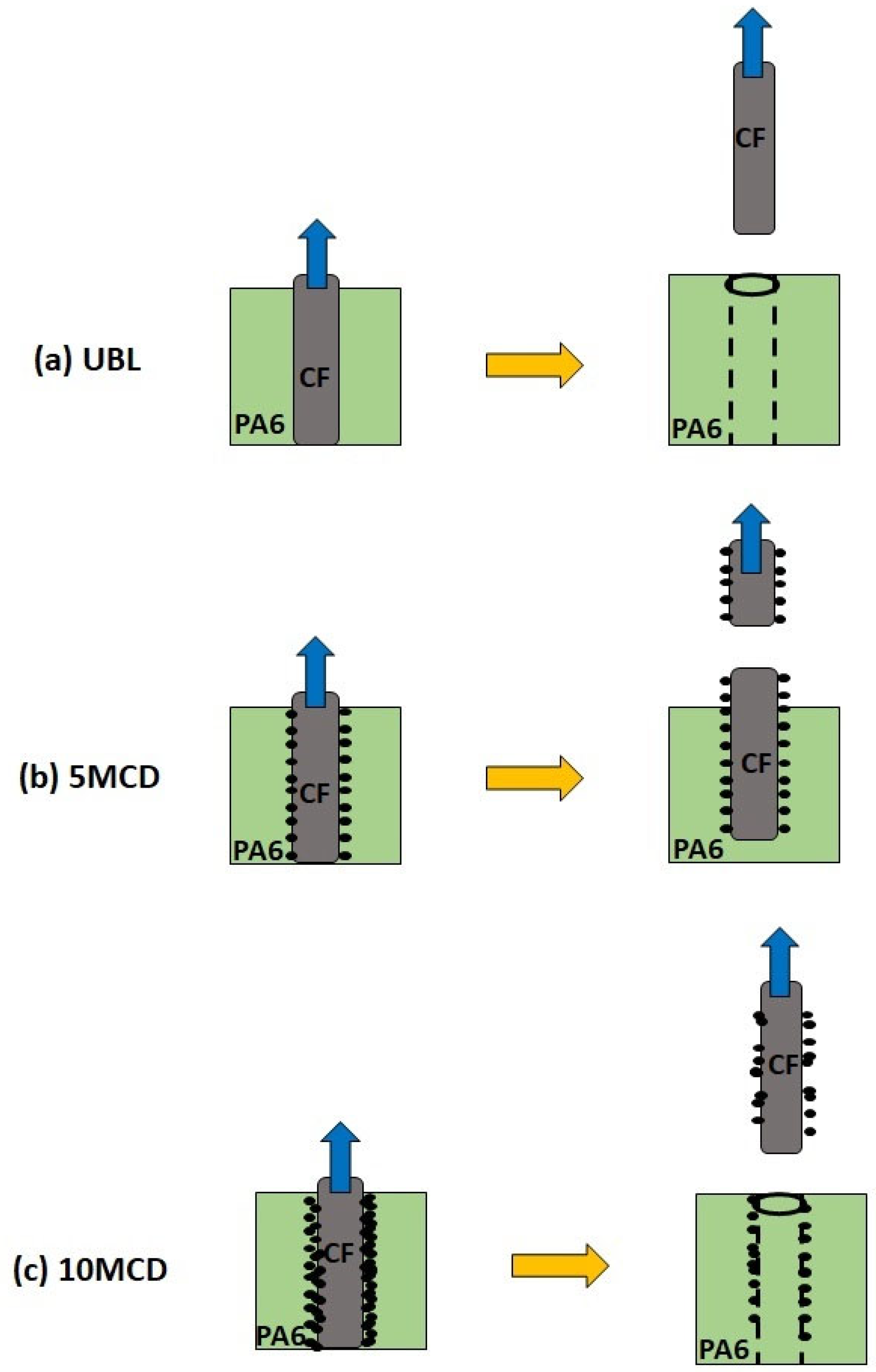
2. Exploring the Effect of Carbon Soot Deposition on Fiber–Matrix Bonding
3. Preparation and Characterization of CNO Particles
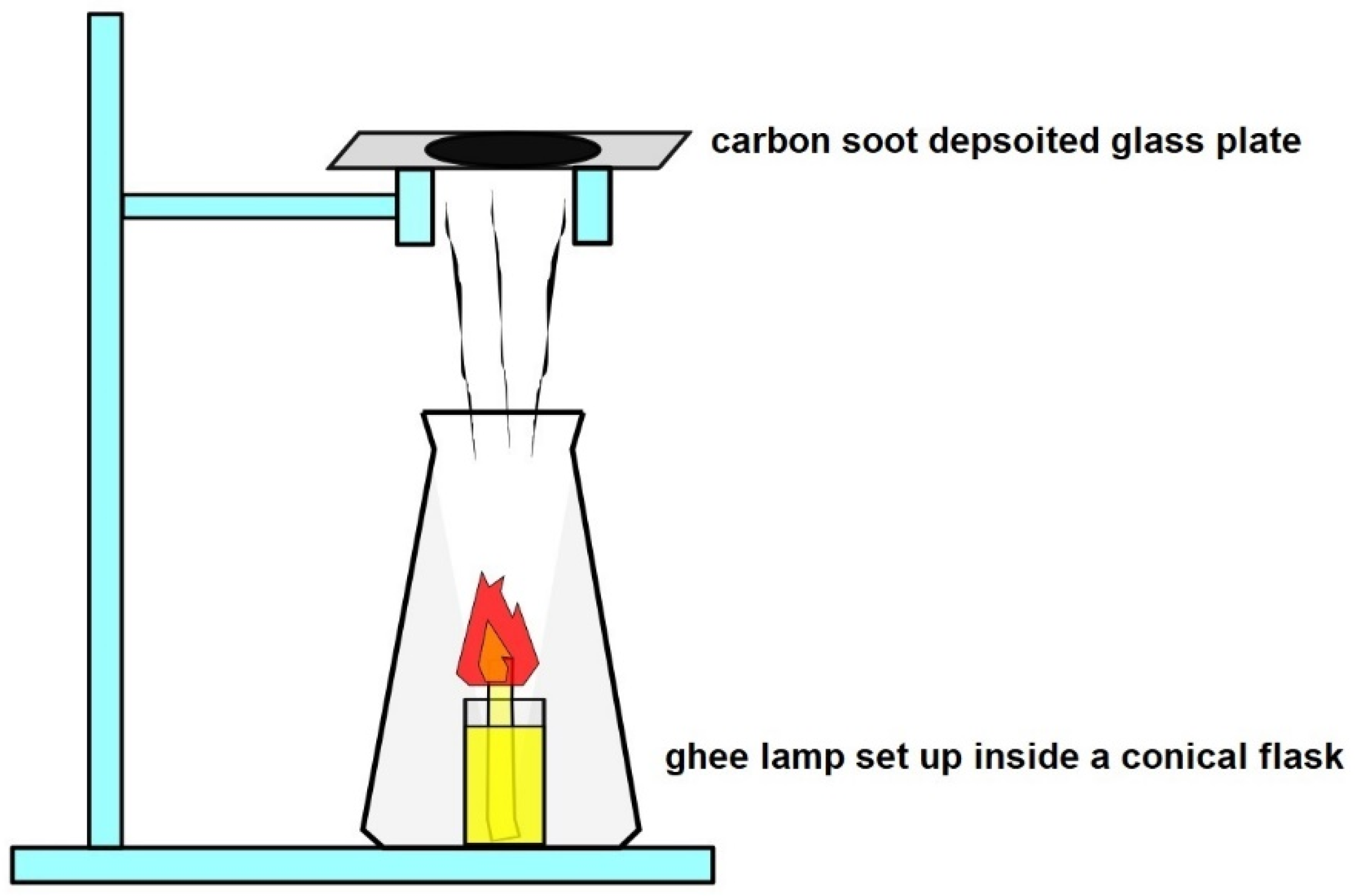
3.1. Flame Synthesis of CNOs
3.2. Characterization of CNO Particles
3.2.1. HRTEM Results on the Morphology of CNOs
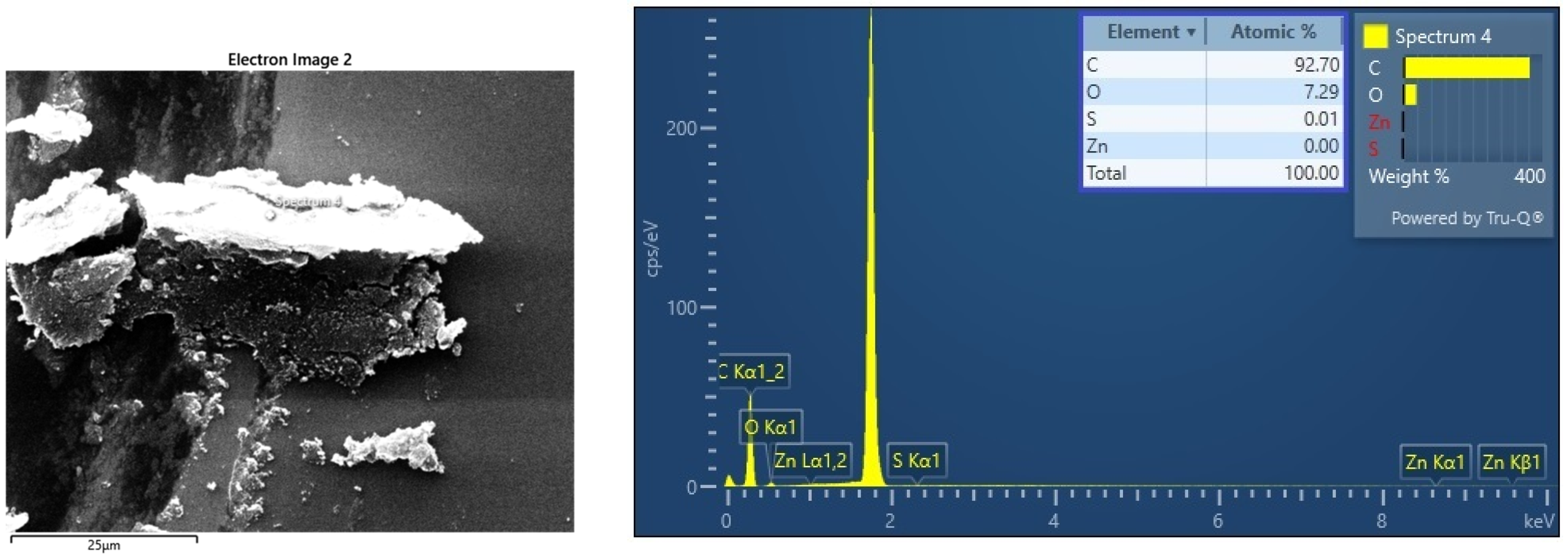
3.2.2. EDX Results on the Chemical Composition of CNOs
3.2.3. FTIR Results on Functional Groups Present in CNOs
3.2.4. XRD Results on the Crystallinity of CNOs
4. CNO Deposition on Carbon Fiber Using a Soot Deposition Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallick, P.K. Thermoplastics and Thermoplastic–Matrix Composites for Lightweight Automotive Structures; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 174–207. [Google Scholar]
- Jianguo, Z.; Gang, D. Mechanical properties of polyimide composite reinforced with carbon nanotubes and carbon fibers. J. Thermoplast. Compos. Mater. 2014, 28, 1250–1259. [Google Scholar] [CrossRef]
- Kim, B.-J.; Cha, S.-H.; Park, Y.-B. Ultra-high-speed processing of nanomaterial-reinforced woven carbon fiber/polyamide 6 composites using reactive thermoplastic resin transfer molding. Compos. Part B Eng. 2018, 143, 36–46. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Zhao, Y. Enhanced interfacial interactions of carbon fiber reinforced PEEK composites by regulating PEI and graphene oxide complex sizing at the interface. Compos. Sci. Technol. 2018, 154, 175–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Yuan, B.; Yuan, Y.; Liew, K.M.; Song, L.; Hu, Y. Preparation of Large-Size Reduced Graphene Oxide-Wrapped Ammonium Polyphosphate and Its Enhancement of the Mechanical and Flame Retardant Properties of Thermoplastic Polyurethane. Ind. Eng. Chem. Res. 2017, 56, 7468–7477. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Y.; Yousefpour, K.; Park, C.; Kumar, V. Evaluating the Lightning Strike Damage Tolerance for CFRP Composite Laminates Containing Conductive Nanofillers. Appl. Compos. Mater. 2022, 29, 1537–1554. [Google Scholar] [CrossRef]
- Tallman, T.N.; Gungor, S.; Wang, K.W.; Bakis, C.E. Damage detection via electrical impedance tomography in glass fiber/epoxy laminates with carbon black filler. Struct. Health Monit. 2014, 14, 100–109. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Chen, G.; Dai, P. Interlaminar fracture toughness and conductivity of carbon fiber/epoxy resin composite laminate modified by carbon black-loaded polypropylene non-woven fabric interleaves. Compos. Struct. 2020, 234, 111649. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, L.; Deng, S.; Zhang, J.; Tang, Y.; Chen, Y. CF/EP composite laminates with carbon black and copper chloride for improved electrical conductivity and interlaminar fracture toughness. Compos. Sci. Technol. 2012, 72, 412–420. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, L.; Wang, D.; Tang, Y.; Mustapha, S.; Chen, Y. Assessment of transverse impact damage in GF/EP laminates of conductive nanoparticles using electrical resistivity tomography. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1587–1598. [Google Scholar] [CrossRef]
- Hu, W.; Yin, H.; Yuan, H.; Tang, Y.; Ren, X.; Wei, Y. Microwave absorption and mechanical properties of glass fiber/polyamide 6 composites containing carbon black by microstructural design. Compos. Sci. Technol. 2023, 233, 109927. [Google Scholar] [CrossRef]
- Markov, A.; Fiedler, B.; Schulte, K. Electrical conductivity of carbon black/fibres filled glass-fibre-reinforced thermoplastic composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1390–1395. [Google Scholar] [CrossRef]
- Mohapatra, D.; Badrayyana, S.; Parida, S. Facile wick-and-oil flame synthesis of high-quality hydrophilic onion-like carbon nanoparticles. Mater. Chem. Phys. 2016, 174, 112–119. [Google Scholar] [CrossRef]
- Iijima, S. High resolution electron microscopy of some carbonaceous materials. J. Microsc. 1980, 119, 99–111. [Google Scholar] [CrossRef]
- Borgohain, R.; Yang, J.; Selegue, J.P.; Kim, D.Y. Controlled synthesis, efficient purification, and electrochemical characterization of arc-discharge carbon nano-onions. Carbon 2014, 66, 272–284. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Shao, G. The formation of onion-like carbon-encapsulated cobalt carbide core/shell nanoparticles by the laser ablation of metallic cobalt in acetone. Carbon 2013, 55, 108–115. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, B.; Zou, H.; Liang, M.; Chen, Y. Enhanced interfacial adhesion between polypropylene and carbon fiber by graphene oxide/polyethyleneimine coating. J. Ind. Eng. Chem. 2017, 51, 129–139. [Google Scholar] [CrossRef]
- Mongwe, T.H.; Matsoso, B.J.; Mutuma, B.K.; Coville, N.J.; Maubane, M.S. Synthesis of chain-like carbon nano-onions by a flame assisted pyrolysis technique using different collecting plates. Diam. Relat. Mater. 2018, 90, 135–143. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Lange, H.; Huczko, A.; Baranowski, P.; Hübers, H.W.; Gemming, T.; Pichler, T.; Büchner, B.; Rümmeli, M.H. One-step catalyst-free generation of carbon nanospheres via laser-induced pyrolysis of anthracene. J. Solid State Chem. 2008, 181, 2796–2803. [Google Scholar] [CrossRef]
- Cortes, L.Q.; Racagel, S.; Lonjon, A.; Dantras, E.; Lacabanne, C. Electrically conductive carbon fiber/PEKK/silver nanowires multifunctional composites. Compos. Sci. Technol. 2016, 137, 159–166. [Google Scholar] [CrossRef]
- Yumitori, S.; Arao, Y.; Tanaka, T.; Naito, K.; Tanaka, K.; Katayama, T. Increasing the interfacial strength in carbon fiber/polypropylene composites by growing CNTs on the fibers. In Proceedings of the Computational Methods and Experimental Measurements XVI, Coruña, Spain, 2–4 July 2013. [Google Scholar]
- Liu, Y.-T.; Song, H.-Y.; Yao, T.-T.; Zhang, W.-S.; Zhu, H.; Wu, G.-P. Effects of carbon nanotube length on interfacial properties of carbon fiber reinforced thermoplastic composites. J. Mater. Sci. 2020, 55, 15467–15480. [Google Scholar] [CrossRef]
- Kim, B.-J.; Cha, S.-H.; Kong, K.; Ji, W.; Park, H.W.; Park, Y.-B. Synergistic interfacial reinforcement of carbon fiber/polyamide 6 composites using carbon-nanotube-modified silane coating on ZnO-nanorod-grown carbon fiber. Compos. Sci. Technol. 2018, 165, 362–372. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periasamy, K.; Darouie, M.; Das, R.; Khatibi, A.A. Investigating the Potential of Ghee Precursor-Derived Carbon Nano Onions for Enhancing Interfacial Bonding in Thermoplastic Composites. Molecules 2024, 29, 928. https://doi.org/10.3390/molecules29050928
Periasamy K, Darouie M, Das R, Khatibi AA. Investigating the Potential of Ghee Precursor-Derived Carbon Nano Onions for Enhancing Interfacial Bonding in Thermoplastic Composites. Molecules. 2024; 29(5):928. https://doi.org/10.3390/molecules29050928
Chicago/Turabian StylePeriasamy, Kailashbalan, Maryam Darouie, Raj Das, and Akbar A. Khatibi. 2024. "Investigating the Potential of Ghee Precursor-Derived Carbon Nano Onions for Enhancing Interfacial Bonding in Thermoplastic Composites" Molecules 29, no. 5: 928. https://doi.org/10.3390/molecules29050928