Valorization of Spent Vetiver Roots for Biochar Generation
Abstract
:1. Introduction
2. Results and Discussion
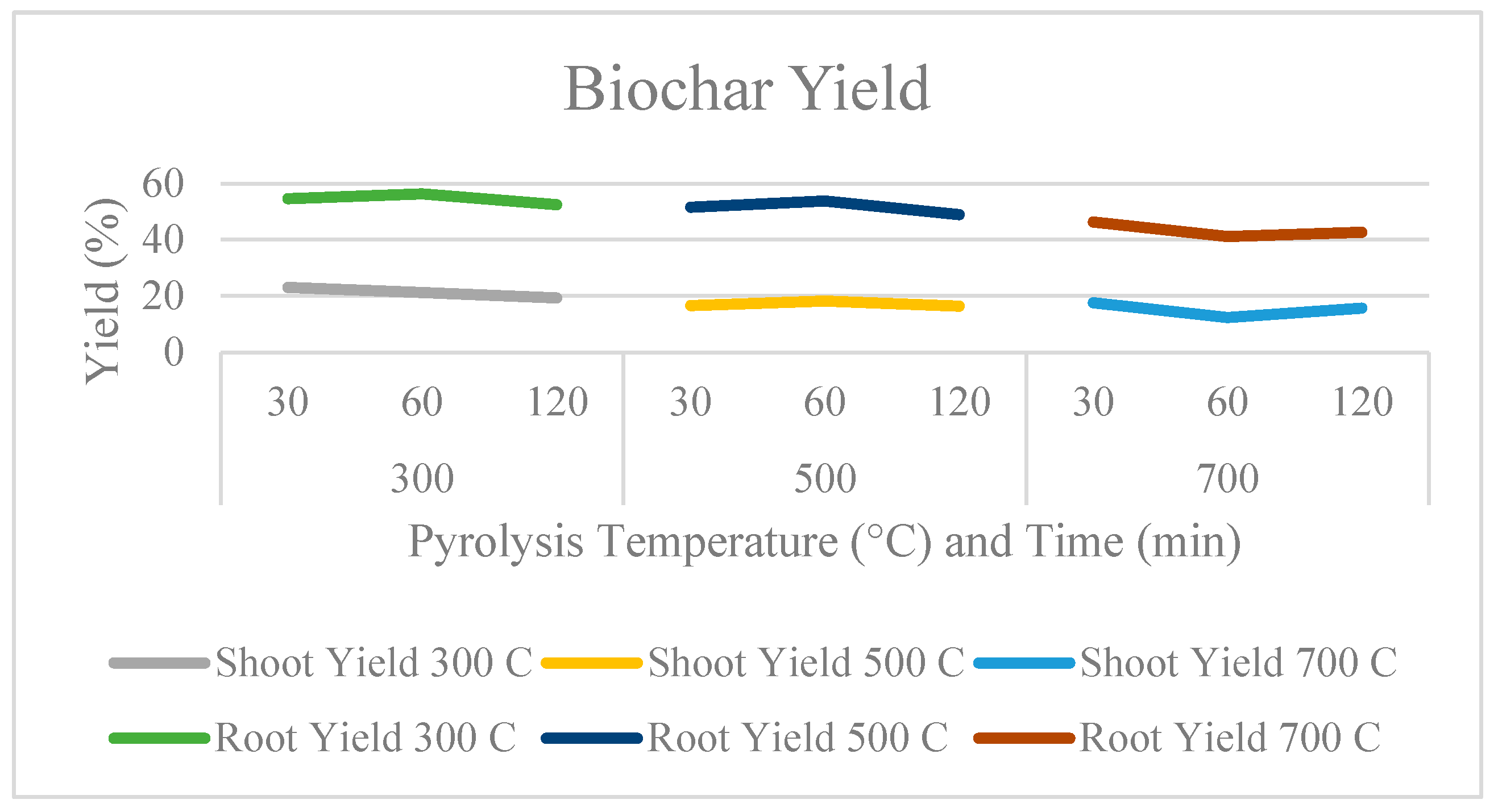
2.1. Effect of Pyrolysis Temperature and Residence Time on the Physical Characteristics of Biochar
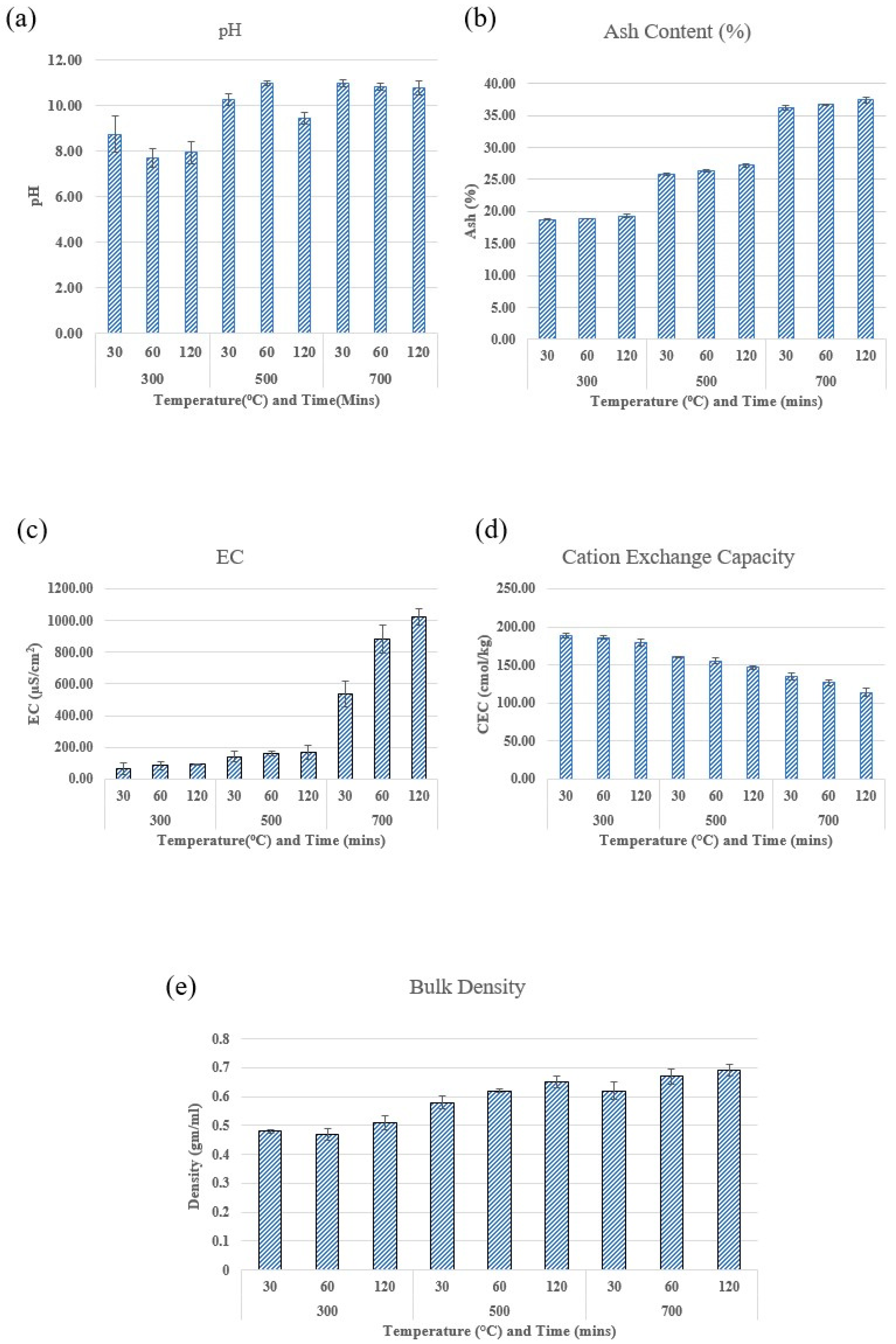
2.2. Effect of Pyrolysis Temperature and Residence Time on the Chemical Characteristics of Biochar
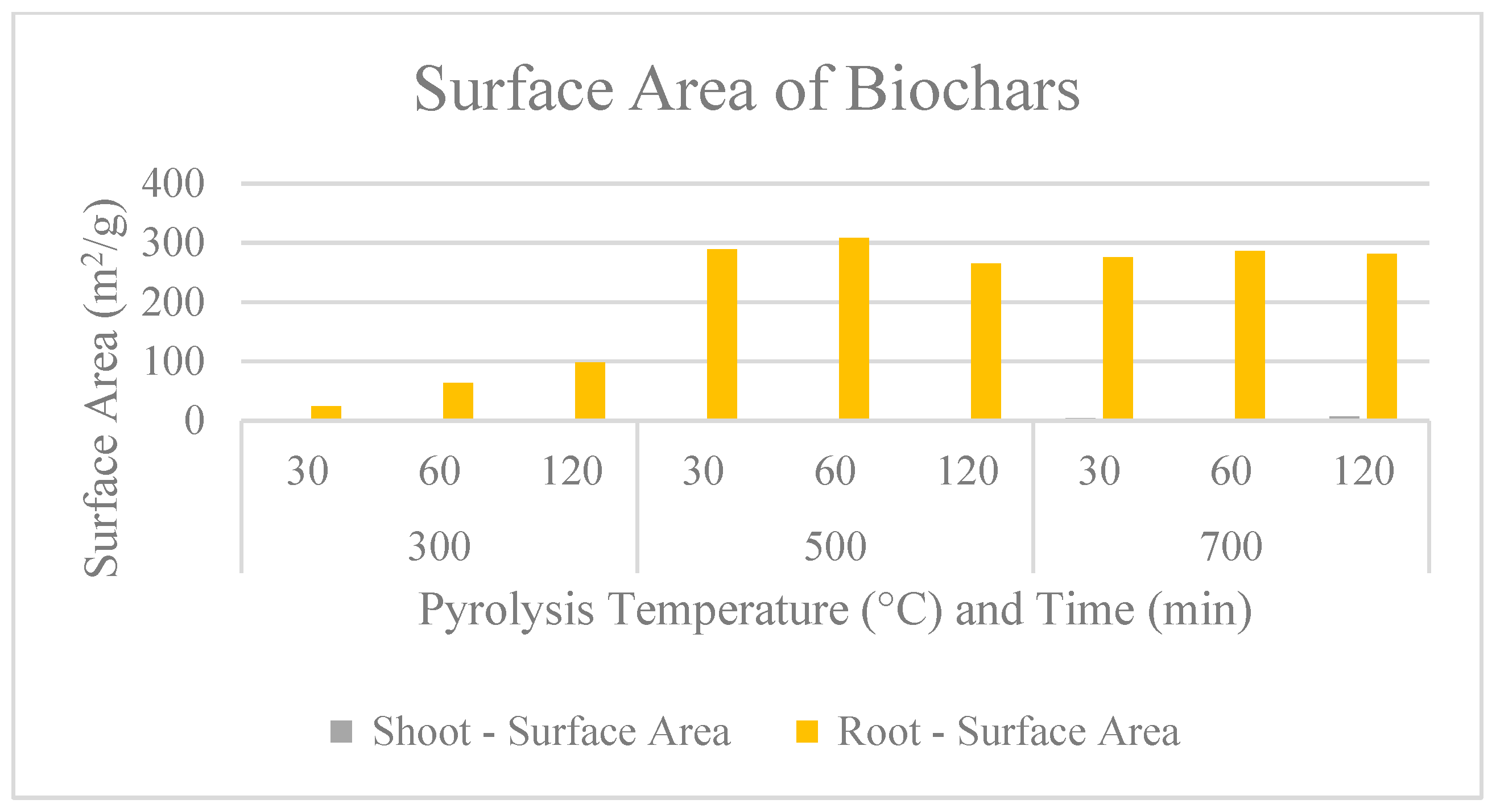
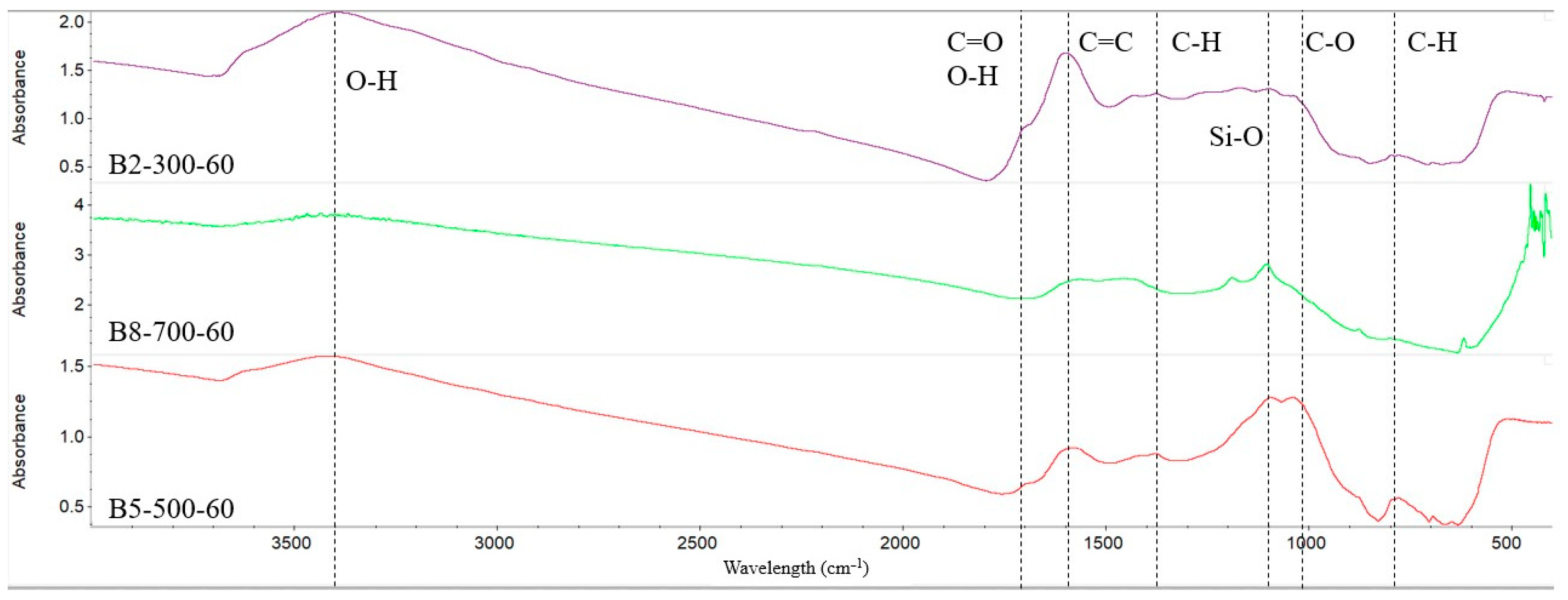
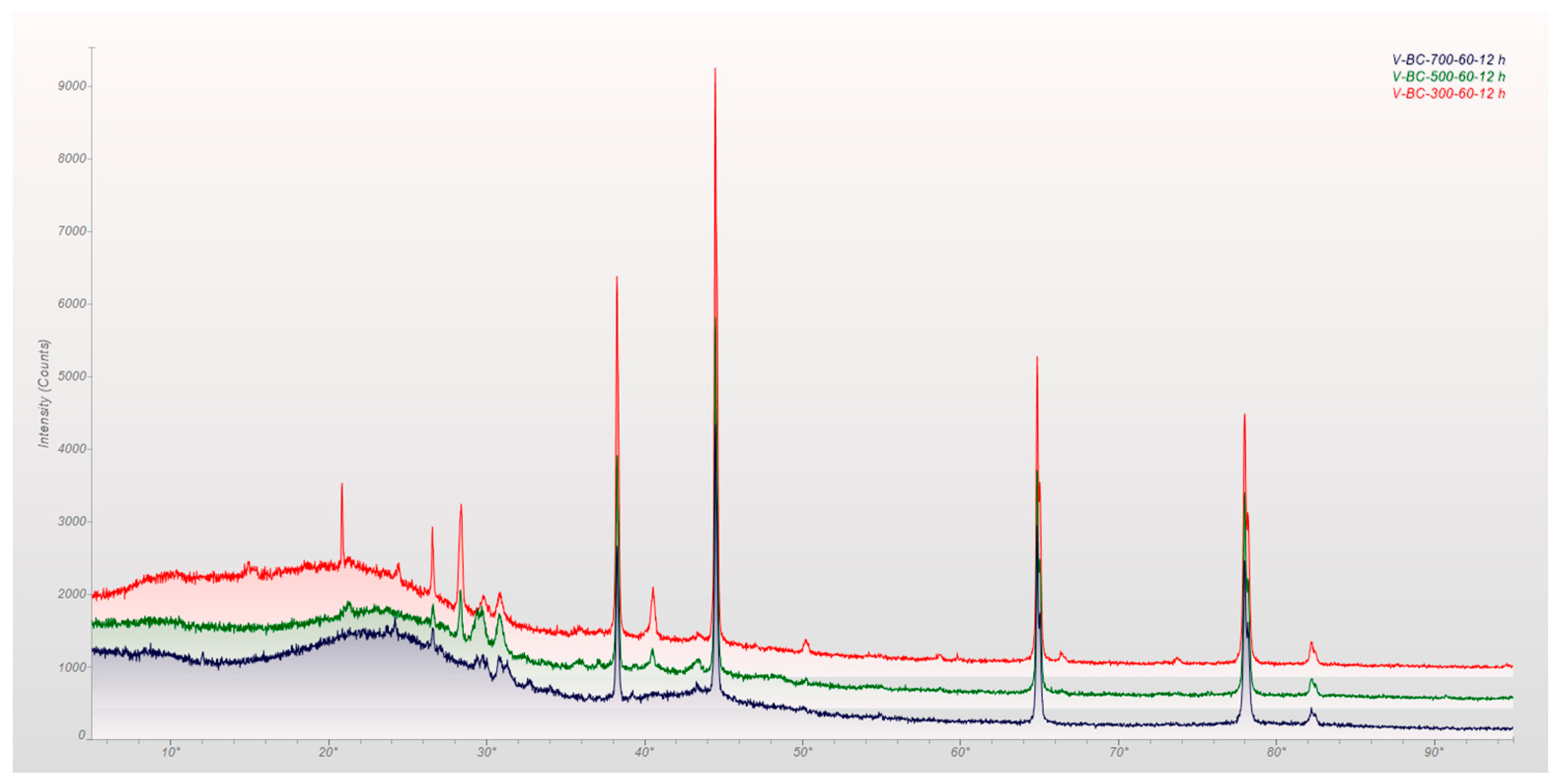
2.3. Surface Characteristics
2.4. Environmental Implications of Vetiver Biochar
2.5. Agronomic Implications of Vetiver Biochar
3. Materials and Methods
3.1. Vetiver Plants
3.2. Vetiver Biochar Generation
3.3. Physical Properties
3.4. Chemical Analysis
3.5. Surface Characterization
3.6. Chemical and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nast, C. Best Vetiver Fragrances for a Smoky, Masculine Scent. Available online: https://www.gq-magazine.co.uk/gallery/best-mens-vetiver-fragrance-guide (accessed on 9 May 2022).
- Lavania, U.C. Other Uses and Utilization of Vetiver: Vetiver Oil. In Proceedings of the Third International Vetiver Conference, Guangzhou, China, 6–9 October 2003; p. 475. [Google Scholar]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal Solid Waste Management and Waste-to-Energy in the Context of a Circular Economy and Energy Recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Hu, Q.; Jung, J.; Chen, D.; Leong, K.; Song, S.; Li, F.; Mohan, B.C.; Yao, Z.; Prabhakar, A.K.; Lin, X.H.; et al. Biochar Industry to Circular Economy. Sci. Total Environ. 2021, 757, 143820. [Google Scholar] [CrossRef] [PubMed]
- Neve, S.; Sarkar, D.; Zhang, Z.; Datta, R. Optimized Production of Second-Generation Bioethanol from a Spent C4 Grass: Vetiver (Chrysopogon zizanioides). Energies 2022, 15, 9597. [Google Scholar] [CrossRef]
- Neve, S.; Du, J.; Barhemat, R.; Meng, W.; Bao, Y.; Sarkar, D. Valorization of Vetiver Root Biochar in Eco-Friendly Reinforced Concrete: Mechanical, Economic, and Environmental Performance. Materials 2023, 16, 2522. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of Biochar, Compost and Biochar–Compost for Soil Quality, Maize Yield and Greenhouse Gas Emissions in a Tropical Agricultural Soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Yrjälä, K.; Ramakrishnan, M.; Salo, E. Agricultural Waste Streams as Resource in Circular Economy for Biochar Production towards Carbon Neutrality. Curr. Opin. Environ. Sci. Health 2022, 26, 100339. [Google Scholar] [CrossRef]
- Masud, M.A.A.; Shin, W.S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D.M.; Iqbal, M.A.; Islam, A.R.M.T.; Malafaia, G. A Critical Review of Sustainable Application of Biochar for Green Remediation: Research Uncertainty and Future Directions. Sci. Total Environ. 2023, 904, 166813. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Woong Chang, S.; Ravindran, B.; Kumar Awasthi, M. Comprehensive Review on Recent Production Trends and Applications of Biochar for Greener Environment. Bioresour. Technol. 2023, 388, 129725. [Google Scholar] [CrossRef]
- Fang, L.; Huang, T.; Lu, H.; Wu, X.-L.; Chen, Z.; Yang, H.; Wang, S.; Tang, Z.; Li, Z.; Hu, B.; et al. Biochar-Based Materials in Environmental Pollutant Elimination, H2 Production and CO2 Capture Applications. Biochar 2023, 5, 42. [Google Scholar] [CrossRef]
- Lyu, H.; Lim, J.Y.; Zhang, Q.; Senadheera, S.S.; Zhang, C.; Huang, Q.; Ok, Y.S. Conversion of Organic Solid Waste into Energy and Functional Materials Using Biochar Catalyst: Bibliometric Analysis, Research Progress, and Directions. Appl. Catal. B Environ. 2024, 340, 123223. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjostrom, K.; Bjornbom, E. Rapid Pyrolysis of Agricultural Residues at High Temperature. Biomass Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 1–13. ISBN 0-203-76226-6. [Google Scholar]
- Novak, J.M.; Lima, I.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Watts, D.W.; Schomberg, H. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on A Loamy Sand. Ann. Environ. Sci. 2009, 3, 12. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Rehrah, D.; Bansode, R.R.; Hassan, O.; Ahmedna, M. Physico-Chemical Characterization of Biochars from Solid Municipal Waste for Use in Soil Amendment. J. Anal. Appl. Pyrolysis 2016, 118, 42–53. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Production of Granular Activated Carbons from Select Agricultural By-Products and Evaluation of Their Physical, Chemical and Adsorption Properties. Bioresour. Technol. 2000, 71, 113–123. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of Sewage Sludge-Derived Biochars from Different Feedstocks and Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.-Y.; Cho, T.-S.; Choi, J.W. Influence of Pyrolysis Temperature on Physicochemical Properties of Biochar Obtained from the Fast Pyrolysis of Pitch Pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of Pyrolysis Temperature on Soybean Stover- and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Pandey, S.D.; Mendonça, F.G.; Rodrigues, M.N.; Faria, B.P.Z.; Campos, J.L.E.; Noronha, I.F.P.C.; Vieira, S.S.; Santos, N.A.V.; Fernandes, L.A.; Sampaio, R.A.; et al. Structural and Elemental Analysis of Biochars in the Search of a Synthetic Path to Mimetize Anthropic Amazon Soils. J. Environ. Manag. 2021, 279, 111685. [Google Scholar] [CrossRef]
- Byrne, C.E.; Nagle, D.C. Carbonization of Wood for Advanced Materials Applications. Carbon 1997, 35, 259–266. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; de Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.A.; Zhu, J.; Muhammad, N.; Sheng, T.; Xu, X. Effect of Synthesis Methods on Magnetic Kans Grass Biochar for Enhanced As(III, V) Adsorption from Aqueous Solutions. Biomass Bioenergy 2014, 71, 299–310. [Google Scholar] [CrossRef]
- Chen, T.; Liu, R.; Scott, N.R. Characterization of Energy Carriers Obtained from the Pyrolysis of White Ash, Switchgrass and Corn Stover—Biochar, Syngas and Bio-Oil. Fuel Process. Technol. 2016, 142, 124–134. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for Environmental Management: Mitigating Greenhouse Gas Emissions, Contaminant Treatment, and Potential Negative Impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar Stability in Soil: Decomposition during Eight Years and Transformation as Assessed by Compound-Specific 14C Analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent Advances in Biochar Applications in Agricultural Soils: Benefits and Environmental Implications. CLEAN Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s Role in Mitigating Soil Nitrous Oxide Emissions: A Review and Meta-Analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Cui, B.; Zhang, K.; Wang, Q.; Liu, X.; Gao, H.; Huang, L. Assessment of Heavy Metal Pollution in Wetland Soils from the Young and Old Reclaimed Regions in the Pearl River Estuary, South China. Environ. Pollut. 2011, 159, 817–824. [Google Scholar] [CrossRef]
- Neve, S.S. Using Vetiver Biochar to Adsorb Lead; GSA: Washington, DC, USA, 2020.
- Sustainable Reuse of Spent Biomass for Environmental Remediation: Vetiver Root Biochar Adsorbs Zinc. Available online: https://acs.digitellinc.com/pl/product/476331 (accessed on 28 November 2023).
- East Coast Student Competition. Available online: https://www.aehsfoundation.org/ecstudentcomp (accessed on 28 November 2023).
- Zhang, H.; Lin, K.; Wang, H.; Gan, J. Effect of Pinus Radiata Derived Biochars on Soil Sorption and Desorption of Phenanthrene. Environ. Pollut. 2010, 158, 2821–2825. [Google Scholar] [CrossRef] [PubMed]
- Neve, S.; Sarkar, D.; Datta, R. Vetiver Root Biochar Amendment Improves the Quality of Copper-Contaminated Sandy Soils. In Proceedings of the ASA, CSSA, SSSA International Annual Meeting, Baltimore, MD, USA, 8 November 2022. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Zhang, H.; Shao, L.; Chen, D.; He, P. Multiscale Visualization of the Structural and Characteristic Changes of Sewage Sludge Biochar Oriented towards Potential Agronomic and Environmental Implication. Sci. Rep. 2015, 5, 9406. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic Values of Greenwaste Biochar as a Soil Amendment. Soil Res. 2007, 45, 629. [Google Scholar] [CrossRef]
- Martinez, J.; Rosa, P.T.V.; Menut, C.; Leydet, A.; Brat, P.; Pallet, D.; Meireles, M.A.A. Valorization of Brazilian Vetiver (Vetiveria Zizanioides (L.) Nash Ex Small) Oil. J. Agric. Food Chem. 2004, 52, 6578–6584. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Miccio, M.; Ruoppolo, G. Torrefaction of Tomato Peel Residues in a Fluidized Bed of Inert Particles and a Fixed-Bed Reactor. Energy Fuels 2016, 30, 4858–4868. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting Biochar Properties and Functions Based on Feedstock and Pyrolysis Temperature: A Review and Data Syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Na Nagara, V.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of Heavy Metals from Stormwater Runoff Using Granulated Drinking Water Treatment Residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Rehrah, D.; Reddy, M.R.; Novak, J.M.; Bansode, R.R.; Schimmel, K.A.; Yu, J.; Watts, D.W.; Ahmedna, M. Production and Characterization of Biochars from Agricultural By-Products for Use in Soil Quality Enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Adsorption of Volatile Organic Compounds by Pecan Shell- and Almond Shell-Based Granular Activated Carbons. Bioresour. Technol. 2003, 90, 175–184. [Google Scholar] [CrossRef]
- Denyes, M.J.; Parisien, M.A.; Rutter, A.; Zeeb, B.A. Physical, Chemical and Biological Characterization of Six Biochars Produced for the Remediation of Contaminated Sites. J. Vis. Exp. 2014, 93, e52183. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Wakakuwa, J.R. Acid Digestion for Sediments, Sludges, Soils, and Solid Wastes. A Proposed Alternative to EPA SW 846 Method 3050. Environ. Sci. Technol. 1989, 23, 898–900. [Google Scholar] [CrossRef]
- Policicchio, A.; Florent, M.; Celzard, A.; Fierro, V.; Jagiello, J.; Bandosz, T.J. Enhancing the Gas Adsorption Capacities of UiO-66 by Nanographite Addition. Microporous Mesoporous Mater. 2020, 309, 110571. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]

| Sample ID | Temperature (°C) | Retention Time (min) | %C | %H | %N | %O | H/C | O/C | %P | %K | %Ca | %Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1-300-30 | 300 | 30 | 61.11 | 3.945 | 2.102 | 21.7 | 0.065 | 0.355 | 0.006 | 0.012 | 0.019 | 0.025 |
| B2-300-60 | 300 | 60 | 63.75 | 4.056 | 2.092 | 24 | 0.064 | 0.377 | 0.008 | 0.013 | 0.021 | 0.029 |
| B3-300-120 | 300 | 120 | 61.99 | 3.9 | 2.033 | 23 | 0.063 | 0.371 | 0.010 | 0.016 | 0.025 | 0.03 |
| B4-500-30 | 500 | 30 | 62.32 | 2.501 | 1.256 | 15.8 | 0.040 | 0.254 | 0.024 | 0.029 | 0.026 | 0.032 |
| B5-500-60 | 500 | 60 | 68.58 | 2.782 | 1.752 | 20.7 | 0.041 | 0.302 | 0.029 | 0.032 | 0.027 | 0.034 |
| B6-500-120 | 500 | 120 | 66.24 | 3.239 | 2.092 | 20.7 | 0.049 | 0.313 | 0.036 | 0.038 | 0.031 | 0.038 |
| B7-700-30 | 700 | 30 | 73.51 | 1.135 | 0.432 | 15.6 | 0.015 | 0.212 | 0.051 | 0.059 | 0.035 | 0.041 |
| B8-700-60 | 700 | 60 | 71.20 | 0.969 | 0.198 | 15 | 0.014 | 0.211 | 0.053 | 0.068 | 0.038 | 0.041 |
| B9-700-120 | 700 | 120 | 84.62 | 1.596 | 0.266 | 14 | 0.019 | 0.165 | 0.057 | 0.072 | 0.043 | 0.05 |
| Feedstock (Temp °C) | Crystalline Phases | Liming Value (% CaCO3) | |
|---|---|---|---|
| Mean | Std. Error | ||
| Vetiver root (300) | Quartz, calcite, merrillite | 2.54 | 0.31 |
| Vetiver root (500) | Quartz, whewellite, sylvite | 3.7 | 0.67 |
| Vetiver root (700) | Quartz, arcanite, sylvite | 5.76 | 0.08 |
| Wheat straw (550) | Quartz, calcite, Mg calcite | 5.7 | 0.1 |
| Wheat straw (700) | Quartz, calcite, Mg calcite | 6.5 | 0.1 |
| Switchgrass (400) | Quartz | 1.9 | 0.2 |
| Switchgrass (550) | Quartz | 3 | 0.2 |
| Rice husk (550) | Quartz, calcite | 1.5 | 0 |
| Rice husk (700) | Quartz, calcite, sylvite | 1.9 | 0.1 |
| Miscanthus straw (550) | Quartz, calcite | 3.8 | 0.1 |
| Miscanthus straw (700) | Arcanite, calcite | 5.6 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neve, S.; Sarkar, D.; Warke, M.; Bandosz, T.; Datta, R. Valorization of Spent Vetiver Roots for Biochar Generation. Molecules 2024, 29, 63. https://doi.org/10.3390/molecules29010063
Neve S, Sarkar D, Warke M, Bandosz T, Datta R. Valorization of Spent Vetiver Roots for Biochar Generation. Molecules. 2024; 29(1):63. https://doi.org/10.3390/molecules29010063
Chicago/Turabian StyleNeve, Sameer, Dibyendu Sarkar, Manas Warke, Teresa Bandosz, and Rupali Datta. 2024. "Valorization of Spent Vetiver Roots for Biochar Generation" Molecules 29, no. 1: 63. https://doi.org/10.3390/molecules29010063
APA StyleNeve, S., Sarkar, D., Warke, M., Bandosz, T., & Datta, R. (2024). Valorization of Spent Vetiver Roots for Biochar Generation. Molecules, 29(1), 63. https://doi.org/10.3390/molecules29010063











