Synthesis of CoMoO4 Nanofibers by Electrospinning as Efficient Electrocatalyst for Overall Water Splitting
Abstract
:1. Introduction
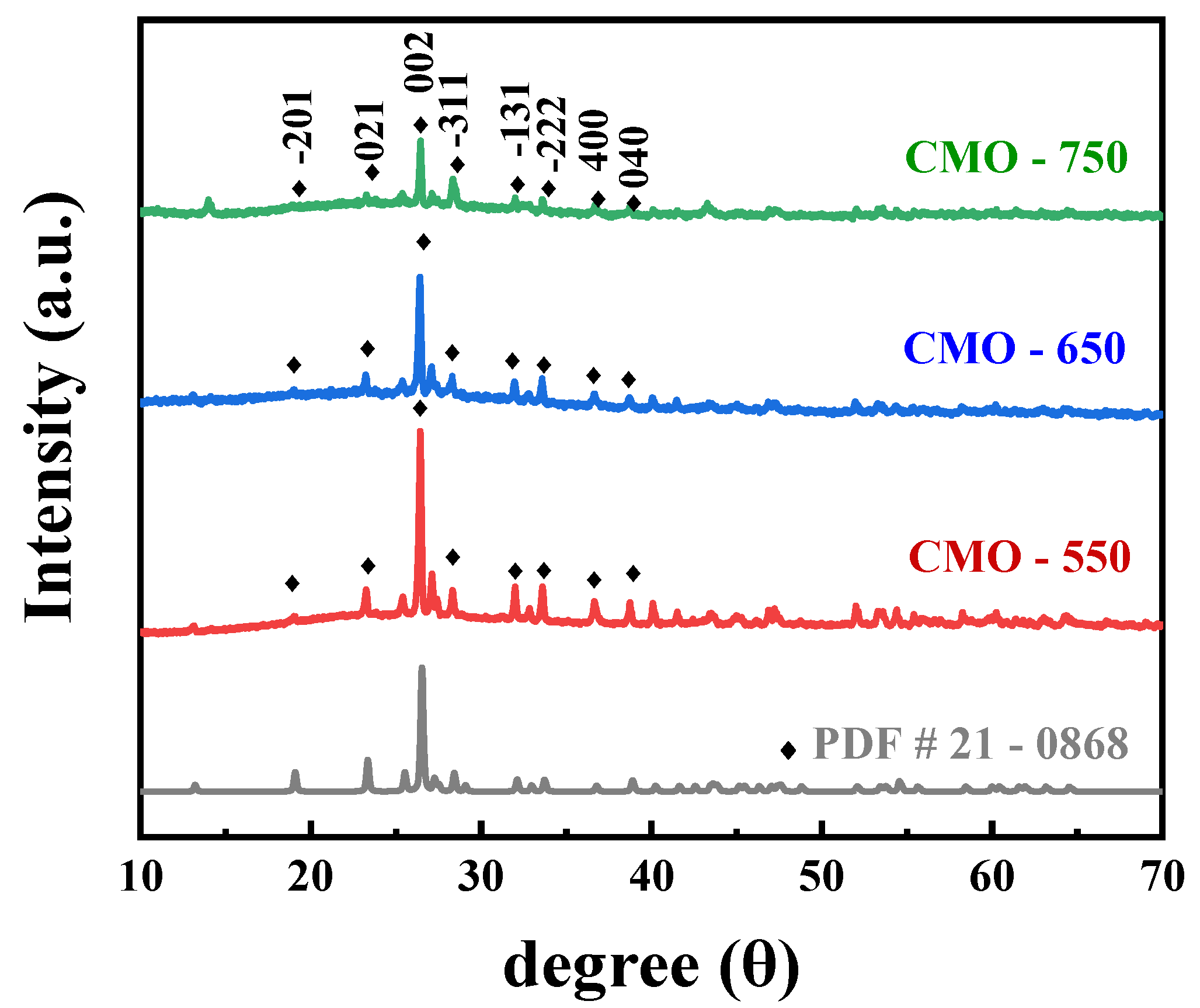
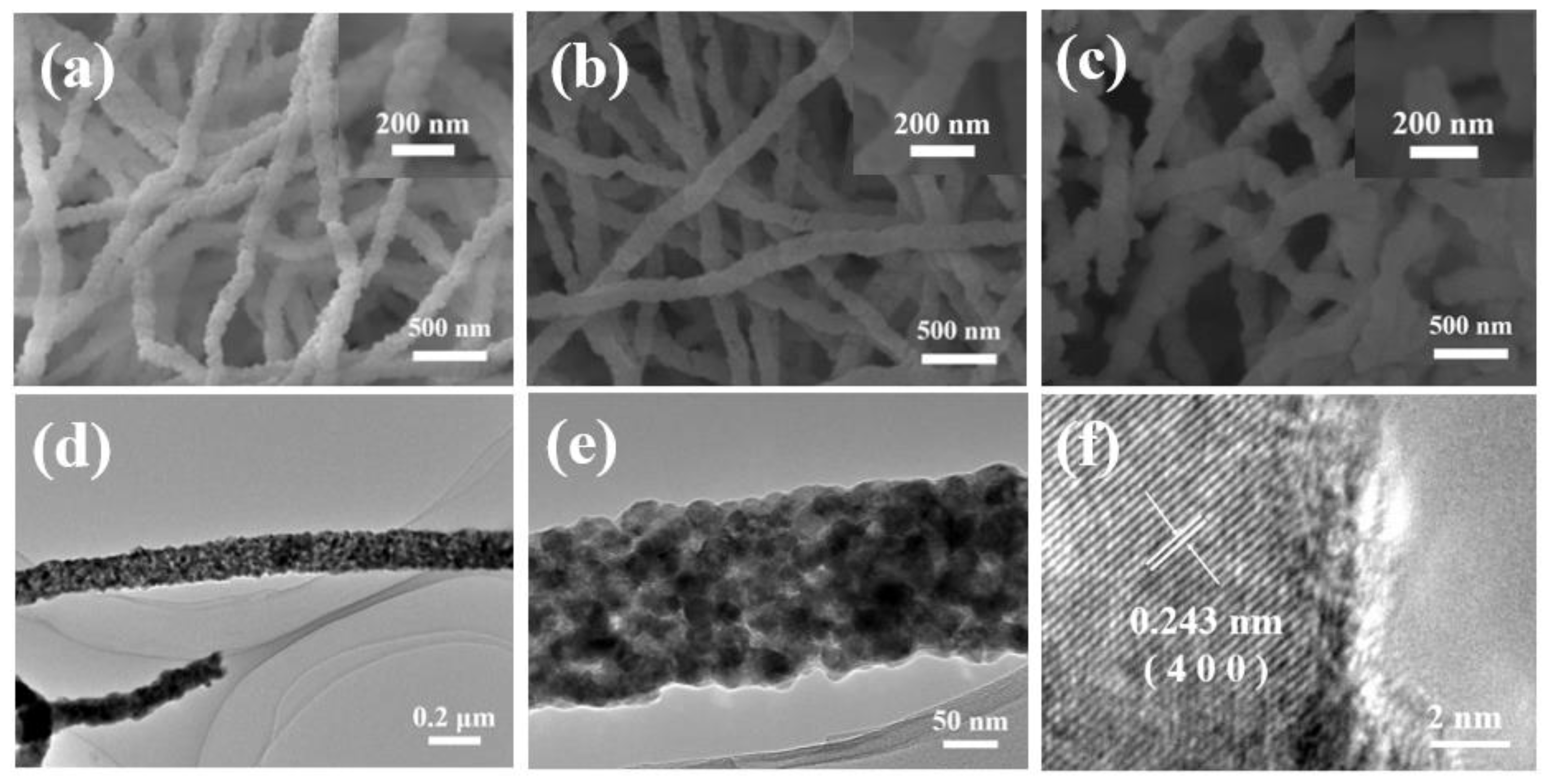
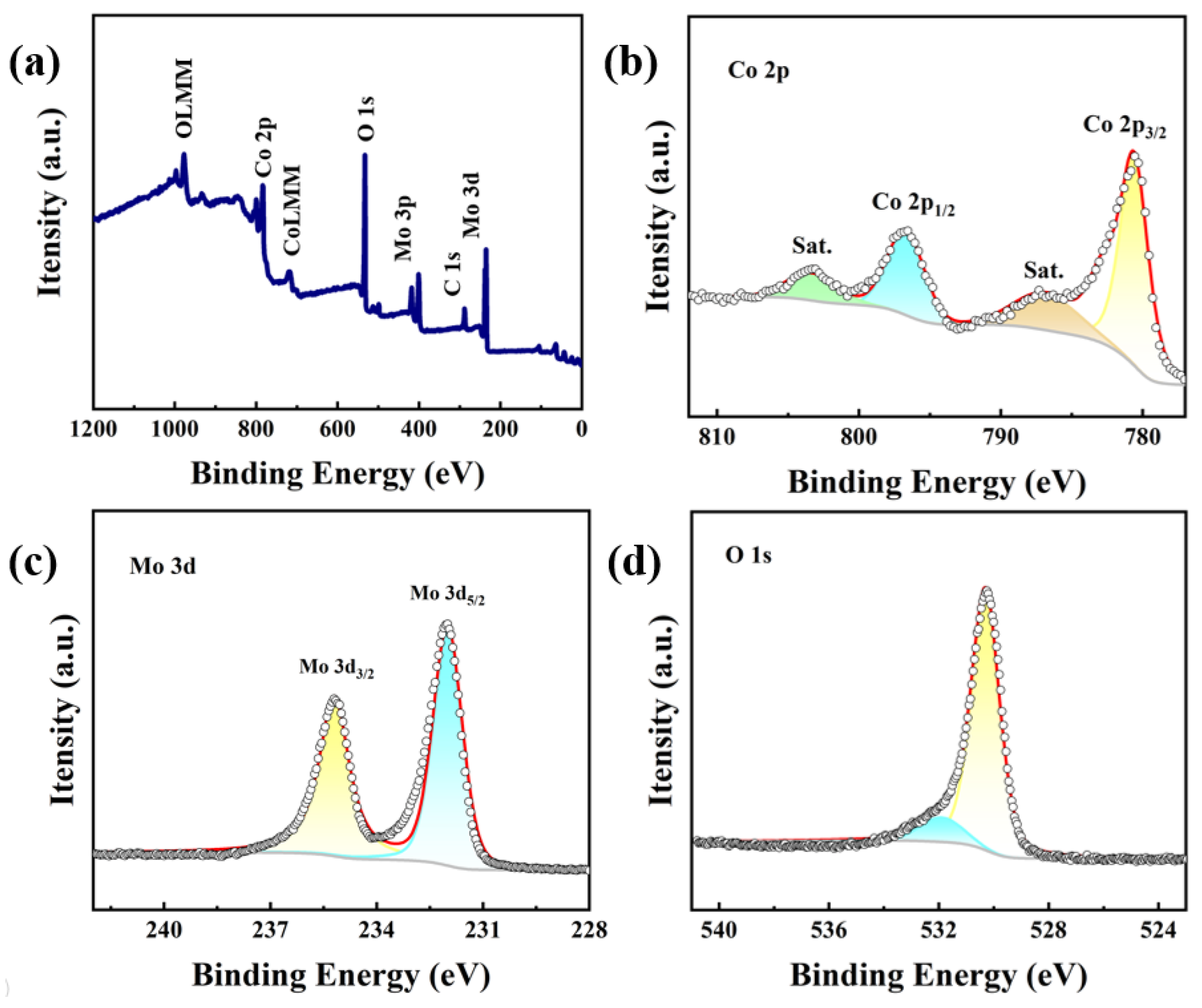
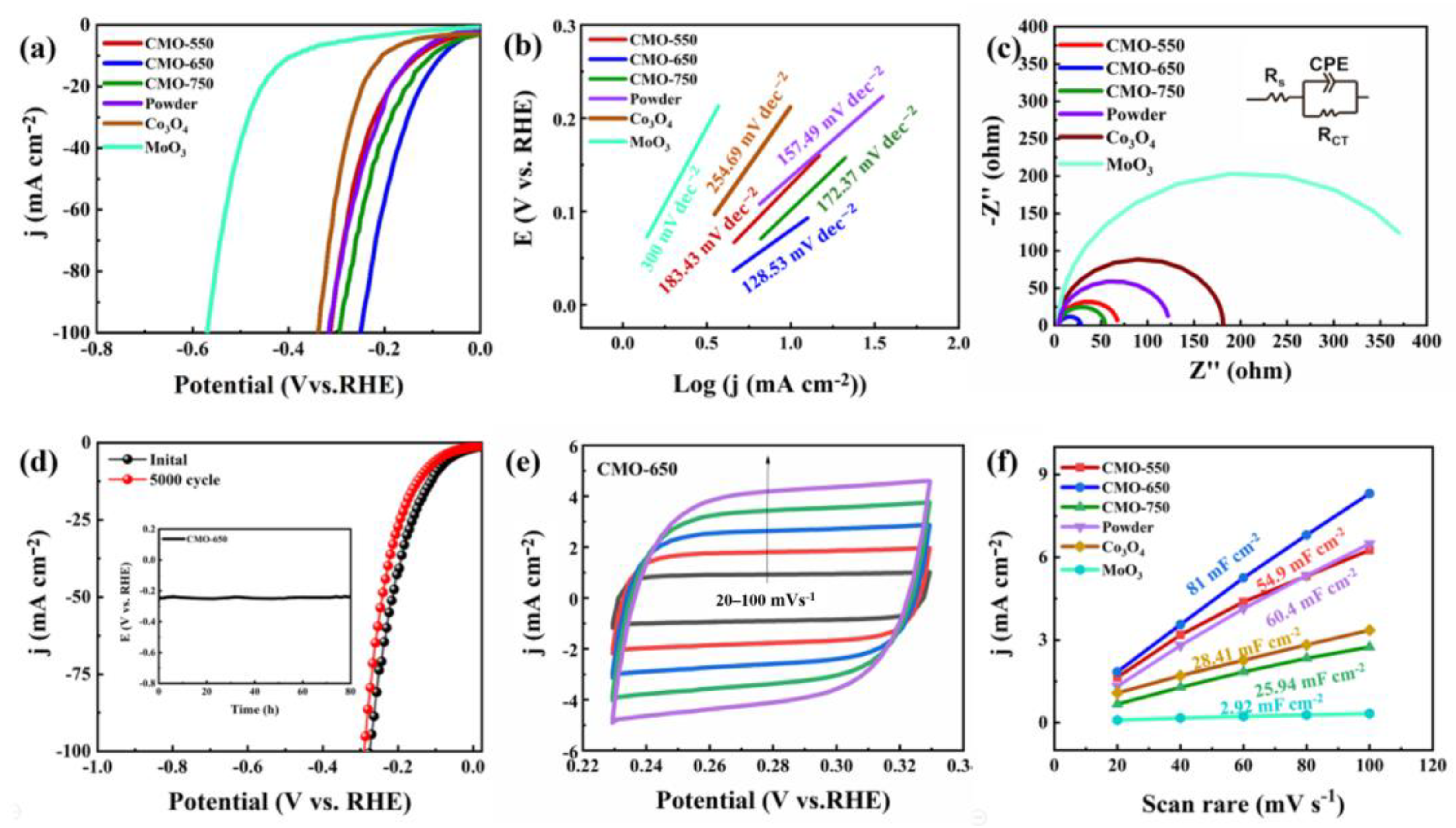
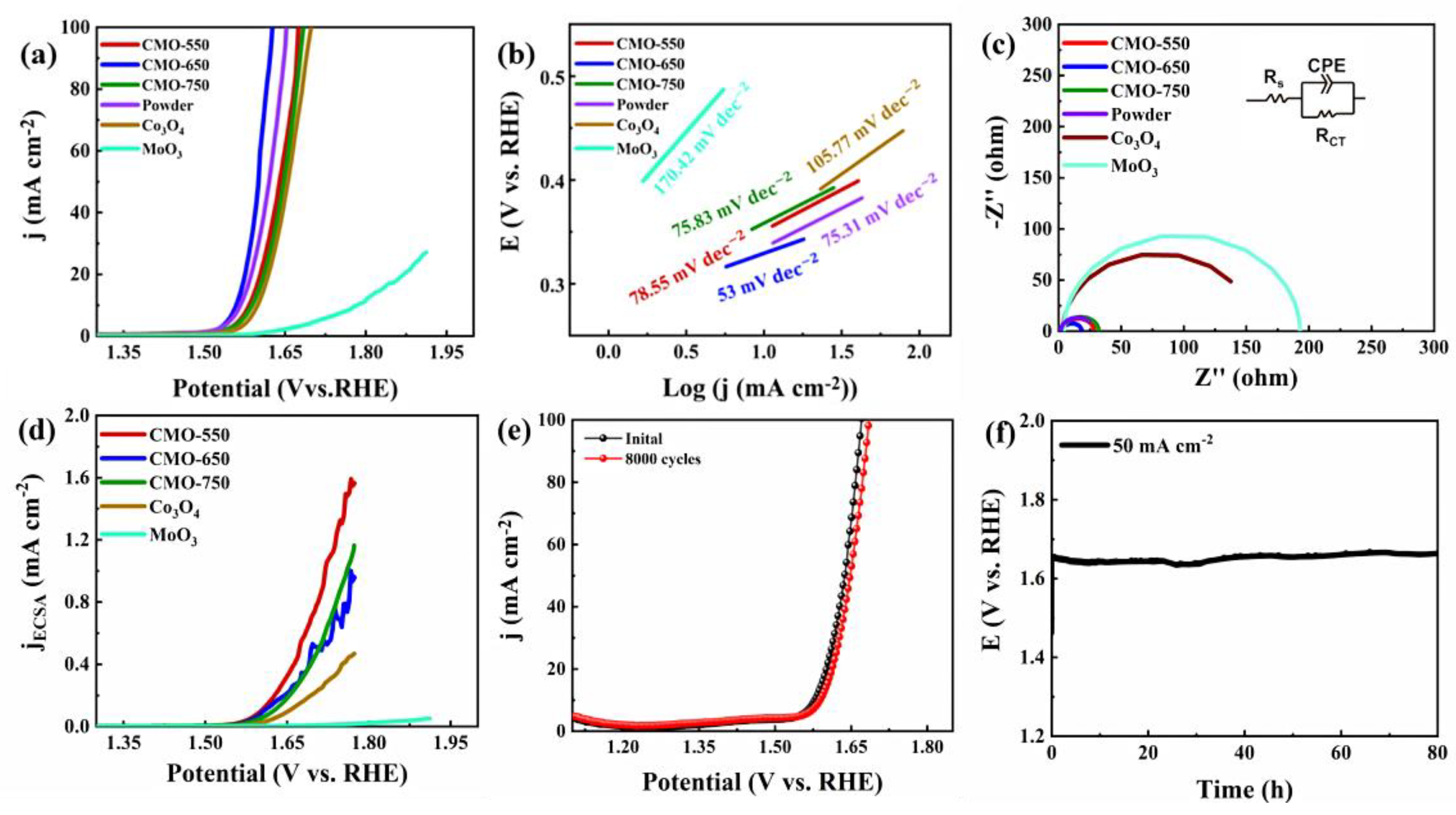
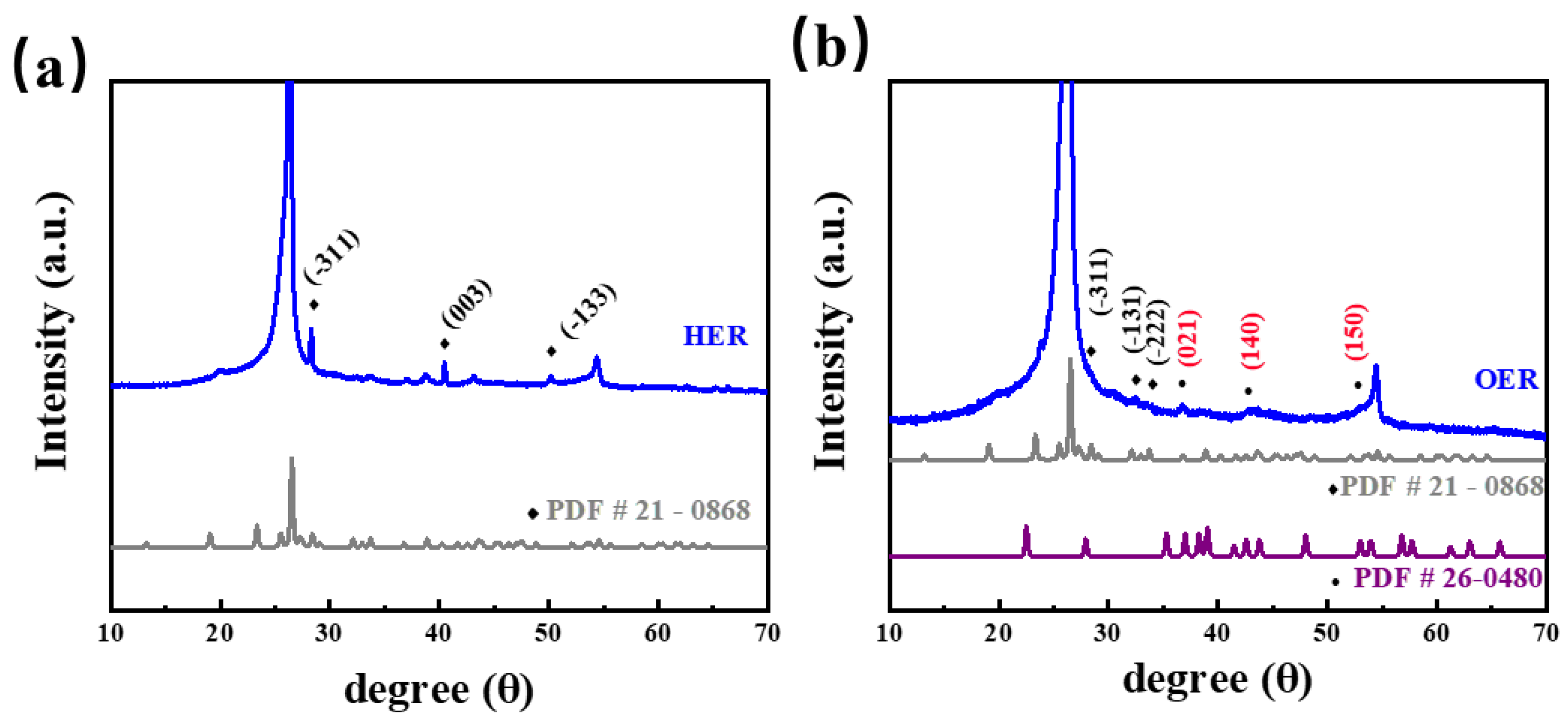
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Synthesis of CoMoO4 Nanofibers
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Guo, Z.; Liu, M.; Wang, Y.; Liu, H.; Wu, L.; Xue, Y.; Cai, N.; Li, H.; Yu, F. CoMoO4 Nanoparticles Decorated Ultrathin Nanoplates Constructed Porous Flower as an Electrocatalyst toward Overall Water Splitting and Zn-Air Batteries. Renew. Energy 2023, 212, 751–760. [Google Scholar] [CrossRef]
- Wang, R.; Li, F.; Ji, J.; Wang, F. CoMoO4 Enhanced Anodized Cobalt Oxide Nanotube as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2022, 579, 152128. [Google Scholar] [CrossRef]
- Zhang, Z.; Ran, J.; Fan, E.; Zhou, S.; Chai, D.-F.; Zhang, W.; Zhao, M.; Dong, G. Mesoporous CoMoO4 Hollow Tubes Derived from POMOFs as Efficient Electrocatalyst for Overall Water Splitting. J. Alloys Compd. 2023, 968, 172169. [Google Scholar] [CrossRef]
- Ma, X.; Wei, B.; Yuan, M.; Li, J.; Liang, S.; Wu, Y.; Dai, D.; Xu, L. Self-Supported Phosphorus-Doped CoMoO4 Rod Bundles for Efficient Hydrogen Evolution. J. Mater. Sci. 2020, 55, 6502–6512. [Google Scholar] [CrossRef]
- Chamani, S.; Sadeghi, E.; Unal, U.; Peighambardoust, N.S.; Aydemir, U. Tuning Electrochemical Hydrogen-Evolution Activity of CoMoO4 through Zn Incorporation. Catalysts 2023, 13, 798. [Google Scholar] [CrossRef]
- González, D.; Heras-Domingo, J.; Sodupe, M.; Rodríguez-Santiago, L.; Solans-Monfort, X. Importance of the Oxyl Character on the IrO2 Surface Dependent Catalytic Activity for the Oxygen Evolution Reaction. J. Catal. 2021, 396, 192–201. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, B.; Lin, Z.; Shen, S.; Xu, A.; Du, Z.; Chen, Y.; Zhong, W. In-Situ Surface Decoration of RuO2 Nanoparticles by Laser Ablation for Improved Oxygen Evolution Reaction Activity in Both Acid and Alkali Solutions. J. Energy Chem. 2021, 54, 510–518. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast Room-Temperature Synthesis of Porous S-Doped Ni/Fe (Oxy)Hydroxide Electrodes for Oxygen Evolution Catalysis in Seawater Splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon Nanotubes Decorated with CoP Nanocrystals: A Highly Active Non-Noble-Metal Nanohybrid Electrocatalyst for Hydrogen Evolution. Angew. Chem. Int. Ed. 2014, 53, 6710–6714. [Google Scholar] [CrossRef]
- Wang, J.; Gao, D.; Wang, G.; Miao, S.; Wu, H.; Li, J.; Bao, X. Cobalt Nanoparticles Encapsulated in Nitrogen-Doped Carbon as a Bifunctional Catalyst for Water Electrolysis. J. Mater. Chem. A 2014, 2, 20067–20074. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Chen, Z.; Lv, C.; Humphrey, M.G.; Zhang, C. Cobalt Phosphide Nanorods as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Nano Energy 2014, 9, 373–382. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmeková, E.; Asefa, T. Cobalt-Embedded Nitrogen-Rich Carbon Nanotubes Efficiently Catalyze Hydrogen Evolution Reaction at All pH Values. Angew. Chem. Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tian, L.; Atkins, S.; Liu, Y.; Murowchick, J.; Chen, X. Converting CoMoO4 into CoO/MoOx for Overall Water Splitting by Hydrogenation. ACS Sustain. Chem. Eng. 2016, 4, 3743–3749. [Google Scholar] [CrossRef]
- Peng, Z.; Jia, D.; Al-Enizi, A.M.; Elzatahry, A.A.; Zheng, G. From Water Oxidation to Reduction: Homologous Ni–Co Based Nanowires as Complementary Water Splitting Electrocatalysts. Adv. Energy Mater. 2015, 5, 1402031. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y. In Situ Cobalt–Cobalt Oxide/N-Doped Carbon Hybrids as Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal–Organic Framework-Based CoP/Reduced Graphene Oxide: High-Performance Bifunctional Electrocatalyst for Overall Water Splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Liu, Z.; Amin, H.M.A.; Peng, Y.; Corva, M.; Pentcheva, R.; Tschulik, K. Facet-Dependent Intrinsic Activity of Single Co3O4 Nanoparticles for Oxygen Evolution Reaction. Adv. Funct. Mater. 2022, 33, 2210945. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Barik, S.; Kharabe, G.P.; Illathvalappil, R.; Singh, C.P.; Kanheerampockil, F.; Walko, P.S.; Bhat, S.K.; Devi, R.N.; Vinod, C.P.; Krishnamurty, S.; et al. Active Site Engineering and Theoretical Aspects of “Superhydrophilic” Nanostructure Array Enabling Efficient Overall Water Electrolysis. Small 2023, 19, 2304143. [Google Scholar] [CrossRef]
- Xie, W.; Yu, T.; Ou, Z.; Zhang, J.; Li, R.; Song, S.; Wang, Y. Self-Supporting Clusters Constituted of Nitrogen-Doped CoMoO4 Nanosheets for Efficiently Catalyzing the Hydrogen Evolution Reaction in Alkaline Media. ACS Sustain. Chem. Eng. 2020, 8, 9070–9078. [Google Scholar] [CrossRef]
- Wang, J.; Xuan, H.; Meng, L.; Yang, J.; Yang, J.; Liang, X.; Li, Y.; Han, P. Facile Synthesis of N, S Co-Doped CoMoO4 Nanosheets as High-Efficiency Electrocatalysts for Hydrogen Evolution Reaction. Ionics 2022, 28, 4685–4695. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Li, Y.; Ma, F.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B.; et al. Anion-Modulation in CoMoO4 Electrocatalyst for Urea-Assisted Energy-Saving Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 33167–33176. [Google Scholar] [CrossRef]
- Zang, M.; Xu, N.; Cao, G.; Chen, Z.; Cui, J.; Gan, L.; Dai, H.; Yang, X.; Wang, P. Cobalt Molybdenum Oxide Derived High-Performance Electrocatalyst for the Hydrogen Evolution Reaction. ACS Catal. 2018, 8, 5062–5069. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, S.; Tong, Y.; Dong, X. Dual-Anions Engineering of Bimetallic Oxides as Highly Active Electrocatalyst for Boosted Overall Water Splitting. J. Colloid Interface Sci. 2022, 623, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Cheng, G.; Ruan, W.; Ma, B.; Yuan, X.; Zhang, X.; Li, Z.; Teng, Y.; Wang, L.; Teng, F. Efficient Hydrogen Production in an Innovative S-Doped CoMoO4-Based Electrolytic Cell: 12.97% Less Energy Consumption. Sustain. Mater. Technol. 2023, 37, e00665. [Google Scholar] [CrossRef]
- Xu, J.; Gu, S.; Fan, L.; Xu, P.; Lu, B. Electrospun Lotus Root-like CoMoO4@Graphene Nanofibers as High-Performance Anode for Lithium Ion Batteries. Electrochim. Acta 2016, 196, 125–130. [Google Scholar] [CrossRef]
- Xie, S.; Wang, H.; Yao, T.; Wang, J.; Wang, C.; Shi, J.-W.; Han, X.; Liu, T.; Cheng, Y. Embedding CoMoO4 Nanoparticles into Porous Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance. J. Colloid Interface Sci. 2019, 553, 320–327. [Google Scholar] [CrossRef]
- Li, F.; Xiao, F.; Yao, T.; Zhu, L.; Liu, T.; Lu, H.; Qian, R.; Liu, Y.; Han, X.; Wang, H. Selenizing CoMoO4 Nanoparticles within Electrospun Carbon Nanofibers towards Enhanced Sodium Storage Performance. J. Colloid Interface Sci. 2021, 586, 663–672. [Google Scholar] [CrossRef]
- Chang, L.; Chen, S.; Fei, Y.; Stacchiola, D.J.; Hu, Y.H. Superstructured NiMoO4 @CoMoO4 Core-Shell Nanofibers for Supercapacitors with Ultrahigh Areal Capacitance. Proc. Natl. Acad. Sci. USA 2023, 120, e2219950120. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, J.; Tian, W.; Hu, Z.; Lv, X.; Zhang, H.; Yue, H.; Zhang, Y.; Ji, J.; Jiang, W. Morphology-Controlled Synthesis of CoMoO4 Nanoarchitectures Anchored on Carbon Cloth for High-Efficiency Oxygen Oxidation Reaction. RSC Adv. 2019, 9, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ren, X.; Ma, H.; Sun, X.; Zhang, Y.; Yan, T.; Wei, Q.; Wu, D. Synthesis of Self-Supported Amorphous CoMoO4 Nanowire Array for Highly Efficient Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10093–10098. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Bondue, C.J.; Eswara, S.; Kaiser, U.; Baltruschat, H. A Carbon-Free Ag–Co3O4 Composite as a Bifunctional Catalyst for Oxygen Reduction and Evolution: Spectroscopic, Microscopic and Electrochemical Characterization. Electrocatalysis 2017, 8, 540–553. [Google Scholar] [CrossRef]
- Zhao, S.; Berry-Gair, J.; Li, W.; Guan, G.; Yang, M.; Li, J.; Lai, F.; Corà, F.; Holt, K.; Brett, D.J.L.; et al. Hydrogen Evolution: The Role of Phosphate Group in Doped Cobalt Molybdate: Improved Electrocatalytic Hydrogen Evolution Performance (Adv. Sci. 12/2020). Adv. Sci. 2020, 7, 2070067. [Google Scholar] [CrossRef]
- Geng, S.; Chen, L.; Chen, H.; Wang, Y.; Ding, Z.-B.; Cai, D.; Song, S. Revealing the Electrocatalytic Mechanism of Layered Crystalline CoMoO4 for Water Splitting: A Theoretical Study from Facet Selecting to Active Site Engineering. Chin. J. Catal. 2023, 50, 334–342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Chang, X.; Li, L.; Zhang, M. Synthesis of CoMoO4 Nanofibers by Electrospinning as Efficient Electrocatalyst for Overall Water Splitting. Molecules 2024, 29, 7. https://doi.org/10.3390/molecules29010007
Fan J, Chang X, Li L, Zhang M. Synthesis of CoMoO4 Nanofibers by Electrospinning as Efficient Electrocatalyst for Overall Water Splitting. Molecules. 2024; 29(1):7. https://doi.org/10.3390/molecules29010007
Chicago/Turabian StyleFan, Jiahui, Xin Chang, Lu Li, and Mingyi Zhang. 2024. "Synthesis of CoMoO4 Nanofibers by Electrospinning as Efficient Electrocatalyst for Overall Water Splitting" Molecules 29, no. 1: 7. https://doi.org/10.3390/molecules29010007
APA StyleFan, J., Chang, X., Li, L., & Zhang, M. (2024). Synthesis of CoMoO4 Nanofibers by Electrospinning as Efficient Electrocatalyst for Overall Water Splitting. Molecules, 29(1), 7. https://doi.org/10.3390/molecules29010007







